Challenges in Diagnosing the Course of the Lingual Nerve for Clinical Practice and Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
- “Lingual nerve” AND “Course”;
- “Lingual nerve” AND “Anatomy”;
- “Lingual nerve” AND “Clinical implications”.
- Studies focusing on the course and anatomy of the lingual nerve;
- Studies published in English;
- Original research, systematic reviews, and meta-analyses.
- Case reports, letters to editors, and non-peer-reviewed articles;
- Studies focusing on surgical techniques without anatomical discussion;
- Articles not available in full text.
2.2. Data Extraction and Analysis
- Challenges in locating LN during clinical and surgical procedures;
- Methods used for anatomical guidance and diagnosis.
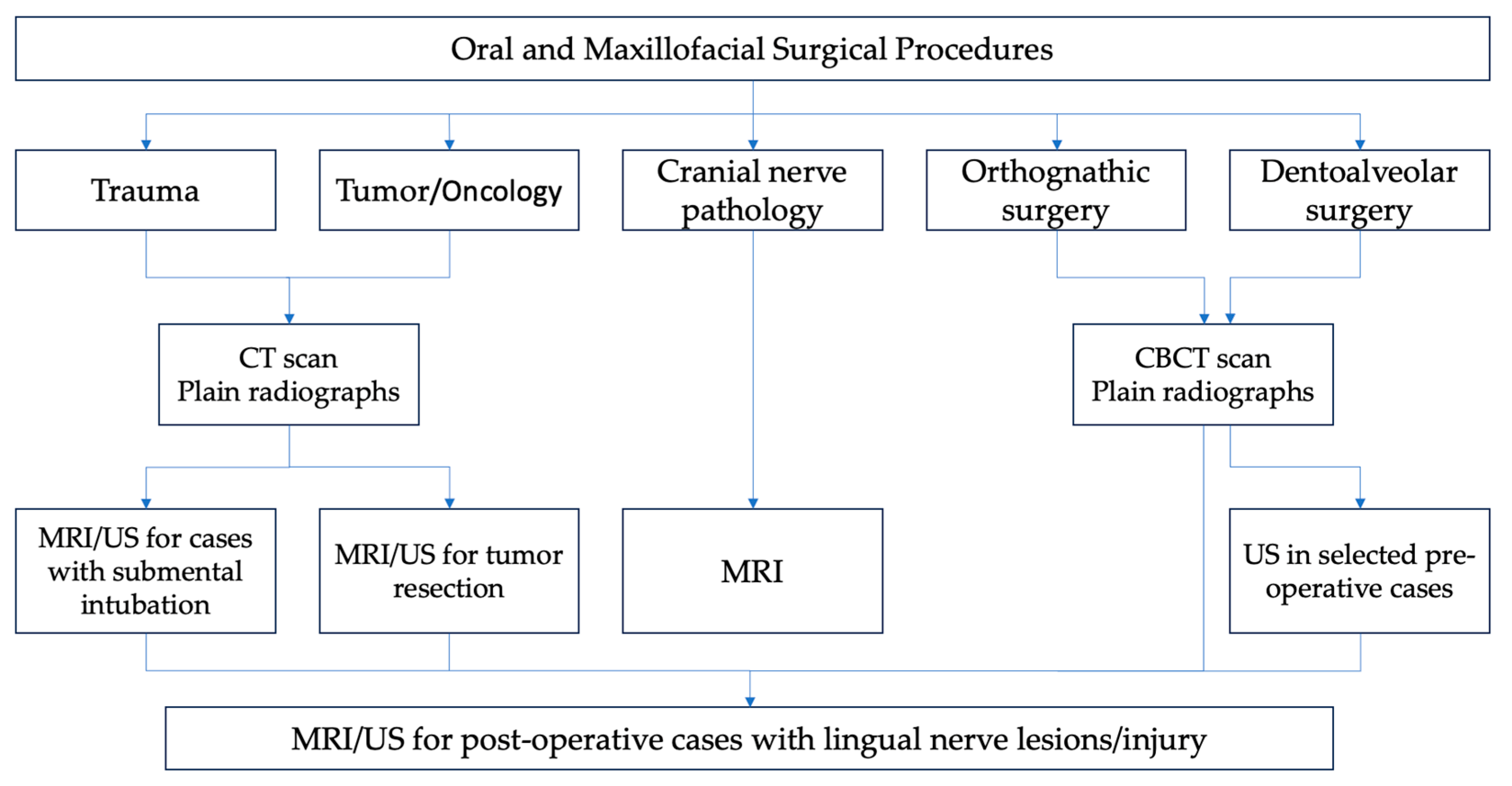
3. Results
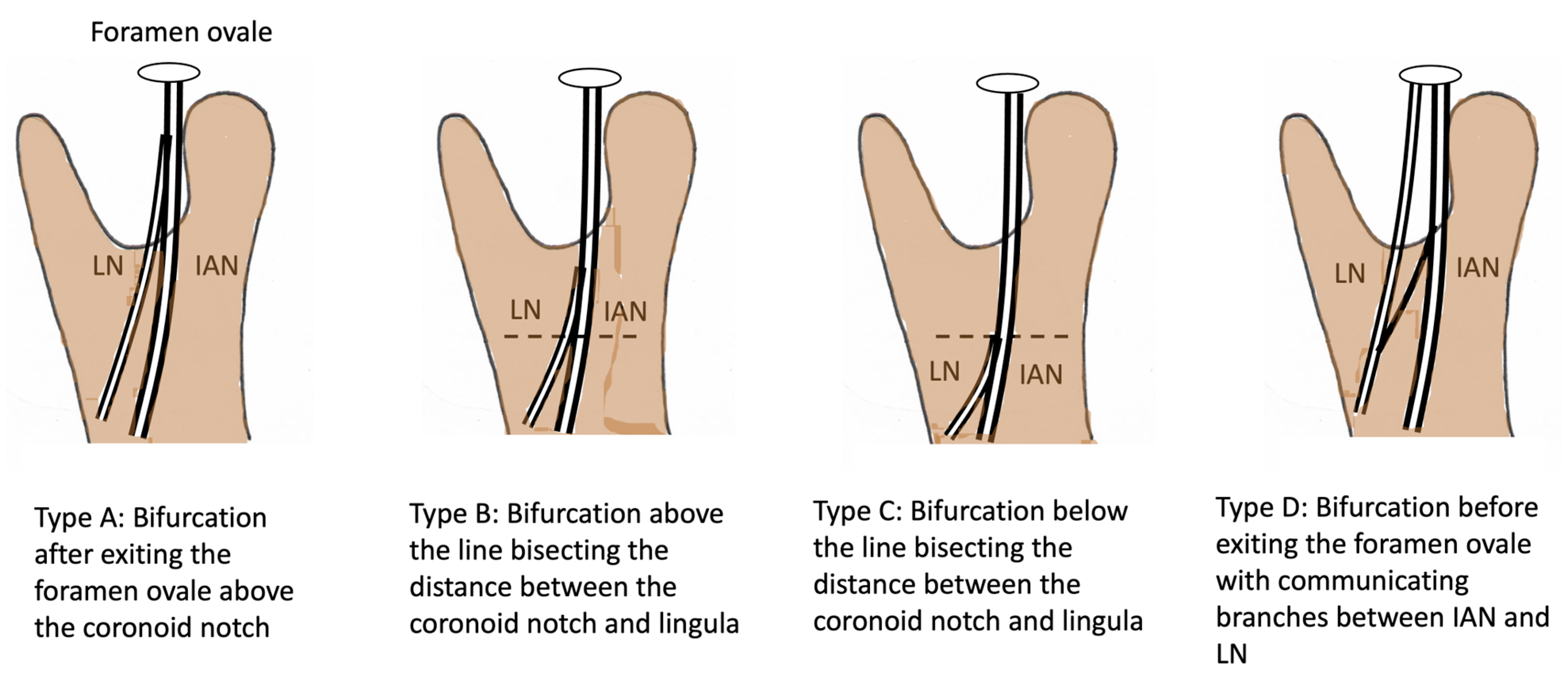
3.1. Challenges Associated with Landmark Identification at the Foramen Ovale
3.2. Challenges in Diagnosing the Lingual Nerve in the Infratemporal Fossa Region
3.3. Challenges in Diagnosing the Lingual Nerve in the Pteryomandibular Space
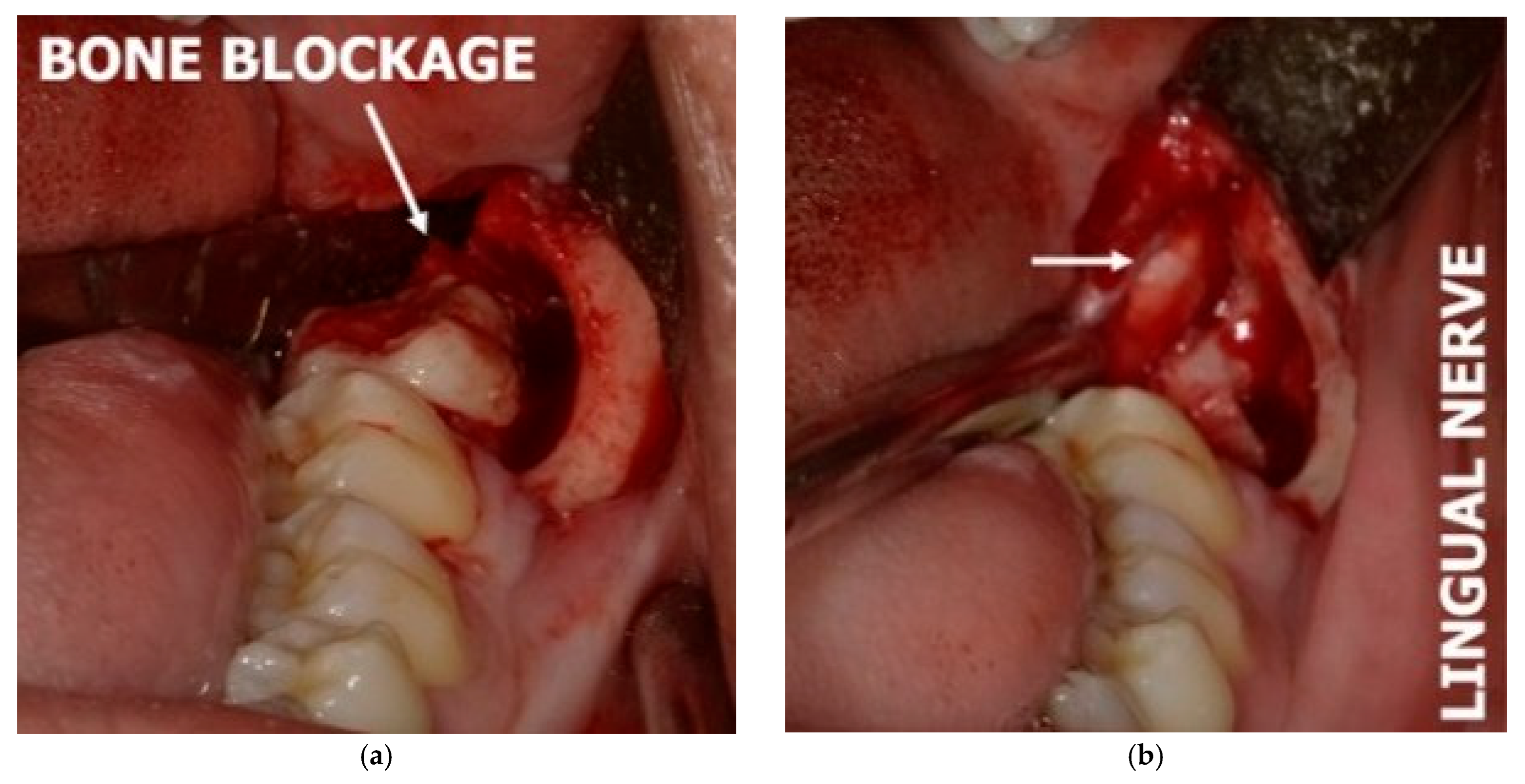
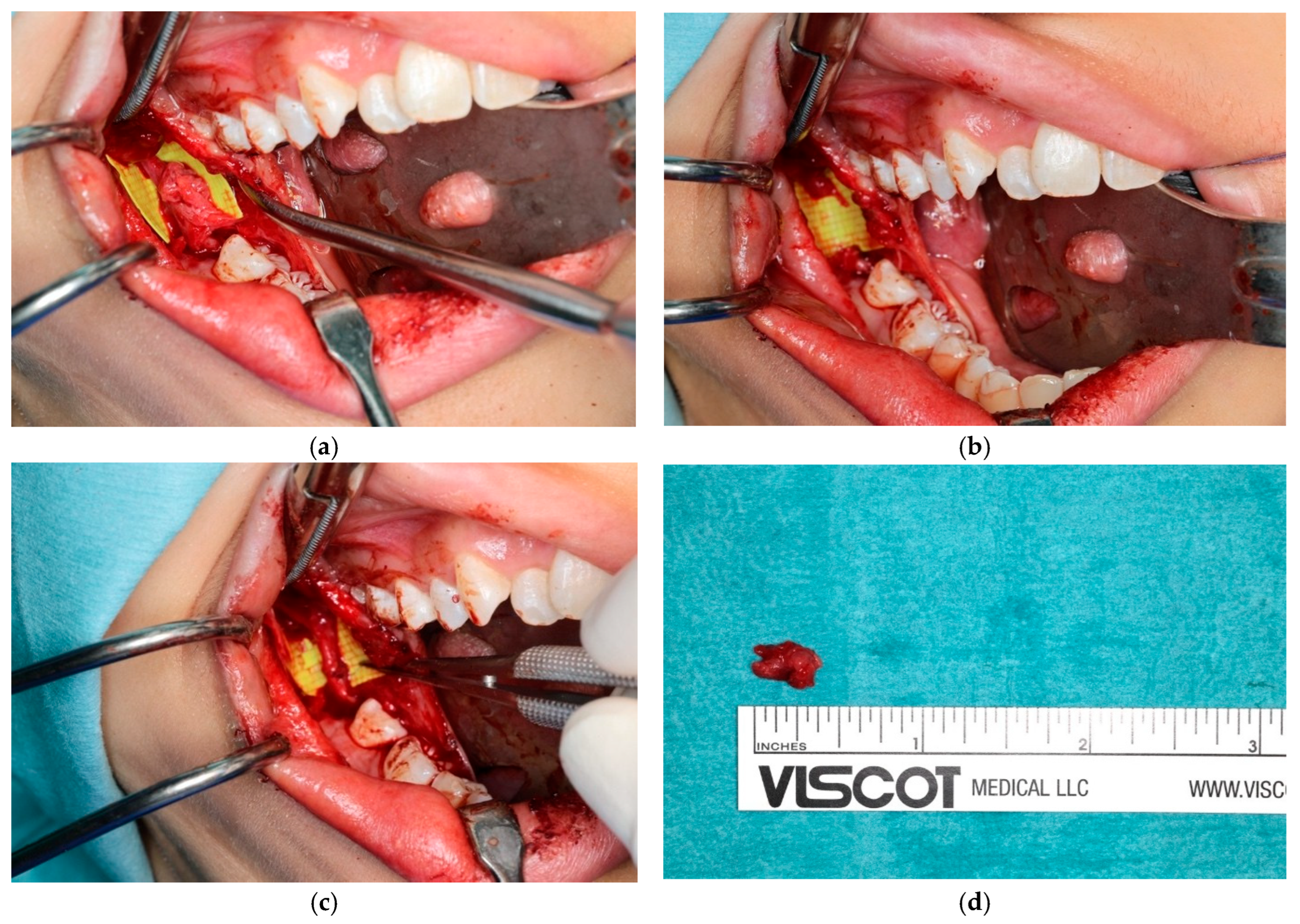
3.4. Challenges in Diagnosing the Lingual Nerve in the Third Molar and Retromolar Regions
3.5. Challenges in Diagnosing the Lingual Nerve in the Premolar/Molar Region
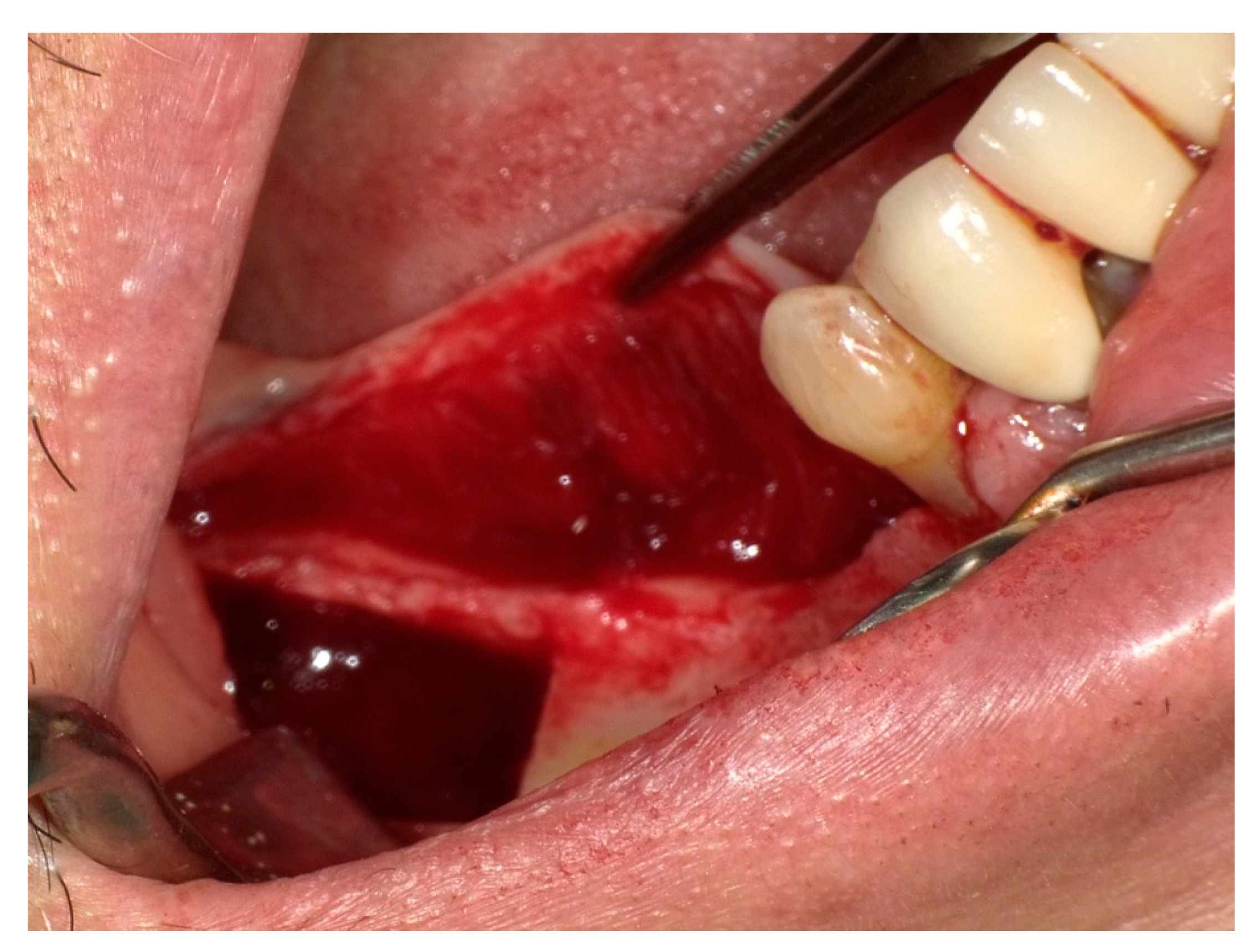
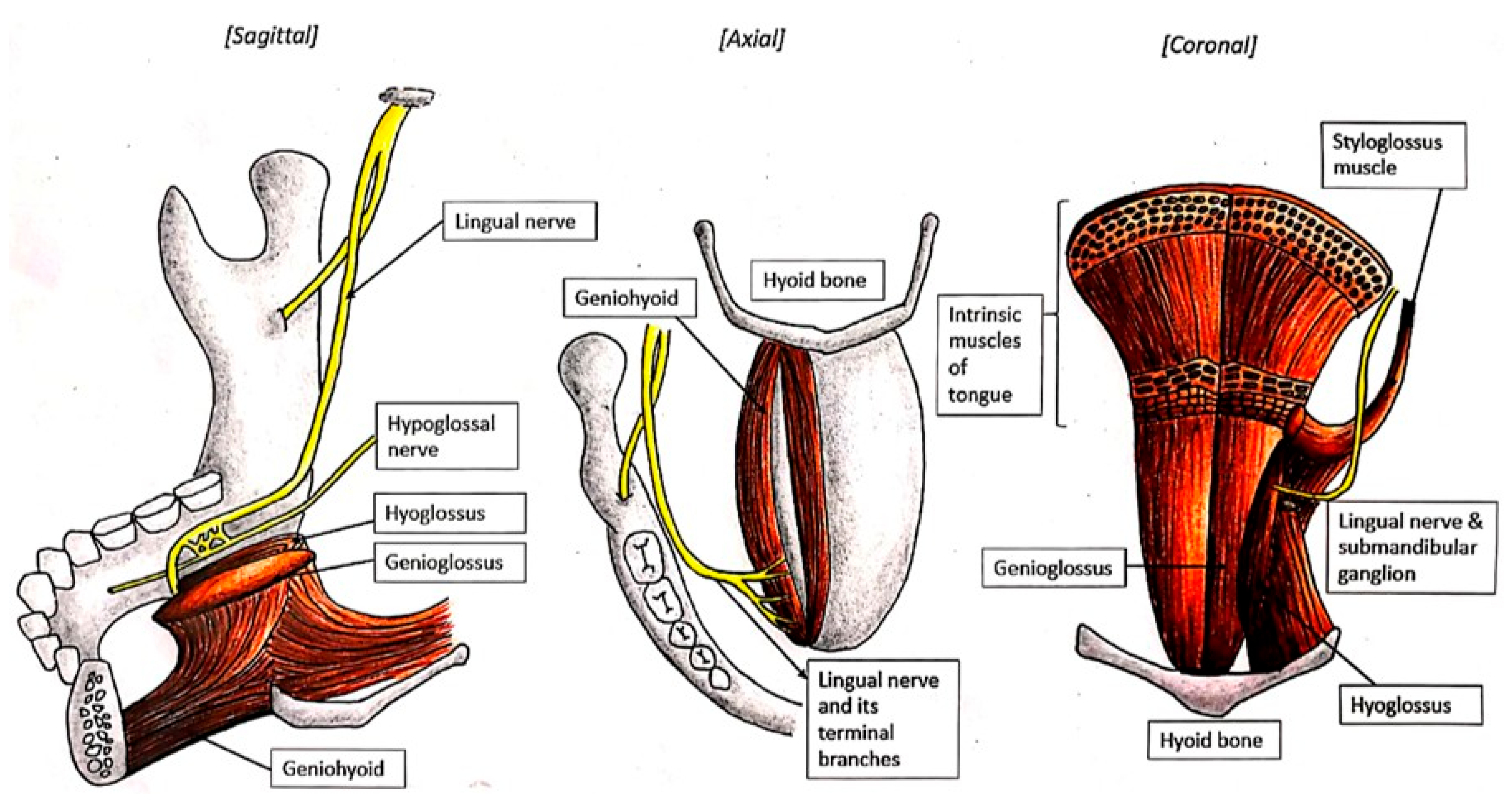
3.6. Challenges in Diagnosing the Lingual Nerve at the Floor of the Mouth
3.7. Challenges in Diagnosing the Lingual Nerve at the Gingiva Anteriorly and Tongue
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| MEDLINE | Medical Literature Analysis and Retrieval System Online |
| Embase | Excerpta Medica dataBASE |
| LN | lingual nerve |
| IAN | inferior alveolar nerve |
| IAN-LN | inferior alveolar nerve and lingual nerve |
| MeSH | Medical Subject Headings |
| CNV3 | cranial nerve V3 |
| US | ultrasound |
| MRI | magnetic resonance imaging |
References
- Ngeow, W.C.; Chai, W.L. Lingual nerve neuropathy. Dent. Update 2019, 46, 775–789. [Google Scholar] [CrossRef]
- Piagkou, M.; Demesticha, T.; Skandalakis, P.; Johnson, E.O. Functional anatomy of the mandibular nerve: Consequences of nerve injury and entrapment. Clin. Anat. 2011, 24, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, J.; Jackson, N.; Toriumi, T.; Kageyama, I.; Reina, F.; Carrera, A.; Fukino, K.; Kitagawa, N.; Tubbs, R.S. The sublingual branch of the lingual nerve: Anatomical study and suggestion for a new terminology. Clin. Anat. 2023, 36, 900–904. [Google Scholar] [CrossRef]
- Ngeow, W.C.; Choon, Y.F.; Chai, W.L.; Tan, C.C.; Lim, D. The evolution of peripheral nerve treatment for trigeminal neuralgia—Peripheral injections. Med. Health 2021, 16, 34–51. [Google Scholar]
- Garaycochea, O.; Baptista, P.; Calvo-Imirizaldu, M.; Terrasa, D.; Moffa, A.; Casale, M.; Alcalde, J.; O’Connor-Reina, C.; Plaza, G.; Fernandez, S. Surgical anatomy of the lingual nerve for palate surgery: Where is located and how to avoid it. Eur. Arch. Otorhinolaryngol. 2022, 279, 5347–5353. [Google Scholar] [CrossRef] [PubMed]
- Isberg, A.M.; Isacsson, G.; Williams, W.N.; Loughner, B.A. Lingual numbness and speech articulation deviation associated with temporomandibular joint disk displacement. Oral Surg. Oral Med. Oral Pathol. 1987, 64, 9–14. [Google Scholar] [CrossRef]
- Nayak, S.R.; Rai, R.; Krishnamurthy, A.; Prabhu, L.V.; Ranade, A.V.; Mansur, D.I.; Kumar, S. An unusual course and entrapment of the lingual nerve in the infratemporal fossa. Bratisl. Lek. Listy 2008, 109, 525–527. [Google Scholar]
- Mendes, M.B.M.; de Carvalho Leite Leal Nunes, C.M.; de Almeida Lopes, M.C. Anatomical relationship of lingual nerve to the region of mandibular third molar. J. Oral Maxillofac. Res. 2014, 4, e2. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Sahni, P.; Singhvi, A.; Nayak, M.; Tiwari, V. Anomalous Bilateral Communication between the Inferior Alveolar Nerve and the Auriculotemporal Nerve: A Rare Variation. Malays. J. Med. Sci. 2014, 21, 71–74. [Google Scholar]
- Ngeow, W.C.; Chai, W.L. Numbness of the auricle following inferior alveolar nerve block: The forgotten complication. Br. Dent. J. 2009, 207, 19–21. [Google Scholar] [CrossRef]
- McGeachie, J.K. Anatomy of the lingual nerve in relation to possible damage during clinical procedures. Ann. R. Australas. Coll. Dent. Surg. 2002, 16, 109–110. [Google Scholar] [PubMed]
- Baron, R.; Procos, A.; Uys, A. An Anatomic Study of the Lingual Nerve and Associated Branches. Clin. Exp. Dent. Res. 2025, 11, e70051. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.Y.; Hu, K.S.; Chung, I.H.; Lee, E.W. Topographic anatomy of the lingual nerve and variations in communication pattern of the mandibular nerve branches. Surg. Radiol. Anat. 2004, 26, 128–135. [Google Scholar] [CrossRef]
- Erdogmus, S.; Govsa, F.; Celik, S. Anatomic position of the lingual nerve in the mandibular third molar region as potential risk factors for nerve palsy. J. Craniofac. Surg. 2008, 19, 264–270. [Google Scholar] [CrossRef]
- Bokindo, I.K.; Butt, F.; Hassanali, J. Morphology and Morphometry of the Lingual Nerve in Relation to the Mandibular Third Molar. Open J. Stomatol. 2015, 5, 6–11. [Google Scholar] [CrossRef]
- Kocabiyik, N.; Varol, A.; Sencimen, M.; Ozan, H. An unnamed branch of the lingual nerve: Gingival branch. Br. J. Oral Maxillofac. Surg. 2009, 47, 214–217. [Google Scholar] [CrossRef]
- Selvi, F.; Yildirimyan, N.; Zuniga, J.R. Inferior alveolar and lingual nerve injuries: An overview of diagnosis and management. Front. Oral Maxillofac. Med. 2022, 4, 27. [Google Scholar] [CrossRef]
- Møller-Hansen, D.P.; Baad-Hansen, L.; Jensen, S.S. Permanent lingual nerve injury after dental procedures: A retrospective study of 228 patients. Int. J. Oral Maxillofac. Surg. 2024, 53, 860–866. [Google Scholar] [CrossRef]
- Berberi, A.; Le Breton, G.; Mani, J.; Woimant, H.; Nasseh, I. Lingual paresthesia following surgical placement of implants: Report of a case. Int. J. Oral Maxillofac. Implant. 1993, 8, 580–582. [Google Scholar]
- Seward, G.R. Anatomic surgery for salivary calculi: Part III. Calculi in the posterior part of the submandibular duct. Oral Surg. Oral Med. Oral Pathol. 1968, 25, 525–531. [Google Scholar] [CrossRef]
- Hölzle, F.W.; Wolff, K.D. Anatomic position of the lingual nerve in the mandibular third molar region with special consideration of an atrophied mandibular crest: An anatomical study. Int. J. Oral Maxillofac. Surg. 2001, 30, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Al-Amery, S.M.; Nambiar, P.; Naidu, M.; Ngeow, W.C. Variation in Lingual Nerve Course: A Human Cadaveric Study. PLoS ONE 2016, 11, e0162773. [Google Scholar] [CrossRef]
- Günenç Beşer, C.; Erçakmak, B.; Ilgaz, H.B.; Vatansever, A.; Sargon, M.F. Revisiting the relationship between the submandibular duct, lingual nerve and hypoglossal nerve. Folia Morphol. 2018, 77, 521–526. [Google Scholar] [CrossRef]
- Holsinger, F.C.; Bui, D.T. Anatomy, Function, and Evaluation of the Salivary Glands. In Salivary Gland Disorders; Myers, E.N., Ferris, R.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–16. [Google Scholar]
- Rusu, M.C.; Nimigean, V.; Podoleanu, L.; Ivaşcu, R.V.; Niculescu, M.C. Details of the intralingual topography and morphology of the lingual nerve. Int. J. Oral Maxillofac. Surg. 2008, 37, 835–839. [Google Scholar] [CrossRef]
- Touré, G.; Bicchieray, L.; Selva, J.; Vacher, C. The intra-lingual course of the nerves of the tongue. Surg. Radiol. Anat. 2005, 27, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Castelli, W.A.; Huelke, D.F.; Celis, A. Some basic anatomic features in paralingual space surgery. Oral Surg. Oral Med. Oral Pathol. 1969, 27, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Symington, J. The Topographical Anatomy of the Salivary Glands. J. Anat. Physiol. 1912, 46 Pt 2, 173. [Google Scholar]
- Baurmash, H.D. Treating oral ranula: Another case against blanket removal of the sublingual gland. Br. J. Oral Maxillofac. Surg. 2001, 39, 217–220. [Google Scholar] [CrossRef]
- Das, S.; Das, T.P.; Ghosh, P.S. Submental intubation: A journey over the last 25 years. J. Anaesthesiol. Clin. Pharmacol. 2012, 28, 291–303. [Google Scholar] [CrossRef]
- Yang, H.M.; Woo, Y.J.; Won, S.Y.; Kim, D.H.; Hu, K.S.; Kim, H.J. Course and distribution of the lingual nerve in the ventral tongue region: Anatomical considerations for frenectomy. J. Craniofac. Surg. 2009, 20, 1359–1363. [Google Scholar] [CrossRef]
- Zur, K.B.; Mu, L.; Sanders, I. Distribution pattern of the human lingual nerve. Clin. Anat. 2004, 17, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, H.; Tanuma, K.; Yamashita, K.; Saigusa, M.; Niimi, S. Nerve fiber analysis for the lingual nerve of the human adult subjects. Surg. Radiol. Anat. 2006, 28, 59–65. [Google Scholar] [CrossRef]
- Păduraru, D.; Rusu, M.C. The anatomy of the intralingual neural interconnections. Surg. Radiol. Anat. SRA 2013, 35, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.D. Surgical treatment of trifacial neuralgia. Cal. State J. Med. 1909, 7, 334. [Google Scholar]
- Shirres, D.A. Deep injections of alcohol for trifacial neuralgia—One hundred and two cases. Can. Med. Assoc. J. 1912, 2, 772–776. [Google Scholar] [PubMed]
- Hecht, D. The methods and technique of the deep alcohol injections for trifacial neuralgia. JAMA 1907, 49, 1574–1580. [Google Scholar] [CrossRef]
- Cushing, H. The role of deep alcohol injections in the treatment of trigeminal neuralgia. J. Am. Med. Assoc. 1920, 75, 441–443. [Google Scholar]
- Mckenzie, K.G. The surgical treatment of trigeminal neuralgia. Can. Med. Assoc. J. 1925, 15, 1119–1124. [Google Scholar]
- Sweet, W.H. Trigeminal injection with radiographic control. J. Am. Med. Assoc. 1950, 142, 392–396. [Google Scholar] [CrossRef]
- Tsai, P.J.; Lee, M.H.; Chen, K.T.; Huang, W.C.; Yang, J.T.; Lin, M.H.C. Foramen ovale cannulation guided by intraoperative computed tomography with magnetic resonance image fusion plays a role in improving the long-term outcome of percutaneous radiofrequency trigeminal rhizotomy. Acta Neurochir. 2019, 161, 1427–1434. [Google Scholar] [CrossRef]
- Pérez-Bovet, J.; Caro Cardera, J.L.; Rimbau Muñoz, J. Frameless navigation-guided percutaneous rhizotomy of the trigeminal nerve: An appraisal of the literature. Neurosurg. Rev. 2022, 45, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Valerio Pascua, J.E.; Mantilla Farfan, P.; Fernandez, M.P.; Santiago Rea, N.; Borro, M.; Alvarez-Pinzon, A.M. Frame navigation guided percutaneous balloon compression for intractable trigeminal neuralgia secondary to multiple sclerosis. Brain Spine 2024, 4, 102798. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, W.; Zhang, M.; Lin, H.; Zhou, S.; Lei, Y.; Xia, J. Application of virtual navigation with multimodality image fusion in foramen ovale cannulation. Pain Med. 2017, 18, 2181–2186. [Google Scholar] [CrossRef]
- Ostrowski, P.; Bonczar, M.; Wilk, J.; Michalczak, M.; Czaja, J.; Niziolek, M.; Sienkiewicz, J.; Szczepanek, E.; Chmielewski, P.; Iskra, T.; et al. The complete anatomy of the lingual nerve: A meta-analysis with implications for oral and maxillofacial surgery. Clin. Anat. 2023, 36, 905–914. [Google Scholar] [CrossRef]
- Piagkou, M.; Demesticha, T.; Piagkos, G.; Georgios, A.; Panagiotis, S. Lingual nerve entrapment in muscular and osseous structures. Int. J. Oral Sci. 2010, 2, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Loughner, B.A.; Larkin, L.H.; Mahan, P.E. Nerve entrapment in the lateral pterygoid muscle. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 299–306. [Google Scholar] [CrossRef]
- Caillet, H.; Delvalle, A.; Doyon, D.; Sigal, R.; Francke, J.P.; Halimi, P.; Bely, N. Visibility of cranial nerves at MRI. J. Neuroradiol. 1990, 17, 289–302. [Google Scholar] [PubMed]
- Aquilanti, L.; Mascitti, M.; Togni, L.; Contaldo, M.; Rappelli, G.; Santarelli, A. A Systematic Review on Nerve-Related Adverse Effects following Mandibular Nerve Block Anesthesia. Int. J. Environ. Res. Public Health 2022, 19, 1627. [Google Scholar] [CrossRef]
- Garisto, G.A.; Gaffen, A.S.; Lawrence, H.P.; Tenenbaum, H.C.; Haas, D.A. Occurrence of paresthesia after dental local anesthetic administration in the United States. J. Am. Dent. Assoc. 2010, 141, 836–844. [Google Scholar] [CrossRef]
- Morris, C.D.; Rasmussen, J.; Throckmorton, G.S.; Finn, R. The anatomic basis of lingual nerve trauma associated with inferior alveolar block injections. J. Oral Maxillofac. Surg. 2010, 68, 2833–2836. [Google Scholar] [CrossRef]
- Sambrook, P.J.; Goss, A.N. Severe adverse reactions to dental local anaesthetics: Prolonged mandibular and lingual nerve anaesthesia. Aust. Dent. J. 2011, 56, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Krafft, T.C.; Hickel, R. Clinical investigation into the incidence of direct damage to the lingual nerve caused by local anaesthesia. J. Craniomaxillofac. Surg. 1994, 22, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Nishioka, T.; Obata, K.; Takeshita, Y.; Irani, C.; Kunisada, Y.; Yoshioka, N.; Ibaragi, S.; Tubbs, R.S.; Iwanaga, J. Lingual nerve revisited-A comprehensive review Part II: Surgery and radiology. Clin. Anat. 2024; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Stacy, G.C.; Hajjar, G. Barbed needle and inexplicable paresthesias and trismus after dental regional anesthesia. Oral Surg. Oral Med. Oral Pathol. 1994, 77, 585–588. [Google Scholar] [CrossRef]
- Hillerup, S.; Jensen, R. Nerve injury caused by mandibular block analgesia. Int. J. Oral Maxillofac. Surg. 2006, 35, 437–443. [Google Scholar] [CrossRef]
- Pogrel, M.A.; Le, H. Etiology of lingual nerve injuries in the third molar region: A cadaver and histologic study. J. Oral Maxillofac. Surg. 2006, 64, 1790–1794. [Google Scholar] [CrossRef]
- Takasugi, Y.; Furuya, H.; Moriya, K.; Okamoto, Y. Clinical evaluation of inferior alveolar nerve block by injection into the pterygomandibular space anterior to the mandibular foramen. Anesth. Prog. 2000, 47, 125–129. [Google Scholar] [PubMed]
- Watanabe, K.; Tokumine, J.; Nagase, M.; Matsumura, G.; Sawada, R.; Kinjo, S.; Yorozu, T. A new and simplified extraoral approach for inferior alveolar nerve block: A cadaveric study and clinical case reports. J. Anesth. 2024, 38, 806–810. [Google Scholar] [CrossRef]
- Kostares, E.; Kostares, M.; Kostare, G.; Kantzanou, M. Prevalence of lingual sensory impairment following bilateral sagittal split osteotomy: A systematic review and meta-analysis. Oral Maxillofac. Surg. 2024, 28, 1055–1062. [Google Scholar] [CrossRef]
- Boynuyogun, E.; Ülkir, M.; Ilgaz, H.; Demiryürek, D.; Çalış, M. A Cadaveric Study of the Position and Tracking of the Inferior Alveolar Nerve and Lingual Nerve: Relevancy with Sagittal Split Ramus Osteotomy. Turk. J. Plast. Surg. 2025, 33, 68–73. [Google Scholar]
- Shimotakahara, R.; Lee, H.; Mine, K.; Ogata, S.; Tamatsu, Y. Anatomy of the lingual nerve: Application to oral surgery. Clin. Anat. 2019, 32, 635–641. [Google Scholar] [CrossRef]
- Hanzelka, T.; Foltán, R.; Pavlíková, G.; Horká, E.; Šedý, J. The role of intraoperative positioning of the inferior alveolar nerve on postoperative paresthesia after bilateral sagittal split osteotomy of the mandible: Prospective clinical study. Int. J. Oral Maxillofac. Surg. 2011, 40, 901–906. [Google Scholar] [CrossRef]
- McLeod, N.M.H.; Bowe, D.C. Nerve injury associated with orthognathic surgery. Part 3: Lingual, infraorbital, and optic nerves. Br. J. Oral Maxillofac. Surg. 2016, 54, 372–375. [Google Scholar] [CrossRef]
- Lewis, J.E. Modified lingual split technique for extraction of impacted mandibular third molars. J. Oral Surg. 1980, 38, 578–583. [Google Scholar] [PubMed]
- Dias, G.J.; de Silva, R.K.; Shah, T.; Sim, E.; Song, N.; Colombage, S.; Cornwall, J. Multivariate assessment of site of lingual nerve. Br. J. Oral Maxillofac. Surg. 2015, 53, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Behnia, H.; Kheradvar, A.; Shahrokhi, M. An anatomic study of the lingual nerve in the third molar region. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2000, 58, 649–651; discussion 652–653. [Google Scholar] [CrossRef] [PubMed]
- Miloro, M.; Halkias, L.E.; Slone, H.W.; Chakeres, D.W. Assessment of the lingual nerve in the third molar region using magnetic resonance imaging. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1997, 55, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Kiesselbach, J.E.; Chamberlain, J.G. Clinical and anatomic observations on the relationship of the lingual nerve to the mandibular third molar region. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1984, 42, 565–567. [Google Scholar] [CrossRef]
- Pogrel, M.A.; Renaut, A.; Schmidt, B.; Ammar, A. The relationship of the lingual nerve to the mandibular third molar region: An anatomic study. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1995, 53, 1178–1181. [Google Scholar] [CrossRef]
- Benninger, B.; Kloenne, J.; Horn, J.L. Clinical anatomy of the lingual nerve and identification. Br. J. Oral Maxillofac. Surg. 2013, 51, 541–544. [Google Scholar] [CrossRef]
- Pogrel, M.A.; Goldman, K.E. Lingual flap retraction for third molar removal. J. Oral Maxillofac. Surg. 2004, 62, 1125–1130. [Google Scholar] [CrossRef]
- Al-Haj Husain, A.; Solomons, M.; Stadlinger, B.; Pejicic, R.; Winklhofer, S.; Piccirelli, M.; Valdec, S. Visualization of the Inferior Alveolar Nerve and Lingual Nerve Using MRI in Oral and Maxillofacial Surgery: A Systematic Review. Diagnostics 2021, 11, 1657. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, M.; Pranno, N.; Pompa, V.; Barchetti, F.; Pompa, G. High-resolution 3-T MR imaging in the evaluation of the trigeminal nerve course. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 257–264. [Google Scholar]
- Assaf, A.T.; Zrnc, T.A.; Remus, C.C.; Schönfeld, M.; Habermann, C.R.; Riecke, B.; Friedrich, R.E.; Fiehler, J.; Heiland, M.; Sedlacik, J. Evaluation of four different optimized magnetic-resonance imaging sequences for visualization of dental and maxillo-mandibular structures at 3 T. J. Craniomaxillofac. Surg. 2014, 42, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Fujita, A.; Yang, A.; Kanazawa, H.; Buch, K.; Sakai, O.; Sugimoto, H. Visualization of the peripheral branches of the mandibular division of the trigeminal nerve on 3D double-echo steady-state with water excitation sequence. AJNR Am. J. Neuroradiol. 2015, 36, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Burian, E.; Probst, F.A.; Weidlich, D.; Cornelius, C.P.; Maier, L.; Robl, T.; Zimmer, C.; Karampinos, D.C.; Ritschl, L.M.; Probst, M. MRI of the inferior alveolar nerve and lingual nerve—Anatomical variation and morphometric benchmark values of nerve diameters in healthy subjects. Clin. Oral Investig. 2020, 24, 2625–2634. [Google Scholar] [CrossRef]
- Mazza, D.; Di Girolamo, M.; Cecchetti, F.; Baggi, L. Appearance of normal MRI anatomy of the lingual nerve using steady-state free precession sequences at 3-T. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. S1), 19–26. [Google Scholar]
- Al-Haj Husain, A.; Valdec, S.; Stadlinger, B.; Rücker, M.; Piccirelli, M.; Winklhofer, S. Preoperative visualization of the lingual nerve by 3D double-echo steady-state MRI in surgical third molar extraction treatment. Clin. Oral Investig. 2022, 26, 2043–2053. [Google Scholar] [CrossRef]
- Jiang, D.; Hong, J.; Yan, Y.; Huang, H.; You, P.; Huang, W.; Zhao, X.; She, D.; Cao, D. Preoperative evaluation of lingual cortical plate thickness and the anatomical relationship of the lingual nerve to the lingual cortical plate via 3T MRI nerve-bone fusion. Dentomaxillofac. Radiol. 2025, 54, 163–172. [Google Scholar] [CrossRef]
- Cox, B.; Zuniga, J.R.; Panchal, N.; Cheng, J.; Chhabra, A. Magnetic resonance neurography in the management of peripheral trigeminal neuropathy: Experience in a tertiary care centre. Eur. Radiol. 2016, 26, 3392–3400. [Google Scholar] [CrossRef]
- Terumitsu, M.; Matsuzawa, H.; Seo, K.; Watanabe, M.; Kurata, S.; Suda, A.; Nakada, T. High-contrast high-resolution imaging of posttraumatic mandibular nerve by 3DAC-PROPELLER magnetic resonance imaging: Correlation with the severity of sensory disturbance. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 85–94. [Google Scholar] [CrossRef]
- Dessouky, R.; Xi, Y.; Zuniga, J.; Chhabra, A. Role of MR neurography for the diagnosis of peripheral trigeminal nerve injuries in patients with prior molar tooth extraction. AJNR Am. J. Neuroradiol. 2018, 39, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Barootchi, S.; Chan, H.L.; Namazi, S.S.; Wang, H.L.; Kripfgans, O.D. Ultrasonographic characterization of lingual structures pertinent to oral, periodontal, and implant surgery. Clin. Oral Implant. Res. 2020, 31, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Ghabriel, M.; Takezawa, K.; Townsend, G. The lingual nerve: Overview and new insights into anatomical variability based on fine dissection using human cadavers. Odontology 2019, 107, 1–9. [Google Scholar] [CrossRef]
- Sittitavornwong, S.; Babston, M.; Denson, D.; Zehren, S.; Friend, J. Clinical anatomy of the lingual nerve: A review. J. Oral Maxillofac. Surg. 2017, 75, 926.e1–926.e9. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.T.; Koch, M.; Witt, R.L.; Ryan, W.R.; Zenk, J.; Katz, P.; Rahmati, R.; Rassekh, C.; Donato, F.; McCulloch, T.M.; et al. Proposal for standardized ultrasound analysis of the salivary glands: Part 1 submandibular gland. Laryngoscope Investig. Otolaryngol. 2024, 9, e1224. [Google Scholar] [CrossRef] [PubMed]
- Miloro, M.; Kolokythas, A. Inferior alveolar and lingual nerve imaging. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2011, 19, 35–46. [Google Scholar] [CrossRef]
- Fazan, V.P.S.; Rodrigues Filho, O.A.; Matamata, F. Communication between the mylohyoid and lingual nerves: Clinical implications. Int. J. Morphol. 2007, 25, 561–564. [Google Scholar] [CrossRef]
- Shinohara, H.; Mataga, I.; Kageyama, I. Discussion of clinical anatomy of the lingual nerves. Okajimas Folia Anat. Jpn. 2010, 87, 97–102. [Google Scholar] [CrossRef][Green Version]
- Fitzgerald, M.J.T.; Law, M.E. The peripheral connexions between the lingual and hypoglossal nerves. J. Anat. 1958, 92 Pt 2, 178. [Google Scholar]
- Iwanaga, J.; Watanabe, K.; Saga, T.; Tabira, Y.; Nakamura, M.; Fisahn, C.; Tubbs, R.S.; Kusukawa, J.; Yamaki, K.I. Communicating branches between lingual and hypoglossal nerve: Observation using Sihler’s staining technique. Surg. Radiol. Anat. SRA 2017, 39, 741–745. [Google Scholar] [CrossRef]
- Hung, N.K.; Lee, C.H.; Chan, S.M.; Yeh, C.C.; Cherng, C.H.; Wong, C.S.; Wu, C.T. Transient unilateral hypoglossal nerve palsy after orotracheal intubation for general anesthesia. Acta Anaesthesiol. Taiwan. 2009, 47, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Cazenave, L.; Mahiou, P.; Swan, J.; Clavert, P.; Barth, J. Combined hypoglossal and lingual nerve palsy: An unrecognized complication after orotracheal intubation for general anaesthesia. Anaesth. Crit. Care Pain Med. 2024, 43, 101418. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Virador, G.M.; Brahmbhatt, P.; Bhatt, A.A.; Middlebrooks, E.H.; Desai, A.; Agarwal, A.; Vibhute, P.; Gupta, V. High-Resolution 7T MR Imaging of the Trochlear Nerve. AJNR Am. J. Neuroradiol. 2023, 44, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hwang, J.M. Imaging of Cranial Nerves III, IV, VI in Congenital Cranial Dysinnervation Disorders. Korean J. Ophthalmol. 2017, 31, 183–193. [Google Scholar]
- Leung, Y.Y.; McGrath, C.; Cheung, L.K. Trigeminal neurosensory deficit and patient reported outcome measures: The effect on quality of life. PLoS ONE 2013, 8, e77391. [Google Scholar] [CrossRef]
- Smith, J.G.; Elias, L.A.; Yilmaz, Z.; Barker, S.; Shah, K.; Shah, S.; Renton, T. The psychosocial and affective burden of posttraumatic neuropathy following injuries to the trigeminal nerve. J. Orofac. Pain 2013, 27, 293–303. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Cheung, L.K. Risk factors of neurosensory deficits in lower third molar surgery: A literature review of prospective studies. Int. J. Oral Maxillofac. Surg. 2011, 40, 1–10. [Google Scholar] [CrossRef]
- Seddon, H.J. The use of autogenous grafts for the repair of large gaps in peripheral nerves. Br. J. Surg. 1947, 35, 151–167. [Google Scholar] [CrossRef]
- Sunderland, S. The function of nerve fibers whose structure has been disorganized. Anat. Rec. 1951, 109, 503–513. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Cheung, L.K. Longitudinal Treatment Outcomes of Microsurgical Treatment of Neurosensory Deficit after Lower Third Molar Surgery: A Prospective Case Series. PLoS ONE 2016, 11, e0150149. [Google Scholar] [CrossRef][Green Version]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. BioMed Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Zhang, Y.; Gu, X.; Huang, L.; Zhang, K.; Qian, T.; Gu, X. Application of stem cells in peripheral nerve regeneration. Burn. Trauma. 2020, 8, tkaa002. [Google Scholar]
- Leung, Y.Y. Management and prevention of third molar surgery-related trigeminal nerve injury: Time for a rethink. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ngeow, W.C. Scar less: A review of methods of scar reduction at sites of peripheral nerve repair. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 357–366. [Google Scholar] [CrossRef]
- Hong, D.H.; Lim, H.K.; Kim, S.M.; Kim, M.J.; Lee, J.H. Recovery of lingual nerve injury: Retrospective observational study. J. Korean Assoc. Oral Maxillofac. Surg. 2011, 37, 355–364. [Google Scholar] [CrossRef]
- Fujita, S.; Tojyo, I.; Nakanishi, T.; Suzuki, S. Comparison of prognosis in two methods for the lingual nerve repair: Direct suture with vein graft cuff and collagen allograft method. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 6. [Google Scholar] [CrossRef]
- Terumitsu, M.; Matsuzawa, H.; Seo, K.; Nakada, T. Evaluating Fine Structure of the Injured Trigeminal Nerve and Tissue Using 3D Anisotropy Contrast Imaging on a 3. 0-T System. 2013, 18, S115. [Google Scholar]
- Al-Haj Husain, A.; Schönegg, D.; Valdec, S.; Stadlinger, B.; Gander, T.; Essig, H.; Piccirelli, M.; Winklhofer, S. Visualization of Inferior Alveolar and Lingual Nerve Pathology by 3D Double-Echo Steady-State MRI: Two Case Reports with Literature Review. J. Imaging 2022, 8, 75. [Google Scholar] [CrossRef]
- Romano, N.; Federici, M.; Castaldi, A. Imaging of cranial nerves: A pictorial overview. Insights Imaging 2019, 10, 33. [Google Scholar] [CrossRef]
- Bangia, M.; Ahmadzai, I.; Casselman, J.; Politis, C.; Jacobs, R.; Van der Cruyssen, F. Accuracy of MR neurography as a diagnostic tool in detecting injuries to the lingual and inferior alveolar nerve in patients with iatrogenic post-traumatic trigeminal neuropathy. Eur. Radiol. 2024, 34, 4619–4627. [Google Scholar] [CrossRef]
- Peeters, F.; Van der Cruyssen, F.; Casselman, J.W.; Hermans, R.; Renton, T.; Jacobs, R.; Politis, C. The diagnostic value of magnetic resonance imaging in posttraumatic trigeminal neuropathic pain. J. Oral Facial Pain Headache 2021, 35, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Al-Amery, S.M.; Ngeow, W.C.; Nambiar, P.; Naidu, M. A pilot study on the effects of direct contact of two different surgical burs on the cadaveric lingual nerve. Int. J. Oral Maxillofac. Surg. 2018, 47, 1153–1160. [Google Scholar] [CrossRef]
- Reda, R.; Zanza, A.; Cicconetti, A.; Bhandi, S.; Miccoli, G.; Gambarini, G.; Di Nardo, D. Ultrasound Imaging in Dentistry: A Literature Overview. J. Imaging 2021, 7, 238. [Google Scholar] [CrossRef]
- Díaz, L.; Contador, R.; Albrecht, H.; Ibáñez, M.; Urrutia, P.; Bencze, B.; Toro, M.; Sáenz-Ravello, G.; Végh, D. Clinical applications of ultrasound imaging in dentistry: A comprehensive literature review. Dent. Rev. 2024, 4, 100086. [Google Scholar] [CrossRef]
- Tattan, M.; Sinjab, K.; Lee, E.; Arnett, M.; Oh, T.J.; Wang, H.L.; Chan, H.L.; Kripfgans, O.D. Ultrasonography for chairside evaluation of periodontal structures: A pilot study. J. Periodontol. 2020, 91, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.R.; Gaughan, J.P.; Ilyas, A.M. The sensitivity and specificity of ultrasound for the diagnosis of carpal tunnel syndrome: A meta-analysis. Clin. Orthop. Relat. Res. 2011, 469, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qin, J.; Xing, D.; Lu, H.; Yue, R.; Li, S.; Wu, D. Ultrasonic Measurement of Lingual Artery and Its Application for Midline Glossectomy. Ann. Otol. Rhinol. Laryngol. 2020, 129, 856–862. [Google Scholar] [CrossRef]
- Lakha, T.; Kheur, M.; Mühlemann, S.; Kheur, S.; Le, B. Ultrasound and CBCT analysis of blood flow and dimensions of the lingual vascular canal: A case control study. J. Oral Biol. Craniofac. Res. 2021, 11, 40–46. [Google Scholar] [CrossRef]
- Afzelius, P.; Nielsen, M.Y.; Ewertsen, C.; Bloch, K.P. Imaging of the major salivary glands. Clin. Physiol. Funct. Imaging 2016, 36, 1–10. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngeow, W.C.; Tay, H.W.; Sarna, K.; Cheah, C.W.; Raj, M.; Acharya, S.K.; Koo, Z.Z.; Wey, M.C. Challenges in Diagnosing the Course of the Lingual Nerve for Clinical Practice and Research. Diagnostics 2025, 15, 1609. https://doi.org/10.3390/diagnostics15131609
Ngeow WC, Tay HW, Sarna K, Cheah CW, Raj M, Acharya SK, Koo ZZ, Wey MC. Challenges in Diagnosing the Course of the Lingual Nerve for Clinical Practice and Research. Diagnostics. 2025; 15(13):1609. https://doi.org/10.3390/diagnostics15131609
Chicago/Turabian StyleNgeow, Wei Cheong, Hui Wen Tay, Krishan Sarna, Chia Wei Cheah, Mary Raj, Surendra Kumar Acharya, Zhong Zheng Koo, and Mang Chek Wey. 2025. "Challenges in Diagnosing the Course of the Lingual Nerve for Clinical Practice and Research" Diagnostics 15, no. 13: 1609. https://doi.org/10.3390/diagnostics15131609
APA StyleNgeow, W. C., Tay, H. W., Sarna, K., Cheah, C. W., Raj, M., Acharya, S. K., Koo, Z. Z., & Wey, M. C. (2025). Challenges in Diagnosing the Course of the Lingual Nerve for Clinical Practice and Research. Diagnostics, 15(13), 1609. https://doi.org/10.3390/diagnostics15131609







