Impact of Microkeratome Dissection Parameters on Textural Interface Opacities in DSAEK Grafts
Abstract
1. Introduction
2. Materials and Methods
2.1. DSAEK Preparation
2.2. Data Analysis
2.3. Statistical Analysis
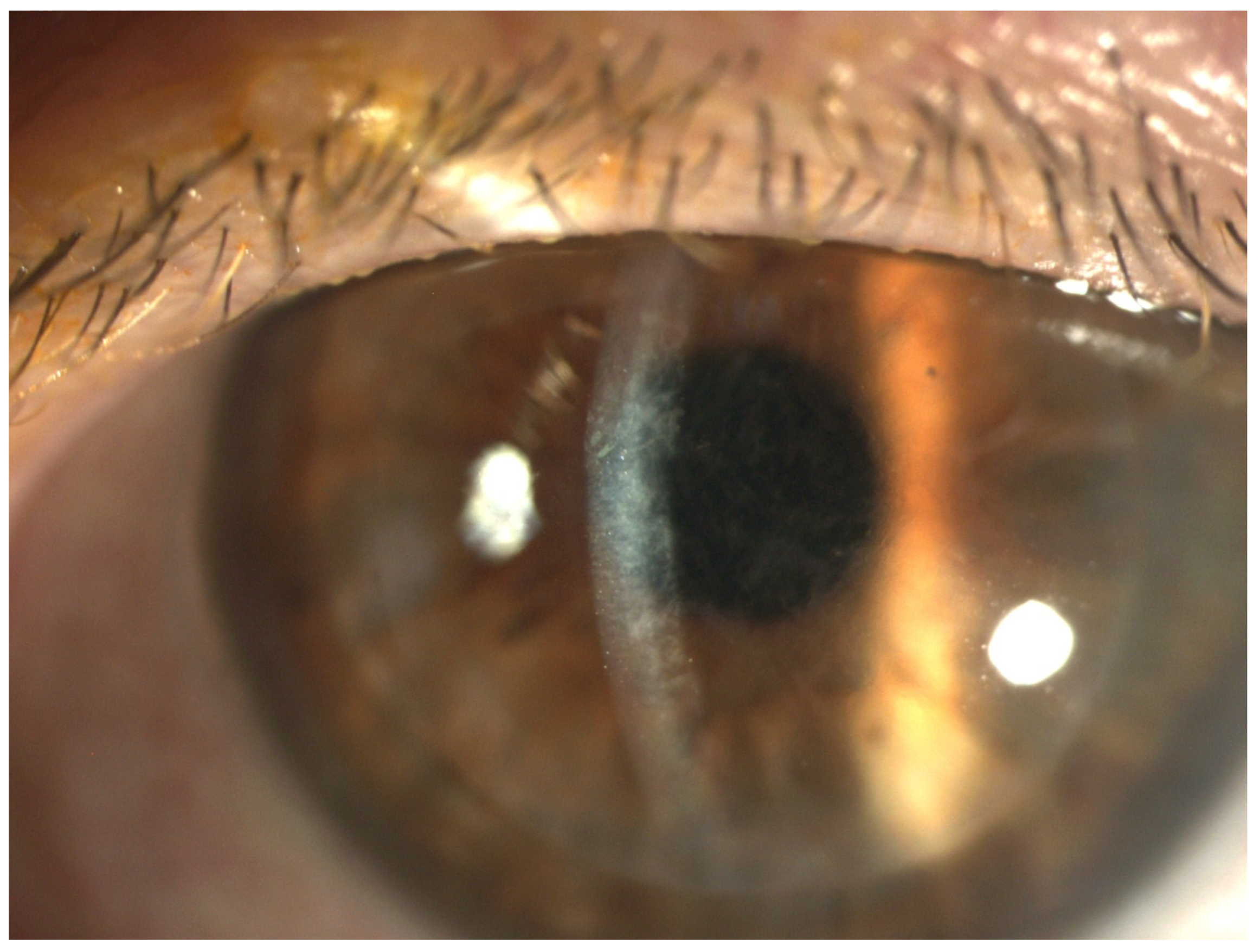
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Meter, W.; Mathews, P.; Philippy, B.; DeMatteo, J. 2023 Eye Banking Statistical Report—Executive Summary. Eye Bank. Corneal Transplant. 2024, 3, e0034. [Google Scholar] [CrossRef]
- Ruzza, A.; Parekh, M.; Avoni, L.; Wojcik, G.; Ferrari, S.; Desneux, L.; Ponzin, D.; Levis, H.J.; Romano, V. Ultra-Thin DSAEK Using an Innovative Artificial Anterior Chamber Pressuriser: A Proof-of-Concept Study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Droutsas, K.; Petrelli, M.; Miltsakakis, D.; Andreanos, K.; Karagianni, A.; Lazaridis, A.; Koutsandrea, C.; Kymionis, G. Visual Outcomes of Ultrathin-Descemet Stripping Endothelial Keratoplasty versus Descemet Stripping Endothelial Keratoplasty. J. Ophthalmol. 2018, 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Busin, M.; Patel, A.K.; Scorcia, V.; Ponzin, D. Microkeratome-Assisted Preparation of Ultrathin Grafts for Descemet Stripping Automated Endothelial Keratoplasty. Investig. Opthalmol. Vis. Sci. 2012, 53, 521. [Google Scholar] [CrossRef]
- Dickman, M.M.; Kruit, P.J.; Remeijer, L.; van Rooij, J.; Van der Lelij, A.; Wijdh, R.H.J.; van den Biggelaar, F.J.H.M.; Berendschot, T.T.J.M.; Nuijts, R.M.M.A. A Randomized Multicenter Clinical Trial of Ultrathin Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) versus DSAEK. Ophthalmology 2016, 123, 2276–2284. [Google Scholar] [CrossRef]
- Clerici, R.; Ceccuzzi, R.; Fausto, R.; Tinelli, C.; Di Palma, M.R.; Mantegna, G.; Riva, I.; Busin, M.; De Angelis, G.; Quaranta, L. Single-Pass Mikrokeratome and Anterior Chamber Pressurizer for the Ultrathin Descemet-Stripping Automated Endothelial Keratoplasty Graft Preparation. Cornea 2021, 40, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.B.; Jacobs, D.S.; Musch, D.C.; Kaufman, S.C.; Reinhart, W.J.; Shtein, R.M. Descemet’s Stripping Endothelial Keratoplasty: Safety and Outcomes. Ophthalmology 2009, 116, 1818–1830. [Google Scholar] [CrossRef]
- Kim, K.; Alder, B.; Vora, G.K.; Carlson, A.N.; Afshari, N.A.; Kuo, A.N.; Kim, T. Textural Interface Opacity after Descemet-Stripping Automated Endothelial Keratoplasty. J. Cataract. Refract. Surg. 2014, 40, 1514–1520. [Google Scholar] [CrossRef]
- Vira, S.; Shih, C.Y.; Ragusa, N.; Sheyman, A.; Feder, R.; Weisenthal, R.W.; Rosenwasser, G.O.D.; Hannush, S.B.; Udell, I.J.; Bouchard, C.S. Textural Interface Opacity After Descemet Stripping Automated Endothelial Keratoplasty. Cornea 2013, 32, e54–e59. [Google Scholar] [CrossRef]
- Kymionis, G.; Voulgari, N.; Kontadakis, G.; Mikropoulos, D.; Petrovic, A.; Droutsas, K. Surgical Management of Post-Descemet Stripping Automated Endothelial Keratoplasty Interface Haze Associated with Interface Deposits. Indian J. Ophthalmol. 2020, 68, 174. [Google Scholar] [CrossRef]
- Kontadakis, G.A.; Palioura, S.; Yoo, S.H. Wavelike Interface Opacities After Descemet-Stripping Automated Endothelial Keratoplasty: 7-Year Follow-Up. Eye Contact Lens Sci. Clin. Pract. 2017, 43, e13–e15. [Google Scholar] [CrossRef]
- Juthani, V.V.; Goshe, J.M.; Srivastava, S.K.; Ehlers, J.P. Association Between Transient Interface Fluid on Intraoperative OCT and Textural Interface Opacity After DSAEK Surgery in the PIONEER Study. Cornea 2014, 33, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.R.; Rosenwasser, G.O.D.; Dubovy, S.R.; Matthews, J.L. Clinicopathologic Correlation of Textural Interface Opacities in Descemet Stripping Automated Endothelial Keratoplasty. Cornea 2014, 33, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Hirst, L.W.; Vandeleur, K.W. Laser in Situ Keratomileusis Interface Deposits. J. Refract. Surg. 1998, 14, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Desautels, J.D.; Quist, T.S.; Skanchy, D.F.; Williams, M.T.; Wallace, R.T. Rainbow Glare after Laser-Assisted in Situ Keratomileusis: A Review of Literature. Clin. Ophthalmol. 2016, 10, 2245–2249. [Google Scholar] [CrossRef]
- Ivarsen, A.; Thøgersen, J.; Keiding, S.R.; Hjortdal, J.Ø.; Møller-Pedersen, T. Plastic Particles at the LASIK Interface. Ophthalmology 2004, 111, 18–23. [Google Scholar] [CrossRef][Green Version]
- Kymionis, G.D.; Kounis, G.A.; Grentzelos, M.A.; Panagopoulou, S.I.; Kandarakis, S.A.; Krasia, M.S. Interface Corneal Stromal Irregularities after Flap Creation Using Femtosecond Laser. Eur. J. Ophthalmol. 2011, 21, 207–209. [Google Scholar] [CrossRef]
- Patel, S.V.; Maguire, L.J.; McLaren, J.W.; Hodge, D.O.; Bourne, W.M. Femtosecond Laser versus Mechanical Microkeratome for LASIK. Ophthalmology 2007, 114, 1482–1490. [Google Scholar] [CrossRef]
- Freidank, S.; Vogel, A.; Linz, N. Mechanisms of Corneal Intrastromal Laser Dissection for Refractive Surgery: Ultra-High-Speed Photographic Investigation at up to 50 Million Frames per Second. Biomed. Opt. Express 2022, 13, 3056. [Google Scholar] [CrossRef]
- Vajpayee, R.B.; Maharana, P.K.; Jain, S.; Sharma, N.; Jhanji, V. Thin Lenticule <scp>D</Scp> Escemet’s Stripping Automated Endothelial Keratoplasty: Single, Slow Pass Technique. Clin. Exp. Ophthalmol. 2014, 42, 411–416. [Google Scholar] [CrossRef]
- Eye Bank Association of America Medical Standards 2023. Eye Bank. Corneal Transplant. 2024, 3, e0027. [CrossRef]
- Chatzea, M.S.; Kymionis, G.D.; Vakalopoulos, D.G.; O’Brien, R.C.; Mora, D.; Llanes, K.; Fout, E.; Buras, W.; Triglia, C.; Tonk, R.S.; et al. Screening and Grading of Textural Interface Opacities in DSAEK Grafts with the M-TIO Scale for Predicting Visual Outcomes. Diagnostics 2025, 15, 1241. [Google Scholar] [CrossRef]
- Wang, Q.; Stoakes, I.M.; Moshirfar, M.; Harvey, D.H.; Hoopes, P.C. Assessment of Pupil Size and Angle Kappa in Refractive Surgery: A Population-Based Epidemiological Study in Predominantly American Caucasians. Cureus 2023, 15, e43998. [Google Scholar] [CrossRef] [PubMed]
- Duggan, M.J.; Rose-Nussbaumer, J.; Lin, C.C.; Austin, A.; Labadzinzki, P.C.; Chamberlain, W.D. Corneal Higher-Order Aberrations in Descemet Membrane Endothelial Keratoplasty versus Ultrathin DSAEK in the Descemet Endothelial Thickness Comparison Trial. Ophthalmology 2019, 126, 946–957. [Google Scholar] [CrossRef]
- Sami Khan, F.; Khalid, M. Examining Higher Order Aberration in Eyes after DSEK. Punjab Univ. J. Math. 2022, 54, 89–101. [Google Scholar] [CrossRef]
- Chaudhury, P.; Singh, P. Role of Pin Hole in Rapid Recognition of Refractive Error as Cause of Diminished Vision. J. Evol. Med. Dent. Sci. 2022, 11, 452–454. [Google Scholar] [CrossRef]
- Melki, S.A.; Safar, A.; Martin, J.; Ivanova, A.; Adi, M. Potential Acuity Pinhole. Ophthalmology 1999, 106, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Narang, P.; Agarwal, A.; Kumar, D.A.; Agarwal, A. Pinhole Pupilloplasty: Small-Aperture Optics for Higher-Order Corneal Aberrations. J. Cataract. Refract. Surg. 2019, 45, 539–543. [Google Scholar] [CrossRef]
- de Freitas Valbon, B.; Ventura, M.P.; da Silva, R.S.; Canedo, A.L.; Velarde, G.C.; Ambrósio, R. Central Corneal Thickness and Biomechanical Changes After Clear Corneal Phacoemulsification. J. Refract. Surg. 2012, 28, 215–219. [Google Scholar] [CrossRef]

| M-TIO Grade | ||||
|---|---|---|---|---|
| Variable, mean ± SD | 0 | 1 | 2 | 3 |
| Pre-cut graft thickness (µm) | 502 ± 54 | 516 ± 61 | 531 ± 63 | 552 ± 78 |
| Post-cut DSAEK lenticule thickness (µm) | 91 ± 19 | 88 ± 19 | 87 ± 16 | 84 ± 16 |
| Difference between the pre-cut graft thickness and post-cut DSAEK lenticule thickness (µm) | 412 ± 57 | 428 ± 62 | 444 ± 62 | 468 ± 78 |
| N | 76 | 224 | 97 | 25 |
| Ordinal Logistic Regression Model | |||
|---|---|---|---|
| N = 422 | Proportional OR (99% CI) | p-Value | |
| Pre-cut Donor Cornea Thickness (µm) | <0.001 | ||
| 1.35 (1.10, 1.67) per 50 µm | |||
| Post-cut DSAEK lenticule thickness (µm) | 0.08 | ||
| 0.67 (0.36, 1.22) per 50 µm | |||
| Difference Pre-cut graft thickness and the post-cut DSAEK lenticule thickness (µm) | <0.001 | ||
| 1.38 (1.13, 1.72) per 50 µm | |||
| Microkeratome head (µm) | <0.001 | ||
| 300 | 115 | 1.60 (0.26, 6.77) | |
| 350 | 270 | 1.98 (0.33, 8.07) | |
| 400 | 20 | 11.73 (1.31, 131.24) | |
| 450 or 500 | 16 | 1 [ref] | |
| Dissection thickness vs. microkeratome head (µm) | <0.001 | ||
| 1.54 (1.22, 1.97) per 50 µm | |||
| Microkeratome | 0.51 | ||
| MORIA 1&2 | 131 | 1 [ref] | |
| MORIA 3&4 | 124 | 0.96 (0.50, 1.85) | |
| MORIA 5&6 | 112 | 1.08 (0.56, 2.05) | |
| MORIA 7&8 (LINEAR MODEL) | 52 | 1.53 (0.63, 4.15) | |
| Donor lens status | 0.67 | ||
| Phakic | 393 | 1 [ref] | |
| Pseudophakic | 25 | 1.31 (0.25, 6.30) | |
| Year | <0.001 | ||
| 2019 | 43 | 1 [ref] | |
| 2020 | 36 | 1.52 (0.35, 6.48) | |
| 2021 | 51 | 1.81 (0.61, 5.62) | |
| 2022 | 108 | 3.93 (1.38, 12.01) | |
| 2023 | 184 | 1.70 (0.67, 4.42) | |
| Ordinal Logistic Regression Model | |||
|---|---|---|---|
| N = 422 | Proportional OR (99% CI) | p-Value | |
| Difference between pre-cut graft thickness and post-cut DSAEK lenticule thickness (µm) | <0.001 | ||
| 1.57 (1.22, 2.06) per 50 µm | |||
| Microkeratome head (µm) | <0.001 | ||
| 300 | 115 | 6.95 (1.04, 36.60) | |
| 350 | 270 | 4.39 (0.76, 19.00) | |
| 400 | 20 | 18.86 (2.35, 175.91) | |
| 450 or 500 | 16 | 1 [ref] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzea, M.S.; Kymionis, G.D.; Vakalopoulos, D.G.; O’Brien, R.C.; Mora, D.; Llanes, K.; Fout, E.; Buras, W.; Triglia, C.; Tonk, R.S.; et al. Impact of Microkeratome Dissection Parameters on Textural Interface Opacities in DSAEK Grafts. Diagnostics 2025, 15, 1608. https://doi.org/10.3390/diagnostics15131608
Chatzea MS, Kymionis GD, Vakalopoulos DG, O’Brien RC, Mora D, Llanes K, Fout E, Buras W, Triglia C, Tonk RS, et al. Impact of Microkeratome Dissection Parameters on Textural Interface Opacities in DSAEK Grafts. Diagnostics. 2025; 15(13):1608. https://doi.org/10.3390/diagnostics15131608
Chicago/Turabian StyleChatzea, Marina S., George D. Kymionis, Dionysios G. Vakalopoulos, Robert C. O’Brien, Daniella Mora, Katrina Llanes, Elizabeth Fout, William Buras, Concetta Triglia, Rahul S. Tonk, and et al. 2025. "Impact of Microkeratome Dissection Parameters on Textural Interface Opacities in DSAEK Grafts" Diagnostics 15, no. 13: 1608. https://doi.org/10.3390/diagnostics15131608
APA StyleChatzea, M. S., Kymionis, G. D., Vakalopoulos, D. G., O’Brien, R. C., Mora, D., Llanes, K., Fout, E., Buras, W., Triglia, C., Tonk, R. S., & Yoo, S. H. (2025). Impact of Microkeratome Dissection Parameters on Textural Interface Opacities in DSAEK Grafts. Diagnostics, 15(13), 1608. https://doi.org/10.3390/diagnostics15131608






