Consistency Analysis of Centiloid Values Across Three Commercial Software Platforms for Amyloid PET Quantification
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Imaging Protocol
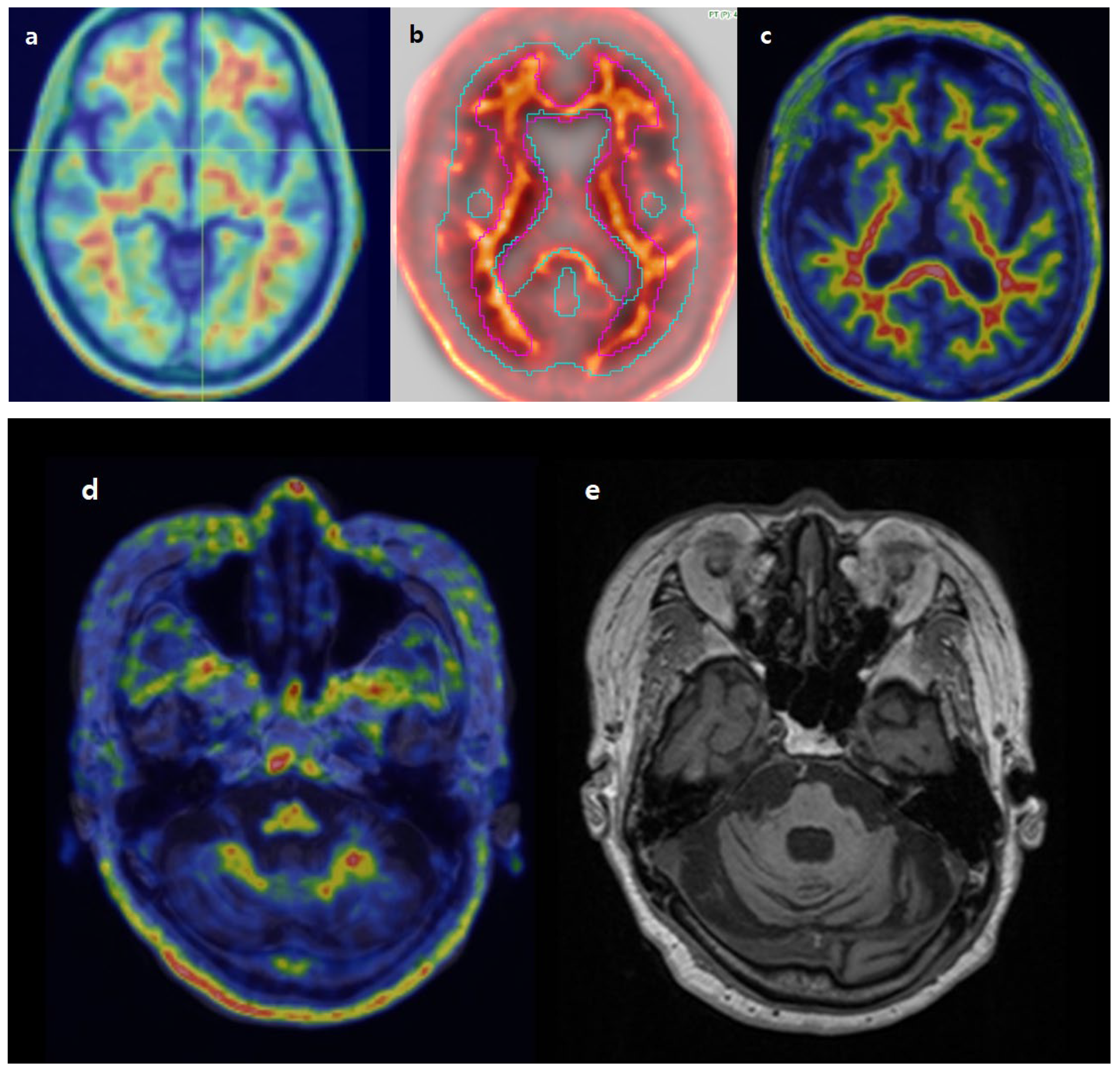
2.3. Imaging Analysis
2.4. Statistical Analysis
3. Results
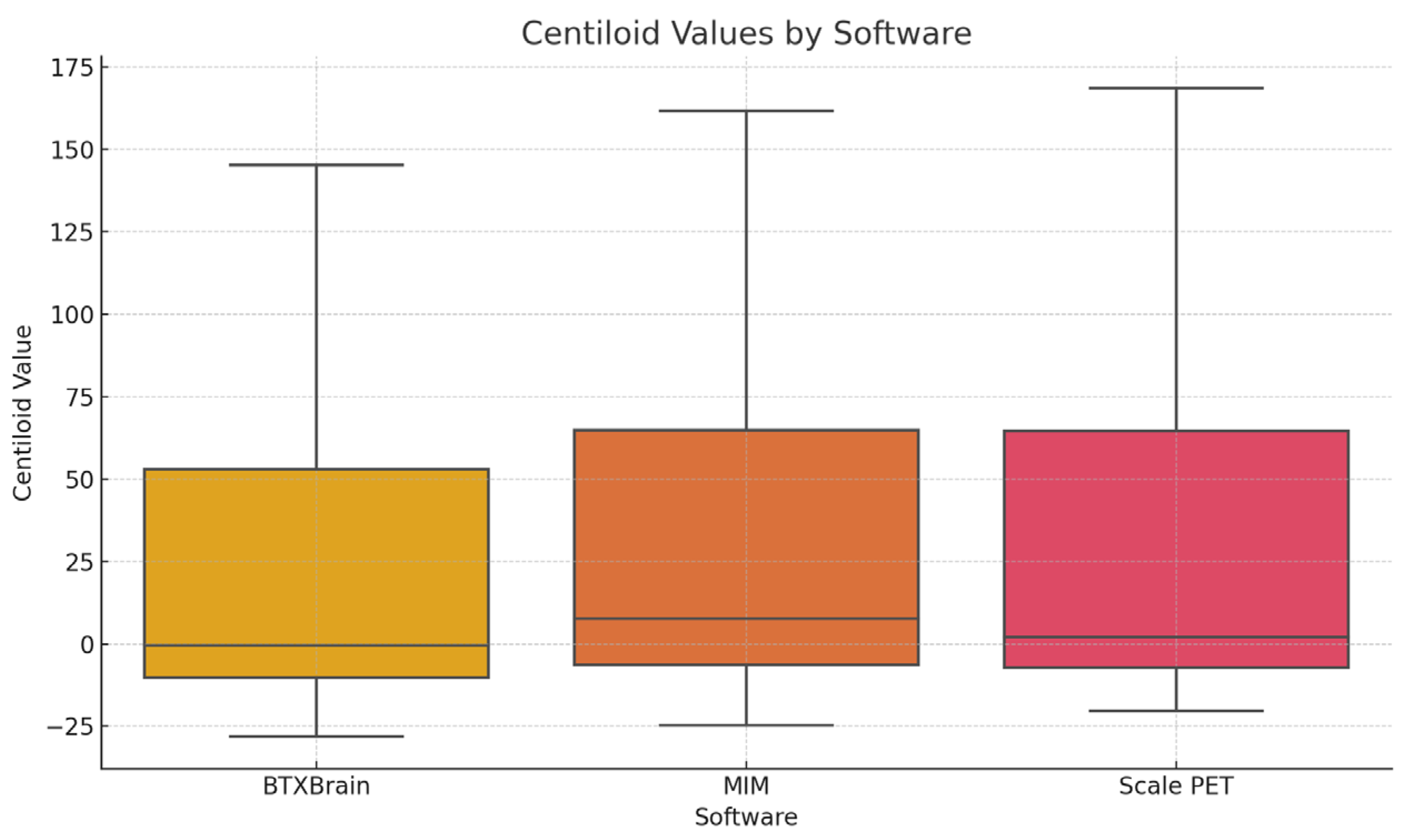
3.1. Demographics
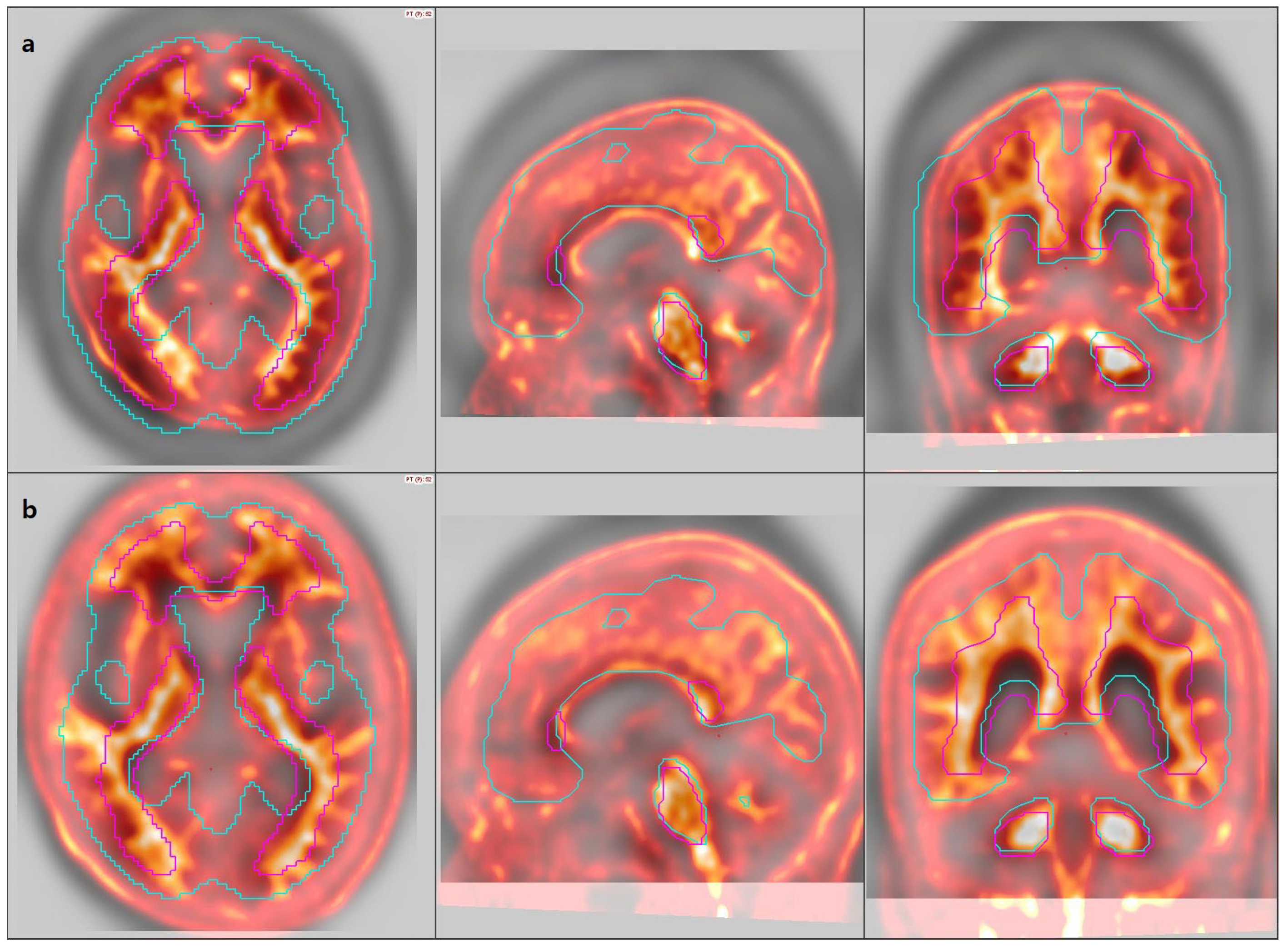
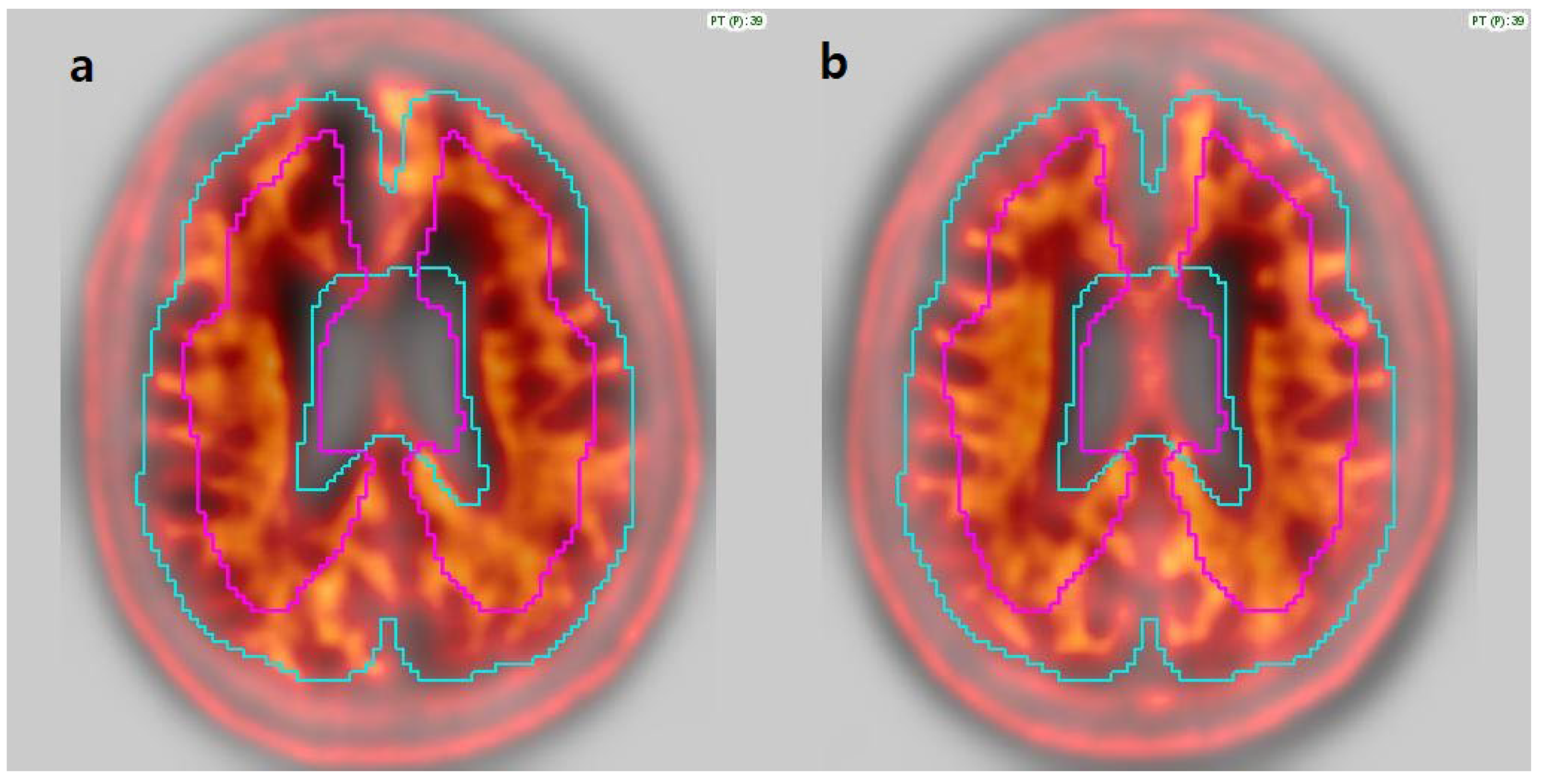
3.2. Image Analysis
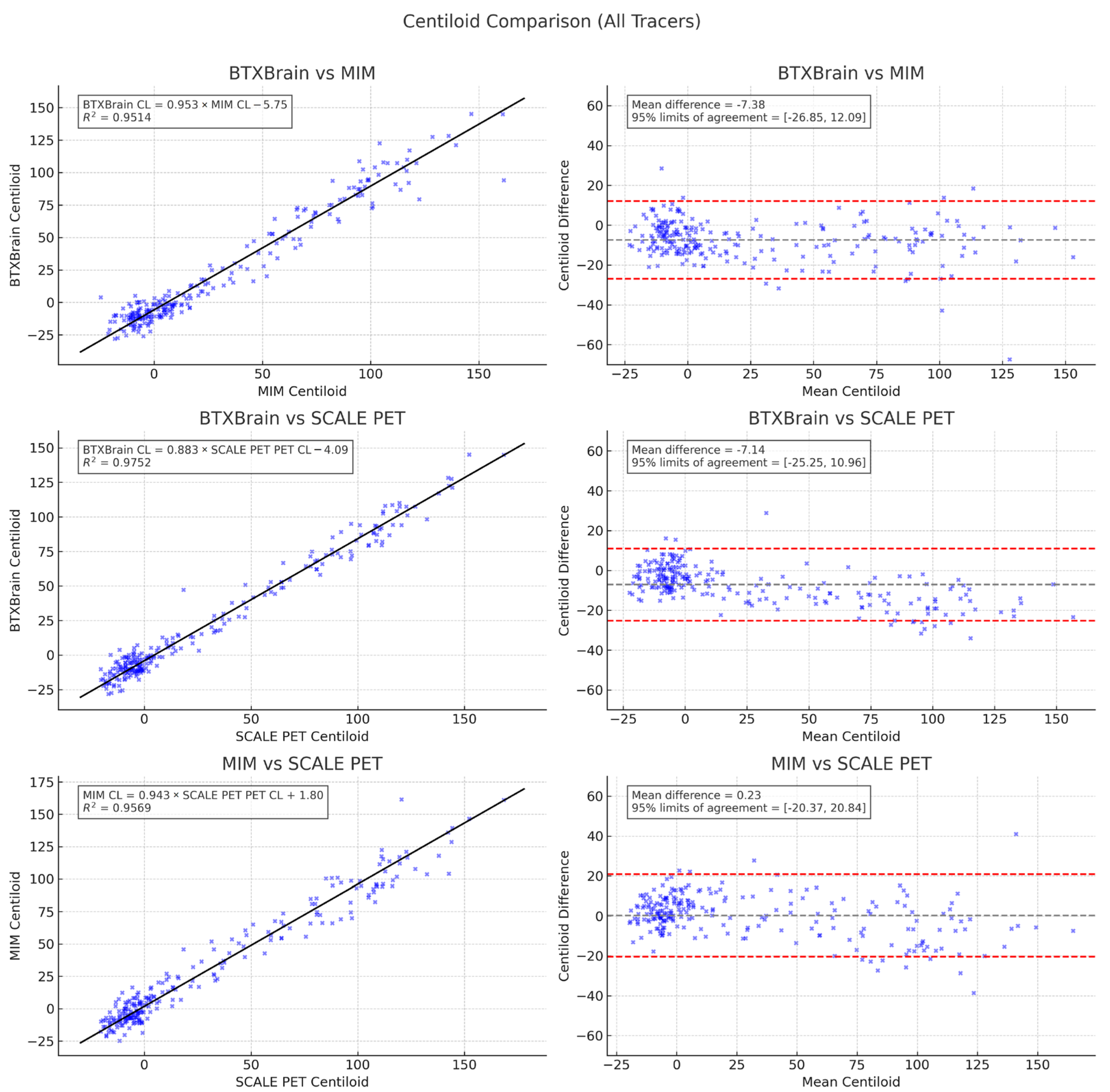
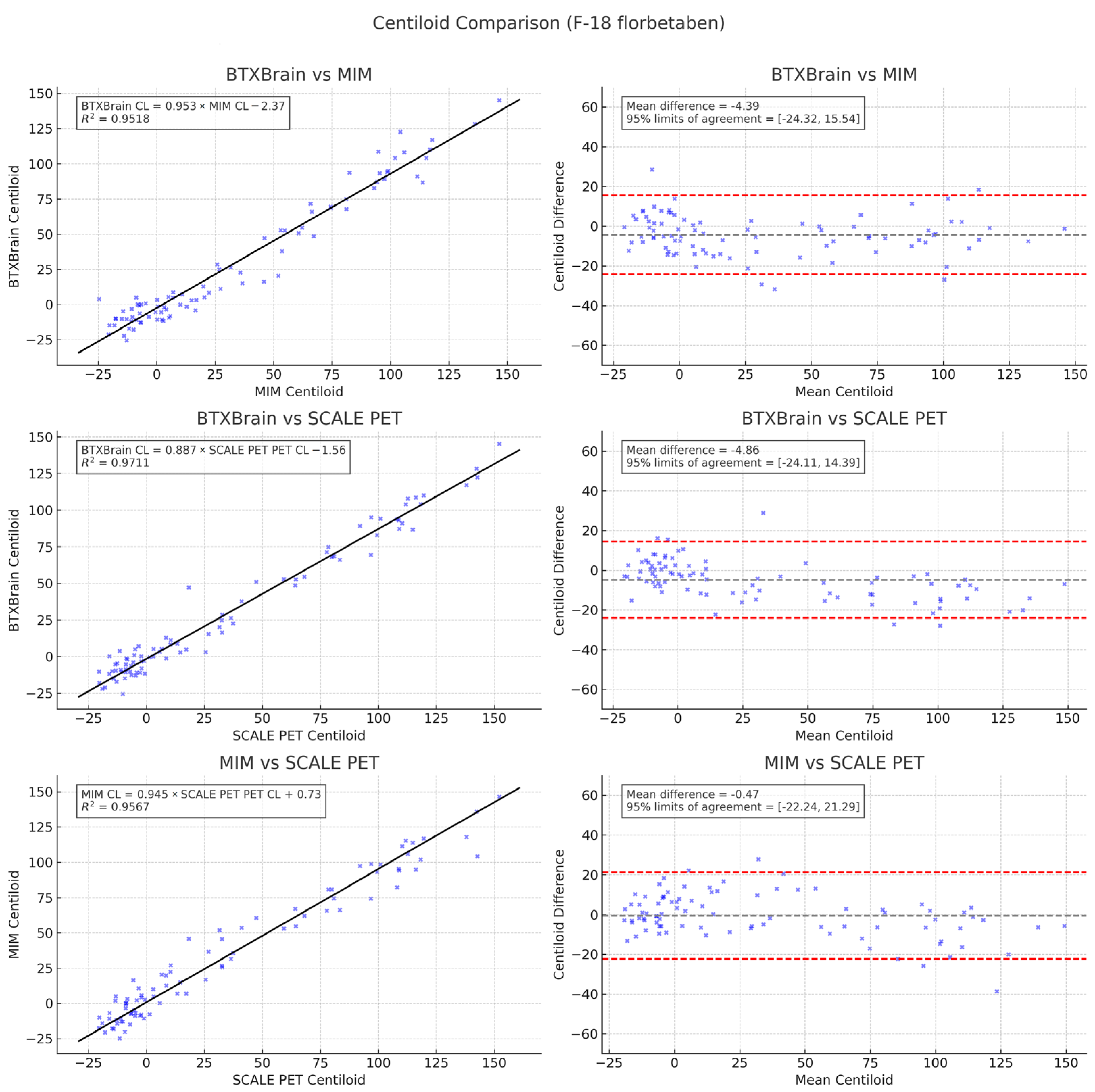
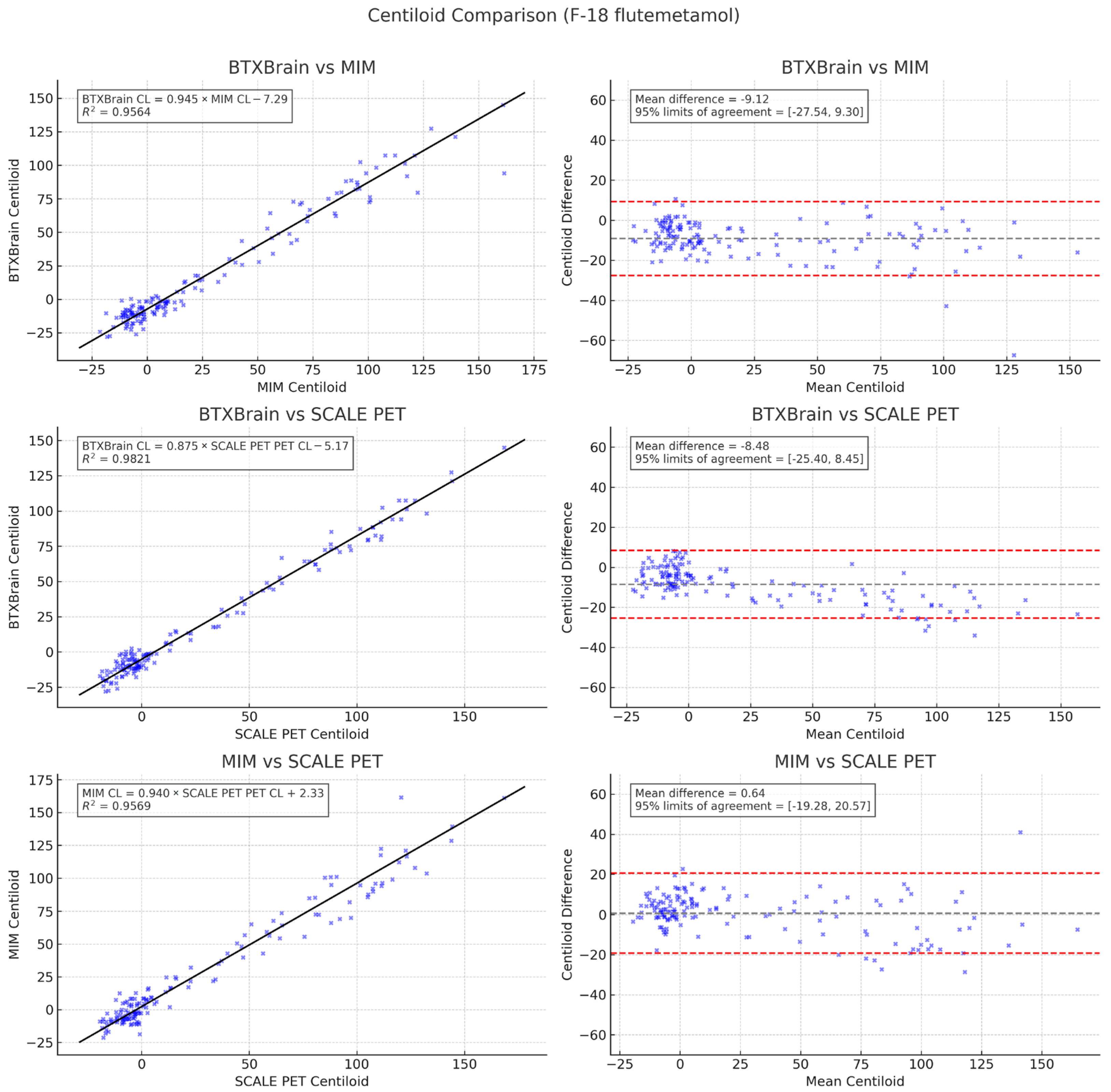
3.3. Agreement Among Quantification Software
3.4. Subgroup Analysis by Radiotracer
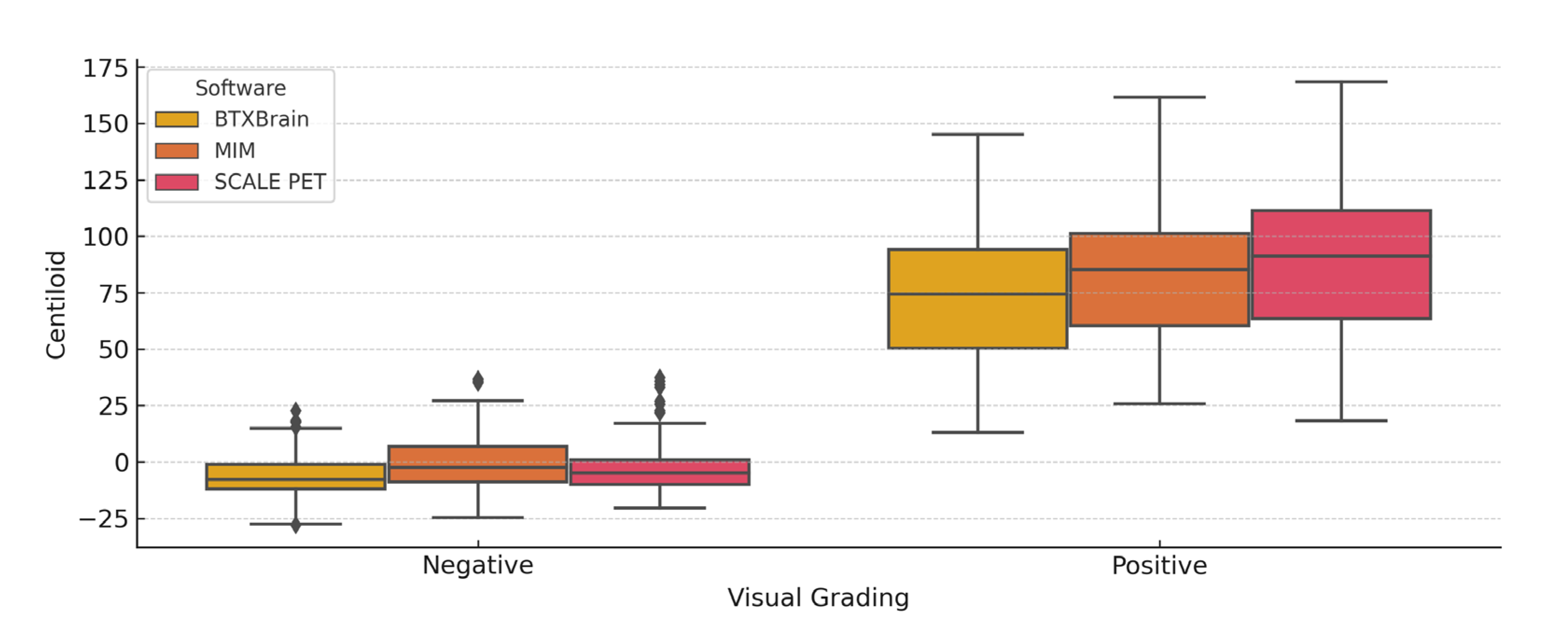
3.5. Centiloid Value Distribution According to Visual Assessment
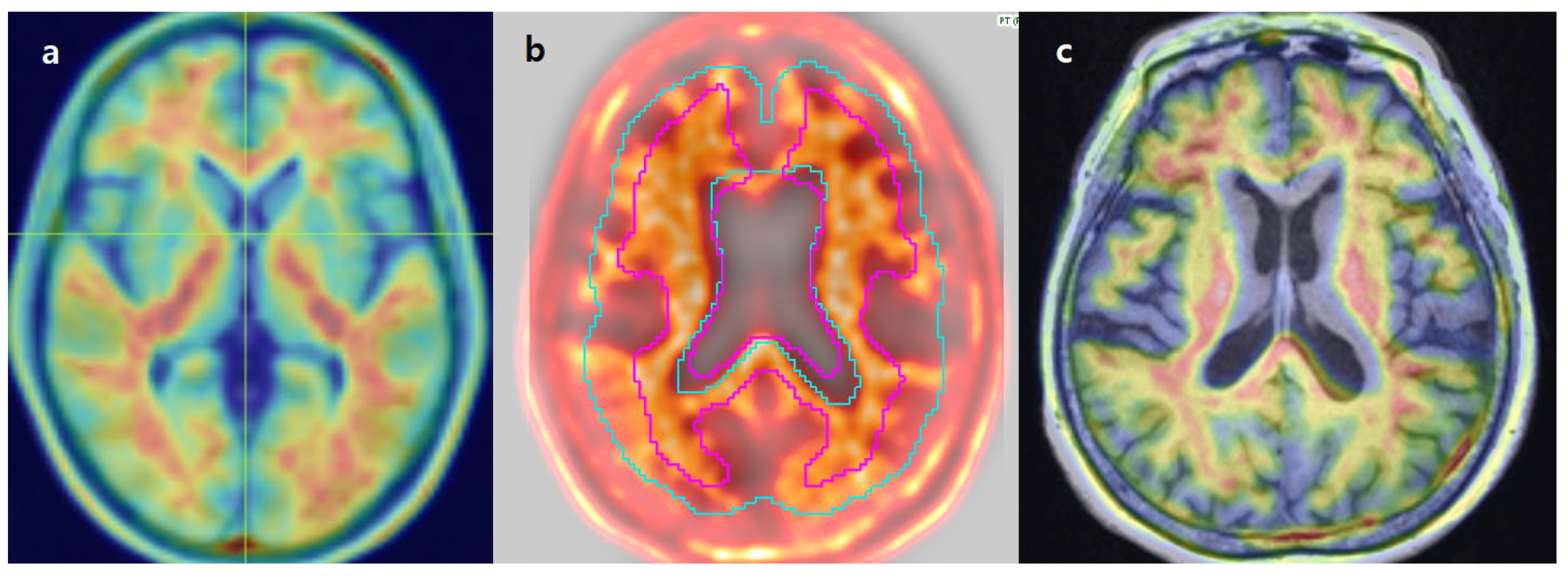
3.6. Review of Individual Discordant Cases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Chapleau, M.; Iaccarino, L.; Soleimani-Meigooni, D.; Rabinovici, G.D. The Role of Amyloid PET in Imaging Neurodegenerative Disorders: A Review. J. Nucl. Med. 2022, 63, 13S–19S. [Google Scholar] [CrossRef]
- Nayate, A.P.; Dubroff, J.G.; Schmitt, J.E.; Nasrallah, I.; Kishore, R.; Mankoff, D.; Pryma, D.A. Use of Standardized Uptake Value Ratios Decreases Interreader Variability of [18F] Florbetapir PET Brain Scan Interpretation. AJNR Am. J. Neuroradiol. 2015, 36, 1237–1244. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Chiao, P.; Klein, G.; Matthews, D.; Thurfjell, L.; Cole, P.E.; Margolin, R.; Landau, S.; Foster, N.L.; Mason, N.S.; et al. The influence of biological and technical factors on quantitative analysis of amyloid PET: Points to consider and recommendations for controlling variability in longitudinal data. Alzheimer’s Dement. 2015, 11, 1050–1068. [Google Scholar] [CrossRef]
- Klunk, W.E.; Koeppe, R.A.; Price, J.C.; Benzinger, T.L.; Devous, M.D.; Jagust, W.J.; Johnson, K.A.; Mathis, C.A.; Minhas, D.; Pontecorvo, M.J.; et al. The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimer’s Dement. 2015, 11, 1–15.e4. [Google Scholar] [CrossRef]
- Kim, D.; Kang, S.K.; Shin, S.A.; Choi, H.; Lee, J.S. Internal and external validations of BTXBrain-Amyloid, AI-powered automated quantification software for amyloid PET. J. Nucl. Med. 2022, 63, 2610. [Google Scholar]
- Lee, J.; Ha, S.; Kim, R.E.Y.; Lee, M.; Kim, D.; Lim, H.K. Development of Amyloid PET Analysis Pipeline Using Deep Learning-Based Brain MRI Segmentation—A Comparative Validation Study. Diagnostics 2022, 12, 623. [Google Scholar] [CrossRef]
- Rowe, C.C.; Doré, V.; Jones, G.; Baxendale, D.; Mulligan, R.S.; Bullich, S.; Stephens, A.W.; De Santi, S.; Masters, C.L.; Dinkelborg, L.; et al. (18)F-Florbetaben PET beta-amyloid binding expressed in Centiloids. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2053–2059. [Google Scholar] [CrossRef]
- Battle, M.R.; Buckley, C.J.; Smith, A.; Van Laere, K.; Vandenberghe, R.; Lowe, V.J. IC-P-173: Utility of PMOD Image Quantification Software for Processing [11C]PIB and [18F]Flutemetamol Images for SUVR Quantitation on The Centiloid Scale. Alzheimer’s Dement. 2016, 12, P126. [Google Scholar] [CrossRef]
- Royse, S.K.; Minhas, D.S.; Lopresti, B.J.; Murphy, A.; Ward, T.; Koeppe, R.A.; Bullich, S.; DeSanti, S.; Jagust, W.J.; Landau, S.M. Validation of amyloid PET positivity thresholds in centiloids: A multisite PET study approach. Alzheimer’s Res. Ther. 2021, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Zhou, J.; Irizarry, M.C.; Kramer, L.D.; Swanson, C.J.; Dhadda, S.; Rabe, M.; Krause, S.; Li, D.J.; Raman, R.; et al. AHEAD 3-45 study design: A global study to evaluate the efficacy and safety of treatment with BAN2401 for 216 weeks in preclinical Alzheimer’s disease with intermediate amyloid (A3 trial) and elevated amyloid (A45 trial). Alzheimer’s Dement. 2020, 16, e044511. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Amasha, A.A.H.; Kasalak, Ö.; Glaudemans, A.W.J.M.; Noordzij, W.; Dierckx, R.A.J.O.; Koopmans, K.-P.; Kwee, T.C. Increased individual workload for nuclear medicine physicians over the past years: 2008–2023 data from The Netherlands. Ann. Nucl. Med. 2025, 39, 273–277. [Google Scholar] [CrossRef]
- Park, K.-S. Application of Artificial Intelligence in Nuclear Neuroimaging. Nucl. Med. Mol. Imaging 2024, 58, 153–157. [Google Scholar] [CrossRef]
- Knesaurek, K.; Warnock, G.; Sideras, P.; Burger, C. Comparison of SUVr calculations in amyloid PET brain imaging. J. Nucl. Med. 2014, 55, 1873. [Google Scholar]
- Kang, S.K.; Kim, D.; Shin, S.A.; Kim, Y.K.; Choi, H.; Lee, J.S. Fast and Accurate Amyloid Brain PET Quantification Without MRI Using Deep Neural Networks. J. Nucl. Med. 2023, 64, 659–666. [Google Scholar] [CrossRef]
- Gillman, A.; Bourgeat, P.; Cox, T.; Villemagne, V.L.; Fripp, J.; Huang, K.; Williams, R.; Shishegar, R.; O’Keefe, G.; Li, S.; et al. Digital detector PET/CT increases Centiloid measures of amyloid in Alzheimer’s disease: A head-to-head comparison of cameras. J. Alzheimer’s Dis. 2025, 103, 1257–1268. [Google Scholar] [CrossRef]
- Carlier, T.; Ferrer, L.; Conti, M.; Bodet-Milin, C.; Rousseau, C.; Bercier, Y.; Bendriem, B.; Kraeber-Bodéré, F. From a PMT-based to a SiPM-based PET system: A study to define matched acquisition/reconstruction parameters and NEMA performance of the Biograph Vision 450. EJNMMI Phys. 2020, 7, 55. [Google Scholar] [CrossRef]
- Kwon, S.J.; Park, H.L. Time to shift to Digital PET/CT? Nucl. Med. Mol. Imaging 2025, 59, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S.; Scipioni, M.; Catana, C. New Horizons in Brain PET Instrumentation. PET Clin. 2024, 19, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Prenosil, G.A.; Sari, H.; Fürstner, M.; Afshar-Oromieh, A.; Shi, K.; Rominger, A.; Hentschel, M. Performance Characteristics of the Biograph Vision Quadra PET/CT System with a Long Axial Field of View Using the NEMA NU 2-2018 Standard. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Navitsky, M.; Joshi, A.D.; Kennedy, I.; Klunk, W.E.; Rowe, C.C.; Wong, D.F.; Pontecorvo, M.J.; Mintun, M.A.; Devous, M.D. Standardization of amyloid quantitation with florbetapir standardized uptake value ratios to the Centiloid scale. Alzheimer’s Dement. 2018, 14, 1565–1571. [Google Scholar] [CrossRef]
- Shekari, M.; Vállez García, D.; Collij, L.E.; Altomare, D.; Heeman, F.; Pemberton, H.; Roé Vellvé, N.; Bullich, S.; Buckley, C.; Stephens, A.; et al. Stress testing the Centiloid: Precision and variability of PET quantification of amyloid pathology. Alzheimer’s Dement. 2024, 20, 5102–5113. [Google Scholar] [CrossRef]
- Anand, K.; Sabbagh, M. Amyloid Imaging: Poised for Integration into Medical Practice. Neurotherapeutics 2017, 14, 54–61. [Google Scholar] [CrossRef]
- Battle, M.R.; Pillay, L.C.; Lowe, V.J.; Knopman, D.; Kemp, B.; Rowe, C.C.; Doré, V.; Villemagne, V.L.; Buckley, C.J. Centiloid scaling for quantification of brain amyloid with [(18)F]flutemetamol using multiple processing methods. EJNMMI Res. 2018, 8, 107. [Google Scholar] [CrossRef]
- Shang, C.; Sakurai, K.; Nihashi, T.; Arahata, Y.; Takeda, A.; Ishii, K.; Ishii, K.; Matsuda, H.; Ito, K.; Kato, T.; et al. Comparison of consistency in centiloid scale among different analytical methods in amyloid PET: The CapAIBL, VIZCalc, and Amyquant methods. Ann. Nucl. Med. 2024, 38, 460–467. [Google Scholar] [CrossRef]

| Group | n (Male/Female) | Age, Median (Range) |
|---|---|---|
| Total | 239 (99/140) | 76 (45–89) |
| Flutemetamol | 151 (67/84) | 76 (45–89) |
| Florbetaben | 88 (32/56) | 77 (54–89) |
| Visually Negative | Visually Positive | p-Value | |
|---|---|---|---|
| BTXBrain | −6.3 ± 9.9 | 73.9 ± 30.4 | <0.001 |
| MIM | −0.2 ± 12.1 | 83.6 ± 30.3 | <0.001 |
| SCALE PET | −3.0 ± 11.3 | 88.2 ± 33.8 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, H.; Lee, N.; Um, Y.H.; Choi, W.H. Consistency Analysis of Centiloid Values Across Three Commercial Software Platforms for Amyloid PET Quantification. Diagnostics 2025, 15, 1599. https://doi.org/10.3390/diagnostics15131599
Yoon H, Lee N, Um YH, Choi WH. Consistency Analysis of Centiloid Values Across Three Commercial Software Platforms for Amyloid PET Quantification. Diagnostics. 2025; 15(13):1599. https://doi.org/10.3390/diagnostics15131599
Chicago/Turabian StyleYoon, Hyukjin, Narae Lee, Yoo Hyun Um, and Woo Hee Choi. 2025. "Consistency Analysis of Centiloid Values Across Three Commercial Software Platforms for Amyloid PET Quantification" Diagnostics 15, no. 13: 1599. https://doi.org/10.3390/diagnostics15131599
APA StyleYoon, H., Lee, N., Um, Y. H., & Choi, W. H. (2025). Consistency Analysis of Centiloid Values Across Three Commercial Software Platforms for Amyloid PET Quantification. Diagnostics, 15(13), 1599. https://doi.org/10.3390/diagnostics15131599





