Comparing FIB-4, VCTE, pSWE, 2D-SWE, and MRE Thresholds and Diagnostic Accuracies for Detecting Hepatic Fibrosis in Patients with MASLD: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Selection Criteria
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Data Analysis
3. Results
3.1. Study, Patient, and Imaging Characteristics
3.2. Diagnostic Accuracy by Modality
3.3. Subgroup Analysis
3.4. Risk of Bias Assessment
4. Discussion
4.1. Limitations
4.2. Future Research
4.3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
Appendix A
| Database | Search Strategy |
|---|---|
| MEDLINE | (exp Non-alcoholic Fatty Liver Disease/OR nonalcoholic fatty liver disease.mp. OR NAFLD.mp. OR metabolic dysfunction-associated steatotic liver disease.mp. OR MASLD.mp.) AND (FIB-4.mp. OR exp Elasticity Imaging Techniques/OR [transient elastography.mp. OR Fibroscan.mp.] OR shear wave elastography.mp. OR SWE.mp. OR pSWE.mp. OR 2D-SWE.mp. OR magnetic resonance elastography.mp. OR MRE.mp.) AND (exp “Sensitivity and Specificity”/OR sensitivity.mp. OR specificity.mp. OR performance.mp. OR exp Data Accuracy/OR accuracy.mp.) |
| EMBASE | (exp nonalcoholic fatty liver/OR nonalcoholic fatty liver disease.mp. OR NAFLD.mp. OR Metabolic dysfunction-associated steatotic liver disease.mp. OR MASLD.mp.) AND (FIB-4.mp. OR exp elastography/OR exp transient elastography/or exp elasticity/OR transient elastography.mp. OR Fibroscan.mp. OR exp elastograph/OR exp shear wave elastography/OR shear wave elastography.mp. OR SWE.mp. OR pSWE.mp. OR 2D-SWE.mp. OR exp. magnetic resonance elastography/OR magnetic resonance elastography.mp. OR MRE.mp.) AND (exp “sensitivity and specificity”/OR sensitivity.mp. OR specificity.mp. OR performance.mp. OR exp performance/OR exp data accuracy/OR exp diagnostic test accuracy study/OR acciracy.mp. OR exp diagnostic accuracy/) |
| Scopus | (TITLE-ABS-KEY ((non-alcoholic AND fatty AND liver AND disease) OR nafld OR (metabolic AND dysfunction-associated AND steatotic AND liver AND disease) OR masld) AND TITLE-ABS-KEY (fib-4 OR (transient AND elastography) OR fibroscan OR (shear AND wave AND elastography) OR swe OR pswe OR 2d-swe OR (magneticAND resonance AND elastography) OR mre) AND TITLE-ABS-KEY (sensitivity OR specificity OR performance OR accuracy)) |
| Cochrane Library | (nonalcoholic fatty liver disease OR nafld OR metabolic dysfunction-associated steatotic liver disease OR masld) AND (FIB-4 OR transient elastography OR fibroscan OR shear wave elastography OR see OR pswe OR 2d-swe OR magnetic resonance elastography OR mre) AND (sensitivity OR specificity OR performance OR accuracy) |
Appendix B
| Author | Year | Country | Study Design | Centers | Total # Patients | Mean Age (Range) | Male Sex (%) | Total Patients |
|---|---|---|---|---|---|---|---|---|
| Chen [11] | 2022 | China | Retrospective | Single-center | 100 | 32 (16–65) | 31 | 100 |
| Lee [12] | 2022 | Republic of Korea | Retrospective | Multicenter | 251 | 44 (34–56) | 53 | 251 |
| Takeuchi [13] | 2018 | Japan | Prospective | Single-center | 71 | 51 (18–82) | 65 | 71 |
| da Silva [14] | 2021 | Brazil | Prospective | Single-center | 108 | 45 | 21 | 108 |
| Duman [15] | 2024 | Turkey | Retrospective | Single-center | 119 | 54 (20–73) | 32 | 119 |
| Cui [16] | 2015 | USA | Prospective | Single-center | 102 | 51 | 41 | 102 |
| Inada [17] | 2022 | Japan | Retrospective | Single-center | 105 | 65 (58–72) | 45 | 105 |
| Tamaki [18] | 2023 | USA and Japan | Both | Multicenter | 806 | NR | 48 | 806 |
| Ogawa [19] | 2018 | Japan | Retrospective | Single-center | 165 | 54 | 58 | 165 |
| Jung [20] | 2021 | USA | Prospective | Multi-center | 238 | 51 (37–65) | 46 | 238 |
| Armandi [21] | 2023 | Italy | Prospective | Single-center | 96 | 50 (20–74) | 62 | 96 |
| Wong [22] | 2010 | France and HK | Prospective | Multicenter | 245 | 51 | 55 | 245 |
| Boursier [23] | 2016 | France | Prospective | Multicenter | 588 | 56 | 57 | 452 |
| Pennisi [24] | 2023 | Italy | Prospective | Single-center | 520 | 52 (25–78) | 65 | 520 |
| Pennisi [25] | 2023 | Multi-country | Retrospective | Multicenter | 1780 | 61 (54–67) | 58 | 1780 |
| Prat [26] | 2019 | United Kingdom | Retrospective | Single-center | 27 | 48 (38–58) | 100 | 27 |
| Arvaniti [27] | 2023 | Greece | Retrospective | Single-center | 38 | 50 (16–69) | 61 | 38 |
| Kao [28] | 2020 | Taiwan | Prospective | Single-center | 123 | 36 | 29 | 123 |
| Castera [29] | 2023 | France | Prospective | Multicenter | 163 | 59 (median) | 58 | 163 |
| Petta [30] | 2019 | Italy, France, Hong Kong, China | Prospective | Multicenter | 968 | 50 | 63 | 968 |
| Staufer [31] | 2019 | Austria | Prospective | Multicenter | 186 | 52 (39–60) | 57 | 186 |
| Boursier [32] | 2023 | France | Prospective | Multicenter | 1051 | 58 [50–66] | 60 | 1051 |
| Noureddin [33] | 2023 | USA | Retrospective | Multicenter | 548 | 58 | 35 | 548 |
| Noureddin [34] | 2021 | USA | Retrospective | Multicenter | 1308 | 57 (median) | NR | 1308 |
| Cheung [35] | 2023 | Malaysia, Hong Kong, China | Prospective | Multicenter | 431 | 48 | 57 | 431 |
| Bhadoria [36] | 2017 | India | Retrospective | Single-center | 779 | 44 | 75 | 779 |
| Anstee [37] | 2019 | Multicenter | Prospective | Multicenter | 3123 | 59 | 58 | 3123 |
| Anstee [38] | 2020 | UK | Prospective | Multicenter | 420 | 58 (44–74) | 52 | 420 |
| Labenz [39] | 2018 | Germany | Prospective | Single-center | 243 | 51 (19–93) | 53 | 243 |
| Arora [40] | 2023 | India, Singapore | Retrospective | Multicenter | 641 | 43 | 55 | 641 |
| Barritt [41] | 2019 | USA | Retrospective | Multicenter | 1549 | 59 | 45 | 1549 |
| Harrison [42] | 2020 | USA | Prospective | Multi-center | 320 | 55 | 38 | 307 |
| Petta [43] | 2015 | Italy | Prospective | Single-center | 179 | 45 (18–72) | 68 | 179 |
| Gabriel-Medina [44] | 2023 | Spain | Prospective | Single-center | 140 | 59 | 42 | 140 |
| Zhang [45] | 2023 | China | Prospective | Single-center | 71 | 46 | 68 | 71 |
| Eddowes [46] | 2019 | UK | Prospective | Multicenter | 356 | 53 (42–64) | 57 | 356 |
| Sanyal [47] | 2023 | Multiple | Not specified | Multicenter | 1434 | 55 | 51 | 3176 |
| Boursier [48] | 2019 | France | Prospective | Multicenter | 938 | 57 (18–80) | 59 | 938 |
| Bertot [49] | 2023 | Australia | Retrospective | Single-center | 271 | 52 (40–64) | 40 | 271 |
| Kobayashi [50] | 2017 | Japan | Retrospective | Single-center | 229 | 56 (45–64) | 46 | 229 |
| Eren [51] | 2022 | Turkey | Retrospective | Multicenter | 560 | 48 (18–71) | 53 | 560 |
| Singh [52] | 2020 | USA | Retrospective | Single-center | 1157 | 51 | 35 | 1157 |
| Marella [53] | 2020 | USA | Retrospective | Single-center | 907 | 47 | 68 | 907 |
| Treeprasertsuk [54] | 2016 | Thailand | Prospective | Single-center | 139 | 41 | 47 | 139 |
| McPherson [55] | 2013 | UK | Retrospective | Single-center | 305 | 48 | 63 | 305 |
| Kolhe [56] | 2019 | India | Retrospective | Single-center | 100 | 46 (18–80) | 53 | 100 |
| Kaya [57] | 2019 | Turkey | Retrospective | Single-center | 463 | 46 | 53 | 463 |
| Nones [58] | 2017 | Brazil | Retrospective | Multicenter | 67 | 55 | 37 | 67 |
| Balakrishnan [59] | 2018 | USA | Cross-sectional | Single-center | 122 | 47 | 20 | 122 |
| Alkayyali [60] | 2020 | Turkey | Retrospective | Single-center | 349 | 48 (38–58) | 43 | 349 |
| Nielsen [61] | 2021 | Denmark | Retrospective | Multicenter | 517 | 55 (54–56) | 52 | 517 |
| Ampuero [62] | 2020 | Spain, France, Italy, Cuba, China | Retrospective | Multicenter | 2452 | 52 (18–57) | 55 | 2452 |
| Sang [63] | 2021 | China, Malaysia, India | Retrospective | Multicenter | 540 | 47 (18–57) | 52 | 540 |
| Shima [64] | 2020 | Japan | Retrospective | Single-center | 278 | 58 (18–57) | 48 | 278 |
| Seko [65] | 2023 | Japan | Retrospective | Multicenter | 371 | 61 (17–85) | 43 | 371 |
| Siddiqui [66] | 2019 | USA | Retrospective | Multicenter | 1904 | 50 (18–57) | 37 | 1904 |
| Li [67] | 2024 | Hong Kong | Retrospective | Single-center | 279 | 52 (18–57) | 55 | 599 |
| Sanyal [68] | 2023 | USA | Retrospective | Multicenter | 1073 | 53 (10–56) | 38 | 1073 |
| Moon [69] | 2023 | Republic of Korea | Retrospective | Single-center | 118 | NR | NR | 118 |
| Zambrano-Huailla [70] | 2020 | Peru, Brazil, Argentina | Retrospective | Multicenter | 379 | 46 (18–75) | 30 | 379 |
| Yang [71] | 2022 | China | Retrospective | Single-center | 309 | 46 (18–57) | 52 | 309 |
| Giammarino [72] | 2022 | USA | Retrospective | Single-center | 244 | NR | NR | 244 |
| Nishikawa [73] | 2016 | Japan | Retrospective | Single-center | 134 | 52 (18–57) | 49 | 134 |
| Kakisaka [74] | 2018 | Japan | Retrospective | Single-center | 125 | 51 | 46 | 125 |
| Schmitz [75] | 2020 | Germany | Prospective | Single-center | 141 | 43 | 27 | 141 |
| Zhang [76] | 2023 | China | Retrospective | Single-center | 105 | 46 (15–69) | 52 | 105 |
| Balakrishnan [77] | 2021 | USA | Retrospective | Single-center | 99 | 47 | 74 | 99 |
| Kariyama [78] | 2022 | Japan | Retrospective | Multicenter | 1059 | 55 (14–87) | 52 | 1059 |
| Mohammed [79] | 2019 | Egypt | Prospective | Single-center | 100 | 47 | 38 | 100 |
| de la Tijera [80] | 2021 | Mexico | Retrospective | Multicenter | 222 | 46 (37–54) | 26 | 222 |
| McPherson [81] | 2015 | UK | Prospective | Multicenter | 634 | 69 (66–72) | 35 | 634 |
| Shah [82] | 2020 | USA | Prospective | Multicenter | 2056 | NR | NR | 2056 |
| Maurice [83] | 2021 | UK, USA, Italy, Canada | Retrospective | Multicenter | 116 | 48 | 93 | 116 |
| Zhou [84] | 2019 | China | Prospective | Single-center | 207 | 42 (18–75) | 73 | 207 |
| Kouvari [85] | 2023 | USA, Italy, Greece, Australia | Prospective | Multicenter | 455 | 53 (51–56) | 52 | 455 |
| Ballestri [86] | 2021 | Italy | Prospective | Single-center | 107 | 48 | 72 | 107 |
| Noureddin [87] | 2022 | USA | Retrospective | Multicenter | 232 | 56 | 60 | 232 |
| Singh [88] | 2022 | India | Prospective | Single-center | 129 | 40 | NR | 129 |
| Prasad [89] | 2020 | India | Retrospective | Single-center | 240 | 39 | 78 | 240 |
| Kim [90] | 2022 | USA | Retrospective | Single-center | 363 | 51 (median) | 46 | 363 |
| Yoneda [91] | 2013 | Japan | Retrospective | Multicenter | 1102 | 60 | NR | 1102 |
| Qadri [92] | 2022 | Finland | Retrospective | Single-center | 378 | 50 (18–75) | 29 | 378 |
| Miller [93] | 2019 | USA | Retrospective | Single-center | 354 | 50 (37–63) | 42 | 354 |
| Drolz [94] | 2021 | Germany | Retrospective | Single-center | 368 | 47 (35–56) | 43 | 368 |
| Meneses [95] | 2020 | Spain | Prospective | Single-center | 50 | 49 (18–57) | 30 | 50 |
| Alqahtani [96] | 2021 | USA | Retrospective | Single-center | 584 | 43 (18–57) | 21 | 584 |
| De Carli [97] | 2020 | Brazil | Retrospective | Single-center | 323 | 37 | 76 | 266 |
| Ito [98] | 2023 | Japan, Taiwan, Korea | Retrospective | Multicenter | 1489 | 46 (18–57) | 54 | 1489 |
| Bril [99] | 2020 | USA | Retrospective | Single-center | 213 | 58 (50–66) | 84 | 213 |
| Satapathy [100] | 2019 | USA | Retrospective | Multicenter | 269 | NR | NR | 269 |
| Moon [101] | 2024 | Republic of Korea | Retrospective | Multicenter | 231 | 46 (18–57) | 54 | 231 |
| Schwenger [102] | 2023 | Canada | Retrospective | Single-center | 170 | 47 (18–57) | 79 | 131 |
| Andrade [103] | 2022 | Brazil | Retrospective | Single-center | 143 | 48 (19–68) | 34 | 143 |
| Aida [104] | 2015 | Japan | Retrospective | Single-center | 148 | 61 (46–70) | 36 | 148 |
| Huang [105] | 2023 | China | Prospective | Single-center | 373 | 31 (18–57) | 34 | 373 |
| McPherson [106] | 2010 | UK | Retrospective | Single-center | 145 | 51 (18–57) | 61 | 145 |
| Kaya [107] | 2020 | Turkey | Retrospective | Single-center | 463 | 46 | 48 | 463 |
| Xun [108] | 2012 | China | Retrospective | Single-center | 152 | 37 (18–57) | 80 | 152 |
| Mikolasevic [109] | 2022 | Croatia | Retrospective | Single-center | 135 | 59 (52–68) | 52 | 135 |
| Younossi [110] | 2023 | USA | Retrospective | Single-center | 463 | 48 | 31 | 463 |
| McPherson [111] | 2012 | UK | Retrospective | Single-center | 123 | 53 (42–64) | 54 | 123 |
| Sanyal [112] | 2023 | Global (including US and Europe) | Retrospective | Multicenter | 2053 | 54 | 62 | 410 |
| Soresi [113] | 2020 | Italy | Retrospective | Single-center | 57 | 42 (18–57) | 28 | 57 |
| Kaya [114] | 2020 | Turkey | Retrospective | Single-center | 107 | 52 (29–71) | 36 | 107 |
| Kawamura [115] | 2013 | Japan | Retrospective | Single-center | 30 | 60 (29–80) | 73 | 30 |
| Kalaiyarasi [116] | 2024 | Singapore | Prospective | Single-center | 16 | 49 (18–57) | 31 | 16 |
| Ishiba [117] | 2021 | Japan | Retrospective | Multicenter | 311 | 58 (16–84) | 59 | 311 |
| Udelsman [118] | 2021 | USA | Retrospective | Single-center | 2465 | 46 (18–57) | 29 | 2465 |
| Zain [119] | 2020 | Malaysia | Retrospective | Single-center | 122 | 50 (18–57) | 50 | 122 |
| Lubner [120] | 2021 | USA | Retrospective | Single-center | 186 | 49 | 40 | 186 |
| Soontornmanokul [121] | 2013 | Thailand | Retrospective | Multicenter | 115 | 51 (18–31) | 50 | 115 |
| Wu [122] | 2021 | China | Retrospective | Single-center | 58 | 41 (18–57) | 85 | 58 |
| Le [123] | 2018 | USA | Retrospective | Single-center | 254 | 50 | 35 | 254 |
| Sumida [124] | 2012 | Japan | Retrospective | Multicenter | 576 | 52 (15) | 51 | 576 |
| Chong [125] | 2023 | Malaysia | Retrospective | Single-center | 196 | 50 (39–61) | 50 | 196 |
| Pérez-Gutiérrez [126] | 2013 | Mexico and Chile | Retrospective | Multicenter | 228 | 49 (36–61) | 49 | 228 |
| Singh [127] | 2017 | USA | Retrospective | Multicenter | 1157 | NR | NR | 1157 |
| Panackel [128] | 2019 | India | Retrospective | Single-center | 113 | 49 (37–61) | 55 | 113 |
| Sanyal [129] | 2023 | USA | Prospective | Multicenter | 1073 | 54 | 38 | 1073 |
| Kolhe [130] | 2018 | India | Retrospective | Single-center | 100 | 44 (31–56) | 42 | 100 |
| Luger [131] | 2016 | Austria | Prospective | Single-center | 46 | 42 (13) | 20 | 46 |
Appendix C
| Author | Modality | Year | Country | Study Design | Centers | Vendor Type | Probe Frequency | Min Number of Acquisitions | Technician Experience | Mean Age (Range) | Male Sex (%) | Total Patients |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medellin [132] | pSWE | 2019 | Canada | Prospective | Single-center | Siemens | NR | 10 | 3–20 years | 57 (45–79) | 51 | 47 |
| da Silva [14] | pSWE | 2021 | Brazil | Prospective | Single-center | Siemens | 4–1 MHz | 6 | >5 years | 45 | 21 | 79 |
| Roccarina [133] | pSWE | 2017 | UK | Prospective | Single-center | Philips | NR | NR | NR | 56 | 59 | 60 |
| Leong [134] | pSWE | 2020 | Malaysia | Prospective | Single-center | Philips | NR | 3 | “Experienced” | 57 (47–67) | 46 | 100 |
| Kapur [135] | pSWE | 2021 | India | Prospective | Single-center | Philips | 1–5 MHz | NR | NR | 39 | 71 | 17 |
| Argalia [136] | pSWE | 2022 | Italy | Prospective | Single-center | Philips | NR | 10 | >years | 52 | 64 | 50 |
| Roccarina [137] | pSWE | 2022 | UK | Retrospective | Single-center | Philips | NR | 10 | “Experienced” | 56 (43–69) | 57 | 159 |
| Taibbi [138] | pSWE | 2021 | Italy | Prospective | Single-center | Samsung | 1–7 MHz | 10 | >15 years | 55 (40–73) | 59 | 46 |
| Cui [139] | pSWE | 2016 | USA | Prospective | Single-center | Siemens | 1–5 MHz | 11 | 0.5 years | 49 | 46 | 125 |
| Cassinotto [140] | pSWE | 2016 | France | Prospective | Multicenter | Siemens | NR | 10 | >2 years | 57 (18–80) | 59 | 236 |
| Tomeno [141] | pSWE | 2009 | Japan | Prospective | Single-center | Siemens | NR | NR | NR | 52 | NR | 50 |
| Yoneda [142] | pSWE | 2010 | Japan | Prospective | Single-center | Siemens | 4 MHz | 10 | “Experienced” | 54 | 51 | NR |
| Braticevici [143] | pSWE | 2013 | Romania | Prospective | Single-center | Siemens | 4-MHz | 10 | NR | 51 (47–90) | 44 | 64 |
| Lee [144] | 2D SWE | 2021 | Republic of Korea | Prospective | Single-center | Canon | 1–8 MHz | 9 | Radiologist NOS | 48 (30–63) | 43 | 102 |
| Zhang [145] | 2D SWE | 2022 | USA | Prospective | Single-center | GE | 1–6 MHz | 10 | >10 years | 51.8 (25–78) | 46 | 100 |
| Furlan [146] | 2D SWE | 2020 | USA | Prospective | Single-center | GE | 2–5 MHz | 10 | >10 years | 50 (24–53) | 42 | 57 |
| Yu Ogino [147] | 2D SWE | 2023 | Japan | Retrospective | Single-center | GE | NR | 6 | >26 years | 51 (37–65) | 61 | 107 |
| Zhou [148] | 2D SWE | 2022 | China | Retrospective | Single-center | SuperSonic Imagine | NR | 5 | >10 years | 46 (18–77) | 47 | 116 |
| Ozturk [149] | 2D SWE | 2020 | USA | Retrospective | Single-center | SuperSonic Imagine | 1–6 MHz | 10 | Variable | 51 (39–62) | 47 | 116 |
| Sharpton [150] | 2D SWE | 2021 | USA | Prospective | Single-center | SuperSonic Imagine | 1–6 MHz | 3 | >1 year | 55 (45–64) | 54 | 114 |
| Taru [151] | 2D SWE | 2024 | Romania | Retrospective | Single-center | SuperSonic Imagine | 1–6 MHz | 5 | NR | NR | NR | 149 |
| Imajo [152] | 2D SWE | 2022 | Japan | Prospective | Single-center | GE | 3–6 MHz | 10 | >6 years | 61 (51–71) | 53 | 201 |
| Herrmann [153] | 2D SWE | 2018 | Multi-country | Retrospective | Multicenter | SuperSonic Imagine | 2–6 MHz | 1 (variable) | NR | 54 (20–83) | 54 | 156 |
| Chen [11] | 2D SWE | 2022 | China | Retrospective | Single-center | SuperSonic Imagine | 1–6 MHz | 3 | NR | 32 (16–65) | 31 | 100 |
| Mendoza [154] | 2D SWE | 2022 | Switzerland | Prospective | Single-center | SuperSonic Imagine | NR | 3 | NR | 53 (25–78) | 43 | 88 |
| Takeuchi [13] | 2D SWE | 2018 | Japan | Prospective | Single-center | SuperSonic Imagine | 1–6 MHz | 5 | >10 years | 51 (18–82) | 65 | 71 |
| Jamialahmadi [155] | 2D SWE | 2019 | Iran | Prospective | Single-center | SuperSonic Imagine | 1–6 MHz | 10 | NR | 39 (27–50) | 20 | 90 |
| Petzold [156] | 2D SWE | 2020 | Germany | Prospective | Single-center | GE | NR | NR | 6 | 53 | 33 | 70 |
| Didenko [157] | 2D SWE | 2019 | Ukraine | Prospective | Single-center | Ultrasign | 2–5 MHz | NR | Sonographer NOS | 47 | 33 | 24 |
| Sugimoto [158] | 2D SWE | 2020 | Japan | Prospective | Single-center | Canon | 3.5 MHz | 10 | >15 years | 53 | 51 | 111 |
| Kalaiyarasi [116] | 2D SWE | 2024 | Singapore | Prospective | Single-center | GE | 3–5 MHz | 5 | >20 years | 49 | 31 | 16 |
| Seo [159] | 2D SWE | 2023 | Republic of Korea | Prospective | Multicenter | Canon | 1–8 MHz | 10 | NR | 36 (27–50) | 51 | 105 |
| Kuroda [160] | 2D SWE | 2021 | Japan | Prospective | Single-center | GE | 4.0 MHz | 10 | Radiologist NOS | 55 [18–80] | 49 | 202 |
| Jang [161] | 2D SWE | 2022 | Republic of Korea | Prospective | Multicenter | Canon | 1–8 MHz | 5 | 8–28 years | 38 (27–54) | 48 | 132 |
| Kim [162] | 2D SWE | 2022 | Republic of Korea | Retrospective | Single-center | Tobshiba | NR | 10 | NR | 51 [25–78] | 47 | 60 |
| Lee [163] | 2D SWE | 2017 | Republic of Korea | Prospective | Single-center | Siemens | 1–5 MHz | NR | 13 years | 56 (53–58) | 44 | 69 |
| Cassinotto [140] | 2D SWE | 2016 | France | Prospective | Multicenter | Siemens | NR | 10 | >2 years | 57 (18–80) | 59 | 236 |
| Duman [15] | VCTE | 2024 | Turkey | Retrospective | Single center | Echosens | NA | NR | NR | 54 (20–73) | 32 | 119 |
| Gabriel-Medina [44] | VCTE | 2024 | Spain | Retrospective | Single center | Echosens | NA | 10 | “Experienced” | 59 | 42 | 140 |
| Mikolasevic [164] | VCTE | 2021 | Croatia | Prospective | Multicenter | Echosens | NA | 10 | “Trained” | 59 | 51 | 179 |
| Eddowes [165] | VCTE | 2016 | UK | Prospective | Multicenter | Echosens | NA | 10 | NR | 53 (39–66) | 57 | 117 |
| Jun Yang [166] | VCTE | 2019 | UK | Prospective | Single-center | Echosens | NA | NR | NR | NR | NR | 373 |
| Petta [167] | VCTE | 2019 | UK | Prospective | Multicenter | Echosens | NA | NR | NR | 53 | 57 | 356 |
| Gitto [168] | VCTE | 2021 | China | Prospective | Single-center | Echosens | NA | 10 | “Skilled” | 58 (24–74) | 40 | 85 |
| Juan Zhu [169] | VCTE | 2020 | Japan | Prospective | Multicenter | Echosens | NA | 10 | “Experienced” | 52 (35–70) | 58 | 122 |
| Wong [170] | VCTE | 2017 | USA | Prospective | Multicenter | Echosens | NA | NR | NR | 52 | 32 | 292 |
| Kawamura [173] | VCTE | 2015 | China | Prospective | Single center | Echosens | NA | 10 | NR | 50 | 54 | 203 |
| Imajo [152] | VCTE | 2017 | Japan | Prospective | Single center | Echosens | NA | 10 | NR | 57 | 50 | 171 |
| Bae [174] | VCTE | 2023 | China | Retrospective | Single center | Echosens | NA | 10 | NR | 46 (18–73) | 68 | 71 |
| Park [174] | VCTE | 2021 | The Netherlands | Prospective | Single center | Echosens | NA | NR | >50 exams | 49.5 (20–74) | 62 | 37 |
| Hockings [208] | VCTE | 2020 | USA | Prospective | Single center | Echosens | NA | 10 | >100 exams | 50 (24–53) | 42 | 59 |
| Chung [175] | VCTE | 2022 | Republic of Korea | Retrospective | Multicenter | Echosens | NA | 10 | >500 exams | 44 (34–56) | 53 | 251 |
| Costa-Silva [214] | VCTE | 2021 | USA | Prospective | Single center | Echosens | NA | 10 | “Trained” | 55 (45–64) | 54 | 114 |
| Yilmaz [176] | VCTE | 2024 | Romania | Retrospective | Single center | Echosens | NA | 10 | NR | NR | NR | 149 |
| Bahl [177] | VCTE | 2022 | Japan | Retrospective | Multicenter | Echosens | NA | NR | NR | NR | NR | 126 |
| Pan [178] | VCTE | 2022 | Japan | Prospective | Single center | Echosens | NA | 10 | 6 years | 61 (51–71) | 53 | 201 |
| Lu [179] | VCTE | 2017 | UK | Prospective | Single center | Echosens | NA | NR | NR | 56 | 59 | 60 |
| Fujii [180] | VCTE | 2022 | Switzerland | Prospective | Single-center | Echosens | NA | 3 | NR | 53 [25–78] | 58 | 102 |
| Machado [181] | VCTE | 2024 | Singapore | Prospective | Single-center | Echosens | NA | 10 | NR | 49 | 5 | 16 |
| Tokushige [182] | VCTE | 2023 | Republic of Korea | Retrospective | Multicenter | Echosens | NA | NR | NR | 36 (27–50) | 51 | 105 |
| Yang [184] | VCTE | 2021 | Japan | Prospective | Single-center | Echosens | NA | NR | “Experienced” | 55 (18–80) | 49 | 202 |
| Ruiz-Fernandez [185] | VCTE | 2022 | Republic of Korea | Retrospective | Single-center | Echosens | NA | NR | 10 | 51 (25–78) | 47 | 60 |
| Del Barrio Azaceta [186] | VCTE | 2016 | Republic of Korea | Prospective | Single-center | Echosens | NA | NR | >1000 exam | 56 (53–58) | 44 | 94 |
| Hernandez-Rocha [187] | VCTE | 2020 | Malaysia | Prospective | Single-center | Echosens | NA | 10 | “Trained” | 57 (47–67) | 46 | 100 |
| Zheng [188] | VCTE | 2021 | Italy | Prospective | Single-center | Echosens | NA | 10 | >15 years | 55 (40–73) | 59 | 46 |
| Chuah [189] | VCTE | 2022 | Italy | Prospective | Single-center | Echosens | NA | 10 | NR | 52 | 64 | 50 |
| Ghanvatkar [190] | VCTE | 2022 | UK | Retrospective | Single center | Echosens | NA | 10 | “Experienced” | 56 | NR | 159 |
| Chu [191] | VCTE | 2017 | USA | Prospective | Single-center | Echosens | NA | 10 | NR | 51 | 43 | 94 |
| Yang [192] | VCTE | 2016 | Japan | Prospective | Multicenter | Echosens | NA | 10 | NR | 58 | 57 | 127 |
| Roh [193] | VCTE | 2018 | Japan | Retrospective | Single-center | Echosens | NA | NR | NR | 54 | 58 | 113 |
| Bob Harrap [194] | VCTE | 2023 | Italy | Prospective | Single center | Echosens | NA | 10 | “Experienced” | 50 (20–74) | 62 | 96 |
| de Ledinghen [195] | VCTE | 2010 | HK, France | Prospective | Multicenter | Echosens | NA | 10 | NR | 51 | 55 | 246 |
| Garteiser [196] | VCTE | 2016 | France | Retrospective | Multicenter | Echosens | NA | 10 | “Experienced” | 56 | 57 | 452 |
| Gaia [197] | VCTE | 2023 | Iran | Prospective | Multicenter | Echosens | NA | 10 | >500 exams | 43 | 62 | 73 |
| Alsaqal [198] | VCTE | 2010 | Malaysia | Prospective | Single-center | Echosens | NA | NR | NR | 49 | 60 | 25 |
| Naveau [199] | VCTE | 2023 | Italy | Retrospective | Single center | Echosens | NA | 10 | “Experienced” | 52 (25–78) | 65 | 520 |
| Jafarov [200] | VCTE | 2017 | Hong Kong | Prospective | Single-center | Echosens | NA | 10 | >50 exams | 52 (41–57) | 55 | 215 |
| Tapper [201] | VCTE | 2023 | Multi-country | Prospective | Multicenter | Echosens | NA | NR | NR | 51 | 58 | 632 |
| Chakraborty [202] | VCTE | 2021 | Brazil | Prospective | Single-center | Echosens | NA | 10 | NR | 36 (20–67) | 31 | 85 |
| Siddiqui [203] | VCTE | 2016 | Republic of Korea | Prospective | Multicenter | Echosens | NA | 10 | “Experienced” | 41 | 61 | 183 |
| Pathik [204] | VCTE | 2019 | UK | Retrospective | Single-center | Echosens | NA | 10 | NR | 47 (37–57) | 81 | 27 |
| Ergelen [205] | VCTE | 2018 | Australia | Prospective | Multi-center | Echosens | NA | 10 | >2000 exams | 46 | 32 | 66 |
| Kosick [206] | VCTE | 2019 | Austria | Prospective | Multicenter | Echosens | NA | 10 | NR | 52 | 57 | 140 |
| Tovo [207] | VCTE | 2023 | China | Retrospective | Single-center | Echosens | NA | NR | NR | 43 (35–59) | 59 | 172 |
| Li [67] | VCTE | 2012 | Canada | Prospective | Multicenter | Echosens | NA | 10 | “Experienced” | 50 (43–57) | 63 | 75 |
| Bhatia [104] | VCTE | 2023 | Greece | Retrospective | Single-center | Echosens | NA | 10 | >10 years | 50 (16–69) | 60 | 38 |
| Koh [105] | VCTE | 2020 | Taiwan | Prospective | Single-center | Echosens | NA | 10 | "Trained" | 36 | 29 | 123 |
| Boursier [48] | VCTE | 2023 | France | Prospective | Multicenter | Echosens | NA | NR | NR | 59 (median) | 58 | 163 |
| Bertot [49] | VCTE | 2022 | Spain | Prospective | Single-center | Echosens | NA | NR | NR | 51 | 40 | 115 |
| Al-Fryan [24] | VCTE | 2019 | Italy, France, HK, China | Prospective | Multicenter | Echosens | NA | 10 | >300 exams | 50 | 63 | 968 |
| Chawla [25] | VCTE | 2023 | Spain | Prospective | Multicenter | Echosens | NA | 10 | NR | 56 | 66 | 1124 |
| Hernandez [26] | VCTE | 2015 | China | Prospective | Multicenter | Echosens | NA | 10 | >300 exams | 57 (47–67) | 46 | 101 |
| Pópulo [27] | VCTE | 2023 | France | Prospective | Multicenter | Echosens | NA | 10 | NR | 58 (50–66) | 60 | 1051 |
| Jeong [28] | VCTE | 2021 | USA | Retrospective | Multicenter | Echosens | NA | NR | NR | 57 (median) | NR | 1308 |
| Kwee [29] | VCTE | 2023 | USA | Retrospective | Multicenter | Echosens | NA | 10 | “Trained” | 58 | 35 | 548 |
| Trifan [30] | VCTE | 2014 | USA | Prospective | Single-center | Echosens | NA | NR | NR | 54 (44–64) | 40 | 94 |
| Gaia-Póvoa [31] | VCTE | 2016 | France | Prospective | Multicenter | Echosens | NA | 10 | “Experienced” | 57 (18–80) | 59 | 223 |
| Boursier [32] | VCTE | 2020 | Malaysia | Retrospective | Single-center | Echosens | NA | 10 | “Trained” | 55 | 45 | 136 |
| Younes [33] | VCTE | 2023 | Malaysia/HK/China | Prospective | Multicenter | Echosens | NA | 10 | NR | 48 | 57 | 396 |
| Marra [34] | VCTE | 2015 | Turkey | Prospective | Single-center | Echosens | NA | 10 | NR | 46 (24–62) | 49 | 87 |
| Liu [35] | VCTE | 2017 | India | Retrospective | Single-center | Echosens | NA | 10 | NR | 44 | 75 | 779 |
| Yoo [36] | VCTE | 2010 | Japan | Prospective | Single-center | Echosens | NA | 10 | “Experienced” | 51 | 46 | 54 |
| Saadeh [37] | VCTE | 2008 | Japan | Prospective | Multicenter | Echosens | NA | 10 | NR | 52 [25–78] | 41 | 97 |
| Watanabe [38] | VCTE | 2019 | Multicenter | Prospective | Multicenter | Echosens | NA | 10 | NR | 59 (53–65) | 58 | 1765 |
| Xiao [39] | VCTE | 2021 | China | Prospective | Single-center | Echosens | NA | 10 | “Experienced” | 40 (32–56) | 51 | 91 |
| Cai [40] | VCTE | 2013 | Malaysia | Prospective | Single-center | Echosens | NA | 10 | NR | 50 (38–62) | 53 | 120 |
| Barritt [41] | VCTE | 2010 | Romania | Prospective | Single-center | Echosens | NA | 10 | NR | 42 (20–69) | 71 | 72 |
| Harrison [42] | VCTE | 2023 | India, Singapore | Retrospective | Multicenter | Echosens | NA | 10 | “Trained” | 43 | 55 | 641 |
| Petta [43] | VCTE | 2009 | France/China | Prospective | Multicenter | Echosens | NA | NR | NR | 51 | 55 | 208 |
| Selvarajah [45] | VCTE | 2018 | Germany | Prospective | Single-center | Echosens | NA | 10 | “Trained” | 51 (19–93) | 53 | 126 |
| Djordjevic [46] | VCTE | 2017 | Republic of Korea | Prospective | Multicenter | Echosens | NA | 10 | >1000 exams | 56 (53–58) | 44 | 94 |
| Sanyal [47] | VCTE | 2021 | France | Prospective | Single-center | Echosens | NA | 10 | >100 exams | 42 ± 11 | 16 | 152 |
| Aziz [50] | VCTE | 2011 | Italy | Prospective | Single-center | Echosens | NA | 10 | “Trained” | 48 (24–65) | 72 | 72 |
| Ruiz [51] | VCTE | 2019 | USA | Retrospective | Multicenter | Echosens | NA | 10 | “Experienced” | 59 | 45 | 1549 |
| Jang [52] | VCTE | 2020 | USA | Prospective | Multi-center | Echosens | NA | 10 | NR | 55 | 62 | 212 |
| Sung [53] | VCTE | 2015 | Italy | Prospective | Multicenter | Echosens | NA | 10 | NR | 45 (18–78) | 70 | 321 |
| Kim [54] | VCTE | 2022 | Sweden | Prospective | Single-center | Echosens | NA | 10 | “Experienced” | 55 (13.09) | 59 | 66 |
| Liang [55] | VCTE | 2014 | France | Prospective | Single-center | Echosens | NA | 10 | NR | 43 | 19 | 100 |
| Zhang [56] | VCTE | 2020 | Turkey | Prospective | Single-center | Echosens | NA | 10 | >15,000 exams | 49 | 59 | 139 |
| Wang [57] | VCTE | 2016 | USA | Prospective | Single-center | Echosens | NA | 10 | >500 exams | 51 | 59 | 120 |
| Cho [58] | VCTE | 2019 | USA | Retrospective | Single-center | Echosens | NA | 10 | NR | 54 | 191 | |
| Toubia [59] | VCTE | 2019 | USA | Prospective | Multicenter | Echosens | NA | 10 | “Trained” | 51 | 32 | 393 |
| Kim [60] | VCTE | 2015 | India | Prospective | Single-center | Echosens | NA | 10 | NR | 42 (18–80) | 46 | 110 |
| Nam [61] | VCTE | 2023 | Multiple | Not specified | Multicenter | Echosens | NA | 8 | NR | 55 | 51 | 1434 |
| Moon [62] | VCTE | 2019 | France | Prospective | Multicenter | Echosens | NA | 10 | NR | 57 (18–80) | 59 | 938 |
| Kim [63] | VCTE | 2023 | Australia | Retrospective | Single-center | Echosens | NA | 10 | “Experienced” | 52 (40–64) | 40 | 125 |
| Park [64] | VCTE | 2016 | Turkey | Prospective | Single-center | Echosens | NA | 10 | NR | 47 (25–78) | 62 | 63 |
| Cho [65] | VCTE | 2019 | Canada | Retrospective | Single-center | Echosens | NA | NR | NR | 55 (50–64) | 54 | 86 |
| Jeong [66] | VCTE | 2024 | Hong Kong | Retrospective | Single | Echosens | NA | 10 | “Experienced” | 52 (18–57) | 55 | 431 |
| Li [67] | VCTE | 2019 | Brazil | Prospective | Multicenter | Echosens | NA | 10 | >500 exams | 55 (45–65) | 26 | 104 |
Appendix D
| Author | Year | Country | Study Design | Centers | Vendor Type | Reader Experience | MRI Field Strength | Pulse Sequence | Driver Amplitude | Segmentation Used | Mean Age (Range) | Male Sex (%) | Total Patients |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duman [15] | 2024 | Turkey | Retrospective | Single-center | Siemens | NR | 1.5T | 2D GRE | NR | Manual ROI | 54 (20–73) | 32 | 119 |
| Troelstra [172] | 2021 | The Netherlands | Prospective | Single-center | Phillips | 3 years | 3T | 2D GRE | 50 Hz | Manual ROI | 49 | 62 | 35 |
| Hockings [208] | 2020 | Sweden | NR | Not specified | NR | NR | NR | NR | NR | NR | NR | NR | 68 |
| Cui [16] | 2015 | USA | Prospective | Single-center | GE | NR | 3T | 2D GRE | NR | Manual ROI | 51 | 41 | 102 |
| Zhang [145] | 2022 | USA | Prospective | Single-center | GE | >1 year | 3T | 2D GRE | 60 Hz | Manual ROI | 52 (25–78) | 46 | 100 |
| Furlan [146] | 2020 | USA | Prospective | Single-center | NR | NR | 1.5T | 2D GRE | NR | NR | 50 (24–53) | 42 | 59 |
| Imajo [152] | 2022 | Japan | Prospective | Single-center | GE | >6 years | 3T | SE-EPI | 60 Hz | Manual ROI | 61 (51–71) | 53 | 201 |
| Kalaiyarasi [116] | 2024 | Singapore | Prospective | Single-center | GE | “Experienced” | 1.5T | 2D GRE | NR | Manual ROI | 49 | 31 | 16 |
| Loomba [209] | 2013 | USA | Prospective | Single-center | NR | NR | NR | NR | NR | NR | 50 | 52 | 52 |
| Loomba [210] | 2014 | USA | Prospective | Single-center | GE | “Trained” | 3T | 2D GRE | 60 Hz | Manual ROI | 50 | 44 | 117 |
| Loomba [211] | 2016 | USA | Prospective | Single-center | GE | “Trained” | 3T | 2D GRE | 60 Hz | Manual ROI | 50 | 44 | 99 |
| Loomba [212] | 2020 | USA | Retrospective | Multicenter | NR | NR | NR | NR | NR | NR | NR | 44 | 296 |
| Li [213] | 2023 | USA | Prospective | Single-center | GE | 5 years | 1.5T | 2D GRE | 60 Hz | Manual ROI | 55 (46–60) | 38 | 104 |
| Cui [139] | 2016 | USA | Prospective | Single-center | GE | NR | 3T | 2D GRE | NR | Manual ROI | 49 (15.4) | 46 | 125 |
| Park [174] | 2017 | USA | Prospective | Single-center | GE | “Trained” | 3T | 2D GRE | 60 Hz | Manual ROI | 51 | 43 | 94 |
| Imajo [175] | 2016 | Japan | Prospective | Multicenter | GE | Radiologist | 3T | SE-EPI | NR | Manual ROI | 58 | 57 | 142 |
| Costa-Silva [214] | 2018 | Brazil | Prospective | Single-center | GE | 15 years | 1.5T | NR | 60 Hz | Manual ROI | 54 (25–76) | 14 | 49 |
| Kim [215] | 2020 | Republic of Korea | Prospective | Single-center | Siemens | 6–25 years | 3T | SE-EPI | NR | NR | 51 | 34 | 47 |
| Hanniman [216] | 2022 | Canada | Prospective | Single-center | GE | Medical student, Fellow | 3T | NR | 60 Hz | Manual ROI | 55 (33–74) | 37 | 49 |
| Alsaqal [198] | 2022 | Sweden | Prospective | Single-center | GE | “Trained” | 3T | 2D GRE | NR | NR | 55 (18–70) | 59 | 64 |
| Naveau [199] | 2023 | USA/Japan | Prospective | Multicenter | GE | “Trained” | 1.5T and 3T | NR | NR | NR | 57 (46–67) | 48 | 806 |
| Jafarov [200] | 2022 | Japan | Retrospective | Single-center | GE | NR | 1.5T | 2D GRE | 60 Hz | Manual ROI | 65 (58–72) | 45 | 105 |
| Tapper [201] | 2018 | Japan | Retrospective | Single-center | NR | NR | NR | NR | NR | NR | 54 | 58 | 111 |
| Chakraborty [202] | 2021 | USA/Japan | Prospective | Multi-center | GE | Radiologist | 3T | NR | 60Hz | Automated ROI | 51 (USA)/56 (Japan) | 46 (USA)/56 (Japan) | 238 (USA)/272 (Japan) |
Appendix E
| Author | Modality | RoB Patient Selection | RoB Index Test | RoB Reference Standard | RoB Flow and Timing | AC Patient Selection | AC Index Test | AC Reference Standard |
|---|---|---|---|---|---|---|---|---|
| Lee | 2D SWE | Low | High | Low | Low | Low | Low | Low |
| Zhang | 2D SWE | Low | High | Low | Low | Low | Low | Low |
| Furlan | 2D SWE | Low | High | Low | Low | Low | Low | Low |
| Yu Ogino | 2D SWE | Low | High | Low | High | High | Low | Low |
| Zhou | 2D SWE | Low | High | Low | Low | Low | High | Low |
| Ozturk | 2D SWE | High | High | Low | Low | Low | Low | Low |
| Sharpton | 2D SWE | High | High | Low | Low | Low | Low | Low |
| Taru | 2D SWE | Low | High | Unclear | Unclear | Low | Unclear | Low |
| Imajo | 2D SWE | Low | High | Low | Low | Low | Low | Low |
| Herrmann | 2D SWE | Low | High | Low | Low | Low | Low | Low |
| Chen | 2D SWE | Low | High | Low | Low | Low | Low | Low |
| Mendoza | 2D SWE | Low | Low | Low | Low | Low | Low | Low |
| Takeuchi | 2D SWE | Low | High | Low | Low | Low | Low | Low |
| Jamialahmadi | 2D SWE | High | High | High | High | High | High | Low |
| Petzold | 2D SWE | Low | High | Low | High | Low | Low | Low |
| Didenko | 2D SWE | Low | Unclear | Low | Low | Low | Low | Low |
| Sugimoto | 2D SWE | High | High | Low | High | High | High | Low |
| Kalaiyarasi | 2D SWE | High | Low | Low | High | High | Low | Low |
| Seo | 2D SWE | High | High | High | Low | High | High | High |
| Kuroda | 2D SWE | Low | High | Low | Low | Low | Low | Low |
| Jang | 2D SWE | Low | Unclear | High | Low | Low | Low | High |
| Kim | 2D SWE | Low | High | Low | Low | Low | Unclear | Low |
| Lee | 2D SWE | Low | High | Low | Low | Low | Low | Low |
| Cassinotto | 2D SWE | Low | High | Low | Low | Low | High | Low |
| Medellin | pSWE | Low | Low | Low | Unlear | Low | Low | Low |
| da Silva | pSWE | Low | High | Low | Low | Low | High | Low |
| Roccarina | pSWE | Low | High | Unclear | Unclear | Unclear | High | Unclear |
| Leong | pSWE | Low | High | Low | Low | Low | High | Low |
| Kapur | pSWE | High | High | High | Yes | Low | High | High |
| Argalia | pSWE | Low | High | Low | Low | Low | High | Low |
| Roccarina | pSWE | Low | High | High | High | Low | High | Low |
| Taibbi | pSWE | Low | High | Low | Low | Low | High | Low |
| Cui | pSWE | Low | High | Low | Low | Low | High | Low |
| Cassinotto | pSWE | Low | High | Low | Low | Low | High | Low |
| Tomeno | pSWE | Low | High | Low | Low | Low | High | Low |
| Yoneda | pSWE | Low | High | Low | High | Low | High | Low |
| Braticevici | pSWE | Low | High | Low | Low | Low | Low | Low |
| Duman | VCTE | Low | High | Low | Low | Low | High | Low |
| Gabriel-Medina | VCTE | Low | High | Low | Low | Low | High | Low |
| Mikolasevic | VCTE | Low | High | Low | Low | Low | High | Low |
| Eddowes | VCTE | Low | High | Low | Low | Low | High | Low |
| Eddowes | VCTE | Low | High | Low | Low | Low | High | Low |
| Eddowes | VCTE | Low | High | Low | Low | Low | High | Low |
| Yu | VCTE | Low | High | Low | Low | Low | High | Low |
| Oeda | VCTE | Low | High | Low | Low | Low | High | Low |
| Siddiqui | VCTE | Low | High | Low | Low | Low | High | Low |
| Wong | VCTE | Low | High | Unclear | Low | Unclear | High | Unclear |
| Seki | VCTE | Low | High | Low | Low | Low | High | Low |
| Zhang | VCTE | Low | High | Low | Low | Low | High | Low |
| Troelstra | VCTE | Low | High | Low | Low | Low | High | Low |
| Furlan | VCTE | Low | High | Low | Low | Low | High | Low |
| Lee | VCTE | Low | High | Unclear | Low | Low | High | Low |
| Sharpton | VCTE | High | High | Low | Low | Low | High | Low |
| Taru | VCTE | Low | High | Low | Low | Low | High | Low |
| Kawamura | VCTE | Low | Low | Low | Low | Low | Low | Low |
| Imajo | VCTE | Low | High | Low | Low | Low | High | Low |
| Roccarina | VCTE | High | High | Low | Low | High | High | Low |
| Mendoza | VCTE | Low | High | Low | Low | Low | High | Low |
| Kalaiyarasi | VCTE | High | High | Low | High | High | High | Low |
| Seo | VCTE | High | High | High | Low | High | High | High |
| Kuroda | VCTE | Low | High | Low | Low | Low | High | Low |
| Kim | VCTE | Low | High | Low | Low | Low | High | Low |
| Lee | VCTE | Low | High | Low | Low | Low | High | Low |
| Leong | VCTE | High | High | High | Low | High | High | Low |
| Taibbi | VCTE | Low | High | Low | Low | Low | High | Low |
| Argalia | VCTE | Yes | High | Yes | Yes | Low | High | Low |
| Roccarina | VCTE | Low | High | Low | Unclear | Low | High | Low |
| Park | VCTE | Low | High | High | Low | Low | High | Low |
| Imajo | VCTE | Yes | High | High | Unclear | Low | High | Low |
| Ogawa | VCTE | High | High | Low | Low | High | High | Low |
| Armandi | VCTE | Low | High | Low | Low | Low | High | Low |
| Wong | VCTE | Low | High | Low | Low | Low | High | Low |
| Boursier | VCTE | Low | High | Low | Low | Low | High | Low |
| Salehi | VCTE | Low | High | Low | Low | Low | High | Low |
| Mahadeva | VCTE | Low | High | Low | Low | Low | High | Low |
| Pennisi | VCTE | Low | Low | Low | Low | Low | Low | Low |
| Loong | VCTE | Low | Unclear | Low | Low | Low | Unclear | Low |
| Vali | VCTE | Low | High | Low | Low | Low | High | Low |
| Filho | VCTE | Low | High | Low | High | Low | High | Low |
| Lee | VCTE | Low | High | Low | Low | Low | High | Low |
| Prat | VCTE | Low | Unclear | Low | Low | Low | Unclear | Low |
| Ooi | VCTE | Low | High | Low | Low | Low | High | Low |
| Staufer | VCTE | Low | High | Low | Low | Low | High | Low |
| Lu | VCTE | High | High | Low | Low | High | High | Low |
| Myers | VCTE | Low | High | Low | Low | Low | High | Low |
| Arvaniti | VCTE | Unclear | High | Low | Low | Unclear | High | Unclear |
| Kao | VCTE | Low | High | Low | Low | Low | High | Low |
| Castera | VCTE | Low | Low | Low | Low | Low | Low | Low |
| Ruiz-Fernandez | VCTE | Low | High | Low | Low | Low | High | Low |
| Petta | VCTE | Low | Low | Low | Low | Low | Low | Low |
| Del Barrio Azaceta | VCTE | Low | Unclear | Low | Unclear | Low | Unclear | Low |
| Shen | VCTE | Low | High | Low | Low | Low | High | Low |
| Boursier | VCTE | Low | Low | Low | Low | Low | Low | Low |
| Noureddin | VCTE | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Noureddin | VCTE | Low | Low | Low | Low | Low | Low | Low |
| Vuppalanchi | VCTE | Unclear | High | Low | High | Unclear | High | Low |
| Cassinotto | VCTE | Low | High | Low | Low | Low | High | Low |
| Chuah | VCTE | Low | Unclear | Low | Low | Low | Unclear | Low |
| Cheung | VCTE | Low | High | Low | Low | Low | High | Low |
| Ergelen | VCTE | Low | High | Low | Low | Low | High | Low |
| Bhadoria | VCTE | Low | High | Low | Low | Low | High | Low |
| Yoneda | VCTE | Low | High | Low | Low | Low | High | Low |
| Yoneda | VCTE | Low | High | Low | Low | Low | High | Low |
| Anstee | VCTE | Low | Low | Low | Low | Low | Low | Low |
| Yang | VCTE | Low | High | Low | Low | Low | High | Low |
| Mahadeva | VCTE | Low | High | Low | Low | Low | High | Low |
| Lupsor | VCTE | Low | High | Low | Low | Low | High | Low |
| Arora | VCTE | Low | High | Low | Low | Low | High | Low |
| de Ledinghen | VCTE | Low | High | Low | Low | Low | High | Low |
| Labenz | VCTE | Low | Unclear | Low | High | Low | Unclear | Low |
| Lee | VCTE | Low | High | Low | Low | Low | High | Low |
| Garteiser | VCTE | Low | High | Low | Low | Low | High | Low |
| Gaia | VCTE | High | High | Low | Low | High | High | Low |
| Barritt | VCTE | Unclear | Unclear | Low | Unclear | Unclear | Unclear | Low |
| Harrison | VCTE | Low | High | Low | Low | Low | High | Low |
| Petta | VCTE | Low | Unclear | Low | Low | Low | Unclear | Low |
| Alsaqal | VCTE | Low | High | Low | Low | Low | High | Low |
| Naveau | VCTE | Low | High | Low | Low | Low | High | Low |
| Jafarov | VCTE | Low | High | Low | Low | Low | High | Low |
| Tapper | VCTE | Low | High | Low | Low | Low | High | Low |
| Chakraborty | VCTE | Low | High | Low | Low | Low | High | Low |
| Siddiqui | VCTE | Low | High | Low | Low | Low | High | Low |
| Pathik | VCTE | Low | High | Low | Low | Low | High | Low |
| Sanyal | VCTE | Low | High | Low | Low | Low | High | Low |
| Boursier | VCTE | Low | High | Low | Low | Low | High | Low |
| Bertot | VCTE | Low | Low | Low | Low | Low | Low | Low |
| Ergelen | VCTE | Unclear | High | Low | Low | Unclear | High | Low |
| Kosick | VCTE | Unclear | High | Unclear | Unclear | Unclear | High | Unclear |
| Li | VCTE | Low | Low | Unclear | Low | Low | Low | Unclear |
| Tovo | VCTE | Low | Low | Low | Low | Low | Low | Low |
| Duman | MRE | Low | High | Low | Low | Low | High | Low |
| Troelstra | MRE | Low | High | Low | Low | Low | High | Low |
| Hockings | MRE | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Cui | MRE | Low | Low | Low | Low | Low | Low | Low |
| Zhang | MRE | Low | High | Low | Low | Low | High | Low |
| Furlan | MRE | Low | High | Low | Unclear | Low | High | Low |
| Imajo | MRE | Low | High | Low | Low | Low | High | Low |
| Kalaiyarasi | MRE | Low | Low | Low | Low | Low | Low | Low |
| Loomba | MRE | Unclear | Unclear | Low | Unclear | Unclear | Unclear | Low |
| Loomba | MRE | Low | High | Low | Low | Low | High | Low |
| Loomba | MRE | Low | Unclear | Low | Low | Low | Unclear | Low |
| Loomba | MRE | Low | Low | Low | Unclear | Low | Low | Low |
| Li | MRE | Low | High | Low | Low | Low | High | Low |
| Cui | MRE | Low | High | Low | Low | Low | High | Low |
| Park | MRE | Low | High | Low | Low | Low | High | Low |
| Imajo | MRE | Low | High | Low | Low | Low | High | Low |
| Costa-Silva | MRE | Low | High | Low | Unclear | Low | High | Low |
| Kim | MRE | Low | High | Low | Low | Low | High | Low |
| Hanniman | MRE | Low | Low | Low | Low | Low | Low | Low |
| Alsaqal | MRE | Low | High | Low | Low | Low | High | Low |
| Tamaki | MRE | Low | Low | Low | Low | Low | Low | Low |
| Inada | MRE | Low | High | Low | Low | Low | High | Low |
| Ogawa | MRE | High | High | Low | Low | High | High | Low |
| Jung | MRE | Low | High | Low | Low | Low | High | Low |
| Chen | FIB-4 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Lee | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Takeuchi | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| da Silva | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Duman | FIB-4 | Unclear | Low | Low | Low | Low | Low | Low |
| Cui | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Inada | FIB-4 | Low | High | Low | Low | High | High | Low |
| Tamaki | FIB-4 | Low | Low | Low | Low | High | High | Low |
| Ogawa | FIB-4 | Unclear | High | Low | Unclear | Unclear | High | Low |
| Jung | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Armandi | FIB-4 | Low | Low | Low | Unclear | Low | Low | Low |
| Wong | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Boursier | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Pennisi | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Pennisi | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Prat | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Arvaniti | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Kao | FIB-4 | Unclear | High | Unclear | Low | Unclear | Unclear | Unclear |
| Castera | FIB-4 | Low | Unclear | Low | Low | Low | Unclear | Low |
| Petta | FIB-4 | Low | Low | Low | Unclear | Low | Low | Low |
| Staufer | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Boursier | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Noureddin | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Noureddin | FIB-4 | Low | Unclear | Low | Low | Low | Unclear | Low |
| Cheung | FIB-4 | Low | Unclear | Low | Low | Low | Unclear | Low |
| Bhadoria | FIB-4 | Low | Unclear | Unclear | Low | Low | Unclear | Unclear |
| Anstee | FIB-4 | Low | Unclear | Low | Unclear | Low | Unclear | Low |
| Anstee | FIB-4 | Low | High | Low | Low | Low | High | Low |
| Labenz | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Arora | FIB-4 | Low | Unclear | Unclear | Low | Low | Unclear | Unclear |
| Barritt | FIB-4 | Unclear | Unclear | Low | Unclear | Unclear | Unclear | Low |
| Harrison | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Petta | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Gabriel-Medina | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Zhang | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Eddowes | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Sanyal | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Sanyal | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Sanyal | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Boursier | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Bertot | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Kobayashi | FIB-4 | Low | Unclear | Low | Low | Low | Unclear | Low |
| Eren | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Singh | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Marella | FIB-4 | High | Low | Low | Unclear | High | Low | Low |
| Treeprasertsuk | FIB-4 | Low | Low | Low | Unclear | Low | Low | Low |
| McPherson | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| McPherson | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Kolhe | FIB-4 | High | Low | Low | Low | High | Low | Low |
| Kaya | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Nones | FIB-4 | Low | Unclear | Low | Low | Low | Unclear | Low |
| Balakrishnan | FIB-4 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Alkayyali | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Alkayyali | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Nielsen | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Ampuero | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Sang | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Shima | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Seko | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Siddiqui | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Li | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Li | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Sanyal | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Moon | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Zambrano-Huailla | FIB-4 | Unclear | Unclear | Low | Low | Unclear | Unclear | Low |
| Yang | FIB-4 | High | Unclear | Low | Unclear | High | Unclear | Low |
| Giamarrino | FIB-4 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Nishikawa | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Kakisaka | FIB-4 | Low | Low | Low | Unclear | Low | Low | Low |
| Schmitz | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Zhang | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Balakrishnan | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Kariyama | FIB-4 | Low | Unclear | Low | Low | Low | Unclear | Low |
| Mohammed | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| de la Tijera | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| McPherson | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Shah | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Maurice | FIB-4 | Low | Unclear | Low | Unclear | Low | Low | Low |
| Zhou | FIB-4 | Low | Low | Low | Unclear | Low | Low | Low |
| Kouvari | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Ballestri | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Noureddin | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Singh | FIB-4 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Prasad | FIB-4 | Unclear | Unclear | Yes | Unclear | Unclear | Unclear | Low |
| Kim | FIB-4 | Low | Low | Low | Unclear | Low | Low | Low |
| Yoneda | FIB-4 | Low | Low | Low | Unclear | Low | Low | Low |
| Qadri | FIB-4 | Low | Unclear | Low | Low | Low | Unclear | Low |
| Miller | FIB-4 | Low | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| Drolz | FIB-4 | Low | Low | Unclear | Unclear | Low | Low | Unclear |
| Meneses | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Alqahtani | FIB-4 | Unclear | Low | Low | Unclear | Unclear | Low | Low |
| De Carli | FIB-4 | Unclear | High | Unclear | High | Unclear | High | Unclear |
| Ito | FIB-4 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Bril | FIB-4 | Low | Low | Low | Unclear | Low | Low | Low |
| Satapathy | FIB-4 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Moon | FIB-4 | Unclear | Low | Low | Unclear | Unclear | Low | Low |
| Schwenger | FIB-4 | High | Low | Low | Unclear | High | Low | Low |
| Andrade | FIB-4 | Low | Unclear | Low | Unclear | Low | Unclear | Low |
| Aida | FIB-4 | Low | Unclear | Unclear | Low | Low | Low | Low |
| Huang | FIB-4 | High | Unclear | Low | Unclear | High | Unclear | Low |
| McPherson | FIB-4 | Unclear | Unclear | Unclear | Low | Unclear | Unclear | Unclear |
| Kaya | FIB-4 | Low | Low | Low | Unclear | Low | Low | Low |
| Xun | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Mikolasevic | FIB-4 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Younossi | FIB-4 | Low | Low | Unclear | Low | Low | Low | Low |
| McPherson | FIB-4 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Sanyal | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Soresi | FIB-4 | Low | High | Low | Low | Low | High | Low |
| Kaya | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Kawamura | FIB-4 | Low | Unclear | Unclear | Low | Low | Low | Low |
| Kalaiyarasi | FIB-4 | Unclear | Low | Low | Unclear | Unclear | Low | Low |
| Ishiba | FIB-4 | Unclear | Low | Unclear | Unclear | Unclear | Low | Unclear |
| Udelsman | FIB-4 | Low | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Zain | FIB-4 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Lubner | FIB-4 | Unclear | Low | Low | Low | Unclear | Low | Low |
| Soontornmanokul | FIB-4 | Unclear | Low | Unclear | Unclear | Unclear | Low | Unclear |
| Wu | FIB-4 | Low | Low | Low | Low | Low | Low | Low |
| Le | FIB-4 | Unclear | Unclear | Low | Unclear | Unclear | Unclear | Low |
| Sumida | FIB-4 | Unclear | Low | Low | Unclear | Unclear | Low | Low |
| Chong | FIB-4 | High | Unclear | Unclear | Unclear | High | Unclear | Unclear |
| Pérez-Gutiérrez | FIB-4 | Unclear | Low | Unclear | Unclear | Unclear | Low | Unclear |
| Singh | FIB-4 | Unclear | Low | Unclear | Unclear | Unclear | Low | Unclear |
| Panackel | FIB-4 | High | Unclear | Unclear | Unclear | High | Unclear | Low |
| Sanyal | FIB-4 | High | Unclear | Low | Unclear | High | Unclear | Low |
| Kolhe | FIB-4 | Low | Unclear | Unclear | Low | Low | Unclear | Unclear |
| Luger | FIB-4 | Unclear | Unlcear | Unclear | Unclear | Low | Low | Low |
References
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Duarte-Rojo, A.; Patel, K.; Asrani, S.K.; Alsawas, M.; Dranoff, J.A.; Fiel, M.I.; Murad, M.H.; Leung, D.H.; Levine, D.; et al. AASLD Practice Guideline on imaging-based noninvasive liver disease assessment of hepatic fibrosis and steatosis. Hepatology 2025, 81, 672–724. [Google Scholar] [CrossRef]
- Kanwal, F.; Shubrook, J.H.; Adams, L.A.; Pfotenhauer, K.; Wong, V.W.-S.; Wright, E.; Abdelmalek, M.F.; Harrison, S.A.; Loomba, R.; Mantzoros, C.S.; et al. Clinical Care Pathway for the Risk Stratification and Management of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2021, 161, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Wilson, S.R.; Rubens, D.; Garcia-Tsao, G.; Ferraioli, G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology 2020, 296, 263–274. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies. The PRISMA-DTA Statement. JAMA 2018, 319, 388–396, Erratum in JAMA 2019, 322, 2026. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.; McGowan, J.; Cogo, E.; Grimshaw, J.; Moher, D.; Lefebvre, C. An evidence-based practice guideline for the peer review of electronic search strategies. J. Clin. Epidemiol. 2009, 62, 944–952. [Google Scholar] [CrossRef]
- Chowdhury, A.B.; Mehta, K.J. Liver biopsy for assessment of chronic liver diseases: A synopsis. Clin. Exp. Med. 2022, 23, 273–285. [Google Scholar] [CrossRef]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom. Imaging 2018, 43, 773–785. [Google Scholar] [CrossRef]
- McGrath, T.A.; McInnes, M.D.; Langer, F.W.; Hong, J.; Korevaar, D.A.; Bossuyt, P.M. Treatment of multiple test readers in diagnostic accuracy systematic reviews-meta-analyses of imaging studies. Eur. J. Radiol. 2017, 93, 59–64. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Chen, G.; Yang, J.-C.; Zhang, G.-X.; Cheng, Z.; Du, X. Evaluation of Six Noninvasive Methods for the Detection of Fibrosis in Chinese Patients with Obesity and Nonalcoholic Fatty Liver Disease. Obes. Surg. 2022, 32, 3619–3626. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Jang, J.Y.; Park, S.Y.; Lee, H.W.; Lee, C.K.; Kim, S.U. Comparison of FibroScan-Aspartate Aminotransferase (FAST) Score and Other Non-invasive Surrogates in Predicting High-Risk Non-alcoholic Steatohepatitis Criteria. Front. Med. 2022, 9, 869190. [Google Scholar] [CrossRef]
- Takeuchi, H.; Sugimoto, K.; Oshiro, H.; Iwatsuka, K.; Kono, S.; Yoshimasu, Y.; Kasai, Y.; Furuichi, Y.; Sakamaki, K.; Itoi, T. Liver fibrosis: Noninvasive assessment using supersonic shear imaging and FIB4 index in patients with non-alcoholic fatty liver disease. J. Med. Ultrason. 2017, 45, 243–249. [Google Scholar] [CrossRef]
- da Silva, R.G.; de Miranda, M.L.Q.; Brant, P.E.d.A.C.; Schulz, P.O.; Nascimento, M.d.F.A.; Schmillevitch, J.; Vieira, A.; de Freitas, W.R.; Szutan, L.A. Acoustic radiation force impulse elastography and liver fibrosis risk scores in severe obesity. Arq. Bras. Endocrinol. Metabol. 2021, 65, 730–738. [Google Scholar] [CrossRef]
- Duman, S.; Kuru, D.; Gumussoy, M.; Kiremitci, S.; Gokcan, H.; Ulas, B.; Ellik, Z.; Ozercan, M.; Er, R.E.; Karakaya, F.; et al. A combination of non-invasive tests for the detection of significant fibrosis in patients with metabolic dysfunction-associated steatotic liver disease is not superior to magnetic resonance elastography alone. Eur. Radiol. 2024, 34, 3882–3888. [Google Scholar] [CrossRef]
- Cui, J.; Ang, B.; Haufe, W.; Hernandez, C.; Verna, E.C.; Sirlin, C.B.; Loomba, R. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: A prospective study. Aliment. Pharmacol. Ther. 2015, 41, 1271–1280. [Google Scholar] [CrossRef]
- Inada, K.; Tamaki, N.; Kurosaki, M.; Kirino, S.; Yamashita, K.; Hayakawa, Y.; Higuchi, M.; Takaura, K.; Kaneko, S.; Maeyashiki, C.; et al. Validation of magnetic resonance elastography plus fibrosis-4 for significant fibrosis in nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2022, 37, 1726–1731. [Google Scholar] [CrossRef]
- Tamaki, N.; Imajo, K.; Sharpton, S.R.; Jung, J.; Sutter, N.; Kawamura, N.; Yoneda, M.; Valasek, M.A.; Behling, C.; Sirlin, C.B.; et al. Two-Step Strategy, FIB-4 Followed by Magnetic Resonance Elastography, for Detecting Advanced Fibrosis in NAFLD. Clin. Gastroenterol. Hepatol. 2022, 21, 380–387.e3. [Google Scholar] [CrossRef]
- Ogawa, Y.; Honda, Y.; Kessoku, T.; Tomeno, W.; Imajo, K.; Yoneda, M.; Kawanaka, M.; Kirikoshi, H.; Ono, M.; Taguri, M.; et al. Wisteria floribunda agglutinin-positive Mac-2-binding protein and type 4 collagen 7S: Useful markers for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2018, 33, 1795–1803. [Google Scholar] [CrossRef]
- Jung, J.; Loomba, R.R.; Imajo, K.; Madamba, E.; Gandhi, S.; Bettencourt, R.; Singh, S.; Hernandez, C.; A Valasek, M.; Behling, C.; et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut 2020, 70, 1946–1953. [Google Scholar] [CrossRef]
- Armandi, A.; Rosso, C.; Younes, R.; Leeming, D.J.; Karsdal, M.A.; Caviglia, G.P.; Pérez-Diaz-Del-Campo, N.; D’amato, D.; Abdulle, A.; Nicolosi, A.; et al. Cross-Sectional and Longitudinal Performance of Non-Invasive Tests of Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2023, 12, 650. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Vergniol, J.; Wong, G.L.-H.; Foucher, J.; Chan, H.L.-Y.; Le Bail, B.; Choi, P.C.-L.; Kowo, M.; Chan, A.W.-H.; Merrouche, W.; et al. Diagnosis of Fibrosis and Cirrhosis Using Liver Stiffness Measurement in Nonalcoholic Fatty Liver Disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef]
- Boursier, J.; Vergniol, J.; Guillet, A.; Hiriart, J.-B.; Lannes, A.; Le Bail, B.; Michalak, S.; Chermak, F.; Bertrais, S.; Foucher, J.; et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J. Hepatol. 2016, 65, 570–578. [Google Scholar] [CrossRef]
- Pennisi, G.; Enea, M.; Pandolfo, A.; Celsa, C.; Antonucci, M.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Tulone, A.; et al. AGILE 3+ Score for the Diagnosis of Advanced Fibrosis and for Predicting Liver-related Events in NAFLD. Clin. Gastroenterol. Hepatol. 2022, 21, 1293–1302.e5. [Google Scholar] [CrossRef]
- Pennisi, G.; Enea, M.; Falco, V.; Aithal, G.P.; Palaniyappan, N.; Yilmaz, Y.; Boursier, J.; Cassinotto, C.; de Lédinghen, V.; Chan, W.K.; et al. Noninvasive assessment of liver disease severity in patients with nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes. Hepatology 2023, 78, 195–211. [Google Scholar] [CrossRef]
- Prat, L.I.; Roccarina, D.; Lever, R.; Lombardi, R.; Rodger, A.; Hall, A.; Luong, T.V.; Bhagani, S.; Tsochatzis, E.A. Etiology and Severity of Liver Disease in HIV-Positive Patients with Suspected NAFLD: Lessons From a Cohort with Available Liver Biopsies. Am. J. Ther. 2019, 80, 474–480. [Google Scholar] [CrossRef]
- Arvaniti, P. FibroMeter scores are predictive noninvasive markers of advanced and significant liver fibrosis in patients with chronic viral hepatitis or metabolic dysfunction-associated steatotic liver disease. Ann. Gastroenterol. 2023, 36, 661–669. [Google Scholar] [CrossRef]
- Kao, W.-Y.; Chang, I.-W.; Chen, C.-L.; Su, C.-W.; Fang, S.U.; Tang, J.-H.; Chang, C.-C.; Chang, Y.-J.; Wang, W. Fibroscan-Based Score to Predict Significant Liver Fibrosis in Morbidly Obese Patients with Nonalcoholic Fatty Liver Disease. Obes. Surg. 2020, 30, 1249–1257. [Google Scholar] [CrossRef]
- Castéra, L.; Vidal-Trecan, T.; Khoury, T.; Julla, J.-B.; Paradis, V.; Riveline, J.-P.; Valla, D.; Gautier, J.-F. Head-to-head comparison of Agile 3+, LSM-VCTE, NFS, and FIB-4 scores for detecting advanced fibrosis in patients with type 2 diabetes seen in diabetes clinic. J. Hepatol. 2023, 78, S681–S682. [Google Scholar] [CrossRef]
- Petta, S.; Wong, V.W.-S.; Bugianesi, E.; Fracanzani, A.L.; Cammà, C.; Hiriart, J.-B.; Wong, G.L.-H.; Vergniol, J.; Chan, A.W.-H.; Giannetti, A.; et al. Impact of Obesity and Alanine Aminotransferase Levels on the Diagnostic Accuracy for Advanced Liver Fibrosis of Noninvasive Tools in Patients with Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2019, 114, 916–928. [Google Scholar] [CrossRef]
- Staufer, K.; Halilbasic, E.; Spindelboeck, W.; Eilenberg, M.; Prager, G.; Stadlbauer, V.; Posch, A.; Munda, P.; Marculescu, R.; Obermayer-Pietsch, B.; et al. Evaluation and comparison of six noninvasive tests for prediction of significant or advanced fibrosis in nonalcoholic fatty liver disease. United Eur. Gastroenterol. J. 2019, 7, 1113–1123. [Google Scholar] [CrossRef]
- Boursier, J.; Canivet, C.M.; Costentin, C.; Lannes, A.; Delamarre, A.; Sturm, N.; Le Bail, B.; Michalak, S.; Oberti, F.; Hilleret, M.-N.; et al. Impact of Type 2 Diabetes on the Accuracy of Noninvasive Tests of Liver Fibrosis with Resulting Clinical Implications. Clin. Gastroenterol. Hepatol. 2022, 21, 1243–1251.e12. [Google Scholar] [CrossRef]
- Noureddin, M.; Mena, E.; Vuppalanchi, R.; Samala, N.; Wong, M.; Pacheco, F.; Polanco, P.; Sakkal, C.; Antaramian, A.; Chang, D.; et al. Increased accuracy in identifying NAFLD with advanced fibrosis and cirrhosis: Independent validation of the Agile 3+ and 4 scores. Hepatol. Commun. 2023, 7, e0055. [Google Scholar] [CrossRef]
- Noureddin, M.; Truong, E.; Chang, D.; Gornbein, J.; Mena, E. Simple combined serum-imaging non-invasive testing formulas to assess significant and advanced fibrosis in NAFLD patients. Hepatology 2021, 74 (Suppl. S1), 985A–986A. [Google Scholar] [CrossRef]
- Cheung, J.T.K.; Zhang, X.; Wong, G.L.; Yip, T.C.; Lin, H.; Li, G.; Leung, H.H.; Lai, J.C.; Mahadeva, S.; Mustapha, N.R.N.; et al. MAFLD fibrosis score: Using routine measures to identify advanced fibrosis in metabolic-associated fatty liver disease. Aliment. Pharmacol. Ther. 2023, 58, 1194–1204. [Google Scholar] [CrossRef]
- Bhadoria, A.; Jindal, A.; Rastogi, A.; Sharma, C.; Bhardwaj, A.; Kumar, G.; Sharma, M.; Sarin, S. Non invasive diagnostic modalities to predict advanced hepatic fibrosis in patients with non alcoholic fatty liver disease: A retrospective study. J. Hepatol. 2017, 66, S658–S659. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Lawitz, E.J.; Alkhouri, N.; Wong, V.W.-S.; Romero-Gomez, M.; Okanoue, T.; Trauner, M.; Kersey, K.; Li, G.; Han, L.; et al. Noninvasive Tests Accurately Identify Advanced Fibrosis due to NASH: Baseline Data From the STELLAR Trials. Hepatology 2019, 70, 1521–1530. [Google Scholar] [CrossRef]
- Anstee, Q.; Mantry, P.; Laurent, C.; Shah, D.; Niu, X.; Landgren, H.; Tan, B.; Martins, E.B.; Torstenson, R.; Schattenberg, J.M.; et al. Non-invasive test cut-offs for the identification of advanced liver fibrosis in a diabetes cohort with non-alcoholic steatohepatitis: Data from the phase 3 AURORA study. J. Hepatol. 2020, 73, S524. [Google Scholar] [CrossRef]
- Labenz, C.; Huber, Y.; Kalliga, E.; Nagel, M.; Ruckes, C.; Straub, B.K.; Galle, P.R.; Wörns, M.; Anstee, Q.M.; Schuppan, D.; et al. Predictors of advanced fibrosis in non-cirrhotic non-alcoholic fatty liver disease in Germany. Aliment. Pharmacol. Ther. 2018, 48, 1109–1116. [Google Scholar] [CrossRef]
- Arora, U.; Goyal, R.M.; Teh, K.K.J.; Pei, Y.; Goh, G.B.B.; Lin, S.; Kumar, R.; Biswas, S.; Vaishnav, M.; Swaroop, S.; et al. Poor Performance of Non-invasive Tests for Advanced Fibrosis in Nonalcoholic Fatty Liver Disease: A Multicentric Asian Study. Dig. Dis. Sci. 2023, 68, 4485–4498. [Google Scholar] [CrossRef]
- Barritt, A.S.; Lok, A.S.; Reddy, K.R.; Weiss, L.M.; Firpi, R.J.; Thuluvath, P.J.; Trinh, H.N.; Djedjos, S.; Haubrich, R.; Billin, A.; et al. Routinely available noninvasive tests perform well in identifying patients with advanced fibrosis due to NASH: Data from the target-NASH observational cohort. Hepatology 2019, 70 (Suppl. S1), 1049A. [Google Scholar]
- Harrison, S.A.; Calanna, S.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.; Sejling, A.-S.; Newsome, P.N. Semaglutide for the treatment of non-alcoholic steatohepatitis: Trial design and comparison of non-invasive biomarkers. Contemp. Clin. Trials 2020, 97, 106174. [Google Scholar] [CrossRef]
- Petta, S.; Vanni, E.; Bugianesi, E.; Di Marco, V.; Cammà, C.; Cabibi, D.; Mezzabotta, L.; Craxì, A. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2014, 35, 1566–1573. [Google Scholar] [CrossRef]
- Gabriel-Medina, P.; Ferrer-Costa, R.; Ciudin, A.; Augustin, S.; Rivera-Esteban, J.; Pericàs, J.M.; Selva, D.M.; Rodriguez-Frias, F. Accuracy of a sequential algorithm based on FIB-4 and ELF to identify high-risk advanced liver fibrosis at the primary care level. Intern. Emerg. Med. 2024, 19, 745–756. [Google Scholar] [CrossRef]
- Zhang, F.; Han, Y.; Zheng, L.; Liu, J.; Wu, Y.; Bao, Z.; Liu, L.; Li, W. Association of Non-Invasive Markers with Significant Fibrosis in Patients with Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study. Diabetes, Metab. Syndr. Obesity: Targets Ther. 2023, 16, 2255–2268. [Google Scholar] [CrossRef]
- Eddowes, P.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.; Paradis, V.; Bedossa, P.; Newsome, P.N. Combination of fibroscan and fibrometer (fibrometer VCTE) improves identification of patients with advanced fibrosis in patients with NAFLD. Inflamm. Intest. Dis. 2019, 4, 8–9. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Foucquier, J.; Younossi, Z.M.; Harrison, S.A.; Newsome, P.N.; Chan, W.-K.; Yilmaz, Y.; De Ledinghen, V.; Costentin, C.; Zheng, M.-H.; et al. Enhanced diagnosis of advanced fibrosis and cirrhosis in individuals with NAFLD using FibroScan-based Agile scores. J. Hepatol. 2022, 78, 247–259. [Google Scholar] [CrossRef]
- Boursier, J.; Guillaume, M.; Leroy, V.; Irlès, M.; Roux, M.; Lannes, A.; Foucher, J.; Zuberbuhler, F.; Delabaudière, C.; Barthelon, J.; et al. New sequential combinations of non-invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J. Hepatol. 2019, 71, 389–396. [Google Scholar] [CrossRef]
- Bertot, L.C.; Jeffrey, G.P.; de Boer, B.; Wang, Z.; Huang, Y.; Garas, G.; MacQuillan, G.; Wallace, M.; Smith, B.W.; Adams, L.A. Comparative Accuracy of Clinical Fibrosis Markers, Hepascore and Fibroscan® to Detect Advanced Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2023, 68, 2757–2767. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kumada, T.; Toyoda, H.; Tada, T.; Ito, T.; Kage, M.; Okanoue, T.; Kudo, M. Ability of Cytokeratin-18 Fragments and FIB-4 Index to Diagnose Overall and Mild Fibrosis Nonalcoholic Steatohepatitis in Japanese Nonalcoholic Fatty Liver Disease Patients. Dig. Dis. 2017, 35, 521–530. [Google Scholar] [CrossRef]
- Eren, F.; Kaya, E.; Yilmaz, Y. Accuracy of Fibrosis-4 index and non-alcoholic fatty liver disease fibrosis scores in metabolic (dysfunction) associated fatty liver disease according to body mass index: Failure in the prediction of advanced fibrosis in lean and morbidly obese individuals. Eur. J. Gastroenterol. Hepatol. 2020, 34, 98–103. [Google Scholar] [CrossRef]
- Singh, A.; Gosai, F.; Siddiqui, M.T.; Gupta, M.; Lopez, R.; Lawitz, E.; Poordad, F.; Carey, W.; McCullough, A.; Alkhouri, N. Accuracy of Noninvasive Fibrosis Scores to Detect Advanced Fibrosis in Patients with Type-2 Diabetes with Biopsy-proven Nonalcoholic Fatty Liver Disease. J. Clin. Gastroenterol. 2020, 54, 891–897. [Google Scholar] [CrossRef]
- Marella, H.K.; Reddy, Y.K.; Jiang, Y.; Ganguli, S.; Podila, P.S.; Snell, P.D.; Kovalic, A.J.; Cholankeril, G.; Singal, A.K.; Nair, S.; et al. Accuracy of Noninvasive Fibrosis Scoring Systems in African American and White Patients with Nonalcoholic Fatty Liver Disease. Clin. Transl. Gastroenterol. 2020, 11, 165. [Google Scholar] [CrossRef]
- Treeprasertsuk, S.; Piyachaturawat, P.; Soontornmanokul, T.; Wisedopas-Klaikaew, N.; Komolmit, P.; Tangkijavanich, P. Accuracy of noninvasive scoring systems to assess advanced liver fibrosis in Thai patients with nonalcoholic fatty liver disease. Asian Biomed. 2017, 10, s49–s55. [Google Scholar]
- McPherson, S.; Anstee, Q.M.; Henderson, E.; Day, C.P.; Burt, A.D. Are simple noninvasive scoring systems for fibrosis reliable in patients with NAFLD and normal ALT levels? Eur. J. Gastroenterol. Hepatol. 2013, 25, 652–658. [Google Scholar] [CrossRef]
- Kolhe, K.M.; Amarapurkar, A.; Parikh, P.; Chaubal, A.; Chauhan, S.; Khairnar, H.; Walke, S.; Ingle, M.; Pandey, V.; Shukla, A. Aspartate transaminase to platelet ratio index (APRI) but not FIB-5 or FIB-4 is accurate in ruling out significant fibrosis in patients with non-alcoholic fatty liver disease (NAFLD) in an urban slum-dwelling population. BMJ Open Gastroenterol. 2019, 6, e000288. [Google Scholar] [CrossRef]
- Kaya, E.; Bakir, A.; Yilmaz, Y. Can advanced fibrosis be reliably excluded by simple non-invasive fibrosis scoring systems? Diagnostic performance of FIB-4 and NFS in Turkish patients with biopsy-proven non-alcoholic fatty liver disease. Turk. J. Gastroenterol. 2019, 30, 3–5. [Google Scholar] [CrossRef]
- Nones, R.B.; Ivantes, C.P.; Pedroso, M.L.A. Can FIB4 and NAFLD fibrosis scores help endocrinologists refer patients with non-alcoholic fat liver disease to a hepatologist? Arq. Bras. Endocrinol. Metabol. 2017, 61, 276–281. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Gaba, R.; Jain, S. Clinical fibrosis prediction scores perform poorly among mexican/central american patients with NAFLD. Hepatology 2018, 68 (Suppl. S1), 1304A. [Google Scholar] [CrossRef]
- Alkayyali, T.; Qutranji, L.; Kaya, E.; Bakir, A.; Yilmaz, Y. Clinical utility of noninvasive scores in assessing advanced hepatic fibrosis in patients with type 2 diabetes mellitus: A study in biopsy-proven non-alcoholic fatty liver disease. Acta Diabetol. 2020, 57, 613–618. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Leeming, D.J.; Goodman, Z.; Friedman, S.; Frederiksen, P.; Rasmussen, D.G.K.; Vig, P.; Seyedkazemi, S.; Fischer, L.; Torstenson, R.; et al. Comparison of ADAPT, FIB-4 and APRI as non-invasive predictors of liver fibrosis and NASH within the CENTAUR screening population. J. Hepatol. 2021, 75, 1292–1300. [Google Scholar] [CrossRef]
- Ampuero, J.; Pais, R.; Aller, R.; Gallego-Durán, R.; Crespo, J.; García-Monzón, C.; Boursier, J.; Vilar, E.; Petta, S.; Zheng, M.-H.; et al. Development and Validation of Hepamet Fibrosis Scoring System–A Simple, Noninvasive Test to Identify Patients with Nonalcoholic Fatty Liver Disease with Advanced Fibrosis. Clin. Gastroenterol. Hepatol. 2020, 18, 216–225.e5. [Google Scholar] [CrossRef] [PubMed]
- Sang, C.; Yan, H.; Chan, W.K.; Zhu, X.; Sun, T.; Chang, X.; Xia, M.; Sun, X.; Hu, X.; Gao, X.; et al. Diagnosis of Fibrosis Using Blood Markers and Logistic Regression in Southeast Asian Patients with Non-alcoholic Fatty Liver Disease. Front. Med. 2021, 8, 637652. [Google Scholar] [CrossRef]
- Shima, T.; Sakai, K.; Oya, H.; Katayama, T.; Mitsumoto, Y.; Mizuno, M.; Kanbara, Y.; Okanoue, T. Diagnostic accuracy of combined biomarker measurements and vibration-controlled transient elastography (VCTE) for predicting fibrosis stage of non-alcoholic fatty liver disease. J. Gastroenterol. 2019, 55, 100–112. [Google Scholar] [CrossRef]
- Seko, Y.; Takahashi, H.; Toyoda, H.; Hayashi, H.; Yamaguchi, K.; Iwaki, M.; Yoneda, M.; Arai, T.; Shima, T.; Fujii, H.; et al. Diagnostic accuracy of enhanced liver fibrosis test for nonalcoholic steatohepatitis-related fibrosis: Multicenter study. Hepatol. Res. 2023, 53, 312–321. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Yamada, G.; Vuppalanchi, R.; Loomba, R.; Guy, C.; Brandman, D.; Tonascia, J.; Chalasani, N.; Sanyal, A.J.; Allende, D.; et al. Diagnostic Accuracy of Noninvasive Fibrosis Models to Detect Change in Fibrosis Stage. Clin. Gastroenterol. Hepatol. 2019, 17, 1877–1885.e5. [Google Scholar] [CrossRef]
- Li, G.; Lin, H.; Sripongpun, P.; Liang, L.Y.; Zhang, X.; Wong, V.W.; Wong, G.L.; Kim, W.R.; Yip, T.C. Diagnostic and prognostic performance of the SAFE score in non-alcoholic fatty liver disease. Liver Int. 2023, 44, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Shankar, S.S.; Yates, K.P.; Bolognese, J.; Daly, E.; Dehn, C.A.; Neuschwander-Tetri, B.; Kowdley, K.; Vuppalanchi, R.; Behling, C.; et al. Diagnostic performance of circulating biomarkers for non-alcoholic steatohepatitis. Nat. Med. 2023, 29, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.Y.; Kang, Y.W. Diagnostic performance of serum m2bpgi compared with other serological panels for biop-sy-proven advanced fibrosis and cirrhosis in NAFLD. Hepatol. Int. 2023, 17 (Suppl. S1), S180–S181. [Google Scholar] [CrossRef]
- Zambrano-Huailla, R.; Guedes, L.; Stefano, J.T.; de Souza, A.A.A.; Marciano, S.; Yvamoto, E.; Michalczuk, M.T.; Vanni, D.S.; Rodriguez, H.; Carrilho, F.J.; et al. Diagnostic performance of three non-invasive fibrosis scores (Hepamet, FIB-4, NAFLD fibrosis score) in NAFLD patients from a mixed Latin American population. Ann. Hepatol. 2020, 19, 622–626. [Google Scholar] [CrossRef]
- Yang, X.; Xia, M.; Chang, X.; Zhu, X.; Sun, X.; Yang, Y.; Wang, L.; Liu, Q.; Zhang, Y.; Xu, Y.; et al. A novel model for detecting advanced fibrosis in patients with nonalcoholic fatty liver disease. Diabetes/Metabolism Res. Rev. 2022, 38, e3570. [Google Scholar] [CrossRef] [PubMed]
- Giammarino, A.; Kovalic, A.; Qiu, H.; Bulsara, K.; Khan, S. Accuracy of non-invasive scores in diagnosing nonalcoholic ste-atohepatitis with clinically significant fibrosis in a large cohort of biopsy-proven nonalcoholic fatty liver disease patients. Hepatology 2022, 76 (Suppl. S1), S862. [Google Scholar] [CrossRef]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Kishino, K.; Shimono, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Yoh, K.; Nishimura, T.; et al. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level in non-alcoholic steatohepatitis. Hepatol. Res. 2016, 46, 1194–1202. [Google Scholar] [CrossRef]
- Kakisaka, K.; Suzuki, Y.; Fujiwara, Y.; Abe, T.; Yonezawa, M.; Kuroda, H.; Ishida, K.; Sugai, T.; Takikawa, Y. Evaluation of ballooned hepatocytes as a risk factor for future progression of fibrosis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. 2018, 53, 1285–1291. [Google Scholar] [CrossRef]
- Schmitz, S.M.-T.; Kroh, A.; Ulmer, T.F.; Andruszkow, J.; Luedde, T.; Brozat, J.F.; Neumann, U.P.; Alizai, P.H. Evaluation of NAFLD and fibrosis in obese patients—A comparison of histological and clinical scoring systems. BMC Gastroenterol. 2020, 20, 254. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Shi, H.; Liu, F.; Yu, H.; Shi, H. Exosome GLUT1 derived from hepatocyte identifies the risk of non-alcoholic steatohepatitis and fibrosis. Hepatol. Int. 2023, 17, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Seth, A.; Cortes-Santiago, N.; Jain, S.; Sood, G.K.; El-Serag, H.B.; Thrift, A.P. External Validation of Four Point-of-Care Noninvasive Scores for Predicting Advanced Hepatic Fibrosis in a Predominantly Hispanic NAFLD Population. Dig. Dis. Sci. 2020, 66, 2387–2393. [Google Scholar] [CrossRef]
- Kariyama, K.; Kawanaka, M.; Nouso, K.; Hiraoka, A.; Toyoda, H.; Tada, T.; Ishikawa, T.; Wakuta, A.; Miyake, N.; Murakami, S.; et al. Fibrosis-3 Index: A New Score to Predict Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease Without Age as a Factor. Gastro Hep Adv. 2022, 1, 1108–1113. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Omar, N.M.; Mohammed, S.A.; Amin, A.M.; Gad, D.F. FICK-3 Score Combining Fibrosis-4, Insulin Resistance and Cytokeratin-18 in Predicting Non-alcoholic Steatohepatitis in NAFLD Egyptian Patients. Pak. J. Biol. Sci. 2019, 22, 457–466. [Google Scholar] [CrossRef]
- Higuera-De-La-Tijera, F.; Córdova-Gallardo, J.; Buganza-Torio, E.; Barranco-Fragoso, B.; Torre, A.; Parraguirre-Martínez, S.; Rojano-Rodríguez, M.E.; Quintero-Bustos, G.; Castro-Narro, G.; Moctezuma-Velazquez, C. Hepamet Fibrosis Score in Nonalcoholic Fatty Liver Disease Patients in Mexico: Lower than Expected Positive Predictive Value. Dig. Dis. Sci. 2021, 66, 4501–4507. [Google Scholar] [CrossRef]
- Mcpherson, S.; Hardy, T.; Henderson, E.; Allison, M.; Ratziu, V.; Francque, S.; Burt, A.; Day, C.; Anstee, Q. PWE-120 High rate of false positives for advanced fibrosis when simple non-invasive fibrosis tests are used in older patients (≥ 65 years) with nafld. Gut 2015, 64, A265. [Google Scholar] [CrossRef]
- Shah, D.; Niu, X.; Doshi, J.A.; Marcus, S.; Rodriquez, G.; Tapper, E. Identification of significant fibrosis in patients with non-alcoholic steatohepatitis using non-invasive tests: Determination of optimal thresholds based on cross-sectional analyses of patients screened for a phase 3 randomised controlled trial. J. Hepatol. 2020, 73, S407. [Google Scholar] [CrossRef]
- Maurice, J.B.; Goldin, R.; Hall, A.; Price, J.C.; Sebastiani, G.; Morse, C.G.; Prat, L.I.; Perazzo, H.; Garvey, L.; Ingiliz, P.; et al. Increased Body Mass Index and Type 2 Diabetes Are the Main Predictors of Nonalcoholic Fatty Liver Disease and Advanced Fibrosis in Liver Biopsies of Patients with Human Immunodeficiency Virus Monoinfection. Clin. Infect. Dis. 2020, 73, e2184–e2193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-J.; Ye, F.-Z.; Li, Y.-Y.; Pan, X.-Y.; Chen, Y.-X.; Wu, X.-X.; Xiong, J.-J.; Liu, W.-Y.; Xu, S.-H.; Chen, Y.-P.; et al. Individualized risk prediction of significant fibrosis in non-alcoholic fatty liver disease using a novel nomogram. United Eur. Gastroenterol. J. 2019, 7, 1124–1134. [Google Scholar] [CrossRef]
- Kouvari, M.; Valenzuela-Vallejo, L.; Guatibonza-Garcia, V.; Polyzos, S.A.; Deng, Y.; Kokkorakis, M.; Agraz, M.; Mylonakis, S.C.; Katsarou, A.; Verrastro, O.; et al. Liver biopsy-based validation, confirmation and comparison of the diagnostic performance of established and novel non-invasive steatotic liver disease indexes: Results from a large multi-center study. Metabolism 2023, 147, 155666. [Google Scholar] [CrossRef]
- Ballestri, S.; Mantovani, A.; Baldelli, E.; Lugari, S.; Maurantonio, M.; Nascimbeni, F.; Marrazzo, A.; Romagnoli, D.; Targher, G.; Lonardo, A. Liver Fibrosis Biomarkers Accurately Exclude Advanced Fibrosis and Are Associated with Higher Cardiovascular Risk Scores in Patients with NAFLD or Viral Chronic Liver Disease. Diagnostics 2021, 11, 98. [Google Scholar] [CrossRef]
- Noureddin, M.; Truong, E.; Gornbein, J.A.; Saouaf, R.; Guindi, M.; Todo, T.; Noureddin, N.; Yang, J.D.; Harrison, S.A.; Alkhouri, N. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. J. Hepatol. 2022, 76, 781–787. [Google Scholar] [CrossRef]
- Singh, P.; Duseja, A.; Mehta, M.; De, A.; Mitra, S. Non-invasive fibrosis scores-validation in Indian patients with biopsy-proven non-alcoholic fatty liver disease (NAFLD). Hepatol. Int. 2022, 16 (Suppl. S1), S271. [Google Scholar] [CrossRef]
- Prasad, S.S.; Ranjan, K.C.; Kanta, S.S.; Meher, D. Utility of noninvasive scoring systems of fibrosis for detecting advanced fibrosis in patients with leaner nonalcoholic fatty liver disease (NAFLD). Hepatol. Int. 2020, 14 (Suppl. S1), S360. [Google Scholar]
- Kim, R.G.; Deng, J.; Reaso, J.N.; Grenert, J.P.; Khalili, M. Noninvasive Fibrosis Screening in Fatty Liver Disease Among Vulnerable Populations: Impact of Diabetes and Obesity on FIB-4 Score Accuracy. Diabetes Care 2022, 45, 2449–2451. [Google Scholar] [CrossRef]
- Yoneda, M.; Imajo, K.; Eguchi, Y.; Fujii, H.; Sumida, Y.; Hyogo, H.; Ono, M.; Suzuki, Y.; Kawaguchi, T.; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD); et al. Noninvasive scoring systems in patients with nonalcoholic fatty liver disease with normal alanine aminotransferase levels. J. Gastroenterol. 2013, 48, 1051–1060. [Google Scholar] [CrossRef]
- Qadri, S.; Ahlholm, N.; Lønsmann, I.; Pellegrini, P.; Poikola, A.; Luukkonen, P.K.; Porthan, K.; Juuti, A.; Sammalkorpi, H.; Penttilä, A.K.; et al. Obesity Modifies the Performance of Fibrosis Biomarkers in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2021, 107, e2008–e2020. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Li, N.; Hinton, A.; Mumtaz, K. 1019 Performance of Lab-Based Scoring Tests for the Assessment of Hepatic Fibrosis Compared to the Liver Biopsy Among Patients with Non-Alcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2019, 114, S585–S586. [Google Scholar] [CrossRef]
- Drolz, A.; Wolter, S.; Wehmeyer, M.H.; Piecha, F.; Horvatits, T.; Wiesch, J.S.Z.; Lohse, A.W.; Mann, O.; Kluwe, J. Performance of non-invasive fibrosis scores in non-alcoholic fatty liver disease with and without morbid obesity. Int. J. Obes. 2021, 45, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Meneses, D.; Olveira, A.; Corripio, R.; Méndez, M.d.C.; Romero, M.; Calvo-Viñuelas, I.; Herranz, L.; Vicent, D.; De-Cos-Blanco, A.I. Performance of Noninvasive Liver Fibrosis Scores in the Morbid Obese Patient, Same Scores but Different Thresholds. Obes. Surg. 2020, 30, 2538–2546. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Golabi, P.; Paik, J.M.; Lam, B.; Moazez, A.H.; Elariny, H.A.; Goodman, Z.; Younossi, Z.M. Performance of Noninvasive Liver Fibrosis Tests in Morbidly Obese Patients with Nonalcoholic Fatty Liver Disease. Obes. Surg. 2021, 31, 2002–2010. [Google Scholar] [CrossRef]
- De Carli, M.A.; de Carli, L.A.; Correa, M.B.; Junqueira, G., Jr.; Tovo, C.V.; Coral, G.P. Performance of noninvasive scores for the diagnosis of advanced liver fibrosis in morbidly obese with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2020, 32, 420–425. [Google Scholar] [CrossRef]
- Ito, T.; Nguyen, V.H.; Tanaka, T.; Park, H.; Kawanaka, M.; Arai, T.; Atsukawa, M.; Yoon, E.L.; Tsai, P.-C.; Toyoda, H.; et al. Poor Diagnostic Efficacy of Noninvasive Tests for Advanced Fibrosis in Obese or Younger Than 60 Diabetic NAFLD patients. Clin. Gastroenterol. Hepatol. 2022, 21, 1013–1022.e6. [Google Scholar] [CrossRef]
- Bril, F.; McPhaul, M.J.; Caulfield, M.P.; Clark, V.C.; Soldevilla-Pico, C.; Firpi-Morell, R.J.; Lai, J.; Shiffman, D.; Rowland, C.M.; Cusi, K. Performance of Plasma Biomarkers and Diagnostic Panels for Nonalcoholic Steatohepatitis and Advanced Fibrosis in Patients with Type 2 Diabetes. Diabetes Care 2019, 43, 290–297. [Google Scholar] [CrossRef]
- Satapathy, S.K.; Vilar-Gomez, E.; Brake, D.; Samala, N.; Bickett-Burkhart, K.; Ganguli, S.; Marella, H.K.; Reddy, Y.K.; Snell, P.D.; Podila, P.; et al. Mo1510–Prevalence and Risk Factors for Advanced Liver Histology Among African Americans with Histologically Confirmed Nonalcoholic Fatty Liver Disease (NAFLD). Gastroenterology 2019, 156, S-772. [Google Scholar] [CrossRef]
- Moon, S.Y.; Baek, Y.H.; Jang, S.Y.; Jun, D.W.; Yoon, K.T.; Cho, Y.Y.; Gil Jo, H.; Jo, A.J. Proposal of a Novel Serological Algorithm Combining FIB-4 and Serum M2BPGi for Advanced Fibrosis in Nonalcoholic Fatty Liver Disease. Gut Liver 2023, 18, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Schwenger, K.J.P.; Alali, M.; Ghorbani, Y.; Fischer, S.E.; Jackson, T.D.; Okrainec, A.; Allard, J.P. Reliability of Non-invasive Liver Fibrosis Assessment Tools Versus Biopsy in Pre- and Post-bariatric Surgery Patients with Non-alcoholic Fatty Liver Disease. Obes. Surg. 2022, 33, 247–255. [Google Scholar] [CrossRef]
- Andrade, T.G.; Xavier, L.C.D.; Souza, F.F.; Araújo, R.C. Risk predictors of advanced hepatic fibrosis in patients with nonalcoholic fatty liver disease—A survey in a university hospital in Brazil. Arq. Bras. de Endocrinol. Metabol. 2022, 66, 823–830. [Google Scholar] [CrossRef]
- Aida, Y.; Abe, H.; Tomita, Y.; Nagano, T.; Seki, N.; Sugita, T.; Itagaki, M.; Ishiguro, H.; Sutoh, S.; Aizawa, Y. Serum Immunoreactive Collagen IV detected by Monoclonal Antibodies as a Marker of Severe Fibrosis in Patients with Non- Alcoholic Fatty Liver Disease. J. Gastrointest. Liver Dis. 2015, 24, 61–68. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, S.; Wang, C.; Dong, Z.; Chen, W. Significant fibrosis assessed by liver biopsy among Chinese bariatric surgery patients: A prospective cross-sectional study. Front. Endocrinol. 2023, 14, 1090598. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Stewart, S.F.; Henderson, E.; Burt, A.D.; Day, C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010, 59, 1265–1269. [Google Scholar] [CrossRef]
- Kaya, E.; Bakir, A.; Kani, H.T.; Demirtas, C.O.; Keklikkiran, C.; Yilmaz, Y. Simple Noninvasive Scores Are Clinically Useful to Exclude, Not Predict, Advanced Fibrosis: A Study in Turkish Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gut Liver 2020, 14, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Xun, Y.H.; Fan, J.G.; Zang, G.Q.; Liu, H.; Jiang, Y.M.; Xiang, J.; Huang, Q.; Shi, J.P. Suboptimal performance of simple noninvasive tests for advanced fibrosis in Chinese patients with nonalcoholic fatty liver disease. J. Dig. Dis. 2012, 13, 588–595. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Domislovic, V.; Krznaric-Zrnic, I.; Krznaric, Z.; Virovic-Jukic, L.; Stojsavljevic, S.; Grgurevic, I.; Milic, S.; Vukoja, I.; Puz, P.; et al. The Accuracy of Serum Biomarkers in the Diagnosis of Steatosis, Fibrosis, and Inflammation in Patients with Nonalcoholic Fatty Liver Disease in Comparison to a Liver Biopsy. Medicina 2022, 58, 252. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Felix, S.; Jeffers, T.; Younossi, E.; Goodman, Z.; Racila, A.; Lam, B.P.; Henry, L. The combination of the enhanced liver fibrosis and FIB-4 scores to determine significant fibrosis in patients with nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2023, 57, 1417–1422. [Google Scholar] [CrossRef]
- McPherson, S.; Griffiths, L.; Hardy, T.; Henderson, E.; Burt, A.; Day, C.; Anstee, Q. 1308 THE FIB-4 SCORE RELIABLY EXCLUDES ADVANCED FIBROSIS IN A DIABETIC COHORT WITH NAFLD. J. Hepatol. 2012, 56, S515–S516. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Magnanensi, J.; Majd, Z.; Rosenquist, C.; Vera, D.M.; Almas, J.P.; Connelly, M.A. NIS2+™, an effective blood-based test for the diagnosis of at-risk nonalcoholic steatohepatitis in adults 65 years and older. Hepatol. Commun. 2023, 7, e0223. [Google Scholar] [CrossRef]
- Soresi, M.; Cabibi, D.; Giglio, R.V.; Martorana, S.; Guercio, G.; Porcasi, R.; Terranova, A.; Lazzaro, L.A.; Emma, M.R.; Augello, G.; et al. The Prevalence of NAFLD and Fibrosis in Bariatric Surgery Patients and the Reliability of Noninvasive Diagnostic Methods. BioMed Res. Int. 2020, 2020, 5023157. [Google Scholar] [CrossRef]
- Kaya, E. The utility of noninvasive scores in non-alcoholic fatty liver disease patients with normal and elevated serum transaminases. Hepatol. Forum 2020, 1, 8–13. [Google Scholar] [CrossRef]
- Kawamura, Y.; Saitoh, S.; Arase, Y.; Ikeda, K.; Fukushima, T.; Hara, T.; Seko, Y.; Hosaka, T.; Kobayashi, M.; Sezaki, H.; et al. Three-dimensional magnetic resonance imaging for stringent diagnosis of advanced fibrosis associated with nonalcoholic steatohepatitis. Hepatol. Int. 2013, 7, 850–858. [Google Scholar] [CrossRef]
- Kalaiyarasi, K.; Sanchalika, A.; Min, L.H.; Ming, Y.W.; Vishalkumar, S.; Chao, Y.K.; Keem, L.J.; Sameer, J.; Terence, H.C.W.; Ping, T.Y. Transient Elastography Is the Best-Performing Non-Invasive Test of Liver Fibrosis in Obese Asian Patients with Non-Alcoholic Fatty Liver Disease: A Pilot, Cross-Sectional Study. Medicina 2024, 60, 169. [Google Scholar] [CrossRef]
- Ishiba, H.; Sumida, Y.; Seko, Y.; Tanaka, S.; Hyogo, H.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Yoneda, M.; et al. Type IV Collagen 7S Is the Most Accurate Test For Identifying Advanced Fibrosis in NAFLD with Type 2 Diabetes. Hepatol. Commun. 2020, 5, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Udelsman, B.V.; Corey, K.; Hutter, M.M.; Chang, D.C.; Witkowski, E.R. Use of noninvasive scores for advanced liver fibrosis can guide the need for hepatic biopsy during bariatric procedures. Surg. Obes. Relat. Dis. 2021, 17, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Zain, S.M.; Tan, H.; Mohamed, Z.; Chan, W.; Mahadeva, S.; Basu, R.C.; Mohamed, R. Use of simple scoring systems for a public health approach in the management of non-alcoholic fatty liver disease patients. JGH Open 2020, 4, 1155–1161. [Google Scholar] [CrossRef]
- Lubner, M.G.; Graffy, P.M.; Said, A.; Watson, R.; Zea, R.; Malecki, K.M.; Pickhardt, P.J. Utility of Multiparametric CT for Identification of High-Risk NAFLD. Am. J. Roentgenol. 2021, 216, 659–668. [Google Scholar] [CrossRef]
- Soontornmanokul, T.; Piyachaturawat, P.; Siramolpiwat, S.; Wisedopas, N.; Tangkijvanich, P.; Mahachai, V.; Kullavanijaya, P.; Treeprasertsuk, S. Mo1018 Validation of 4 Noninvasive Scoring Systems to Identify Patients with Advanced Fibrosis in the Thai NAFLD Population. Gastroenterology 2013, 144, S1014. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Kumar, R.; Wang, M.-F.; Singh, M.; Huang, J.-F.; Zhu, Y.-Y.; Lin, S. Validation of conventional non-invasive fibrosis scoring systems in patients with metabolic associated fatty liver disease. World J. Gastroenterol. 2021, 27, 5753–5763. [Google Scholar] [CrossRef] [PubMed]
- Le, P.; Yu, P.-C.; Singh, A.; Nguyen, C.; Singh, T.; McCullough, A.; Rothberg, M. Su1533-Validation of Noninvasive Fibrosis Scores in Prediabetic Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2018, 154, S-1169. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M.; Hyogo, H.; Itoh, Y.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Aoki, N.; Kanemasa, K.; et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012, 12, 2. [Google Scholar] [CrossRef]
- Chong, S.-E.; Chang, F.; Chuah, K.-H.; Sthaneshwar, P.; Mustapha, N.R.N.; Mahadeva, S.; Chan, W.-K. Validation of the Hepamet fibrosis score in a multi-ethnic Asian population. Ann. Hepatol. 2022, 28, 100888. [Google Scholar] [CrossRef]
- Pérez-Gutiérrez, O.Z.; Hernández-Rocha, C.; Candia-Balboa, R.A.; Arrese, M.A.; Benítez, C.; Brizuela-Alcántara, D.C.; Méndez-Sánchez, N.; Uribe, M.; Chávez-Tapia, N.C. Validation study of systems for noninvasive diagnosis of fibrosis in nonalcoholic fatty liver disease in Latin population. Ann. Hepatol. 2013, 12, 416–424. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Karrar, A.; Tokarz, K.; Ulyanov, A.; Stepanova, M.; Younoszai, Z.; Jeffers, T.; Felix, S.C.; Tan, D.; Moin, A.; et al. Signature of Circulating microRNA (miR) Detected by NextSeq Differentiates Non-alcoholic Fatty Liver Disease (NAFLD) Patients with Higher Percentage Hepatic Collagen: A Potential Role for a Prognostic Biomarker: 2017 Category Award (Liver). Am. J. Gastroenterol. 2017, 112, S490–S577. [Google Scholar] [CrossRef]
- Panackel, C.; Jacob, M.; Francis, J.; Raja, K.; Sakpal, M.; Ganjoo, N.; Asthana, S.; Lochan, R.; Saif, R.; Reddy, J.; et al. SAT-295-Diagnostic accuracy of non-invasive Methods (NFS Score and FIB-4) for detecting advanced fibrosis: Avalidation study of NFS score and FIB-4 in a cohort of South Indian patients with NAFLD. J. Hepatol. 2019, 70, e768. [Google Scholar] [CrossRef]
- Sanyal, A.; Shankar, S.; Yates, K.; Bolognese, J.; Daly, E.; Dehn, C.; Neuschwander-Tetri, B.; Kowdley, K.; Vuppalanchi, R.; Behling, C.A.; et al. The Nimble Stage 1 Study Validates Diagnostic Circulating Biomarkers for Nonal-coholic Steatohepatitis. Res. Sq. 2023, Preprint. [Google Scholar] [CrossRef]
- Indian Society of Gastroenterology. Indian J. Gastroenterol. 2018, 37, 1–137. [CrossRef]
- Luger, M.; Kruschitz, R.; Kienbacher, C.; Traussnigg, S.; Langer, F.B.; Schindler, K.; Würger, T.; Wrba, F.; Trauner, M.; Prager, G.; et al. Prevalence of Liver Fibrosis and its Association with Non-invasive Fibrosis and Metabolic Markers in Morbidly Obese Patients with Vitamin D Deficiency. Obes. Surg. 2016, 26, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Medellin, A.; Pridham, G.; Urbanski, S.J.; Jayakumar, S.; Wilson, S.R. Acoustic Radiation Force Impulse and Conventional Ultrasound in the Prediction of Cirrhosis Complicating Fatty Liver: Does Body Mass Index Independently Alter the Results? Ultrasound Med. Biol. 2019, 45, 3160–3171. [Google Scholar] [CrossRef] [PubMed]
- Roccarina, D.; Buzzetti, E.; Misas, M.; Rosselli, M.; Majumdar, A.; Pinzani, M.; Tsochatzis, E. ElastPQ shear-wave elastography compared to fibroscan transient elastography for fibrosis staging in patients with NAFLD. J. Hepatol. 2017, 66, S236. [Google Scholar] [CrossRef]
- Leong, W.L.; Lai, L.L.; Mustapha, N.R.N.; Vijayananthan, A.; Rahmat, K.; Mahadeva, S.; Chan, W.K. Comparing point shear wave elastography (ElastPQ) and transient elastography for diagnosis of fibrosis stage in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2019, 35, 135–141. [Google Scholar] [CrossRef]
- Kapur, S.; Kalra, N.; Bhatia, A.; Duseja, A.; Das, A.; Dhiman, R.K.; Chawla, Y.; Sandhu, M.S. Comparison of Elastography Point Quantification with Transient Elastography in Patients with Chronic Viral Hepatitis and Nonalcoholic Fatty Liver Disease: A Pilot Study. J. Clin. Exp. Hepatol. 2021, 11, 21–29. [Google Scholar] [CrossRef]
- Argalia, G.; Ventura, C.; Tosi, N.; Campioni, D.; Tagliati, C.; Tufillaro, M.; Cucco, M.; Baroni, G.S.; Giovagnoni, A. Comparison of point shear wave elastography and transient elastography in the evaluation of patients with NAFLD. La Radiol. medica 2022, 127, 571–576. [Google Scholar] [CrossRef]
- Roccarina, D.; Prat, L.I.; Pallini, G.; Misas, M.G.; Buzzetti, E.; Saffioti, F.; Aricò, F.M.; Mantovani, A.; Koutli, E.; Goyale, A.; et al. Comparison of point-shear wave elastography (ElastPQ) and transient elastography (FibroScan) for liver fibrosis staging in patients with non-alcoholic fatty liver disease. Liver Int. 2022, 42, 2195–2203. [Google Scholar] [CrossRef]
- Taibbi, A.; Petta, S.; Matranga, D.; Caruana, G.; Cannella, R.; Busè, G.; Di Marco, V.; Midiri, M.; Bartolotta, T.V. Liver stiffness quantification in biopsy-proven nonalcoholic fatty liver disease patients using shear wave elastography in comparison with transient elastography. Ultrasonography 2021, 40, 407–416. [Google Scholar] [CrossRef]
- Cui, J.; Heba, E.; Hernandez, C.; Haufe, W.; Hooker, J.; Andre, M.P.; Valasek, M.A.; Aryafar, H.; Sirlin, C.B.; Loomba, R. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology 2015, 63, 453–461. [Google Scholar] [CrossRef]
- Cassinotto, C.; Boursier, J.; de Lédinghen, V.; Lebigot, J.; Lapuyade, B.; Cales, P.; Hiriart, J.B.; Michalak, S.; Bail, B.L.; Cartier, V.; et al. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 2016, 63, 1817–1827. [Google Scholar] [CrossRef]
- Tomeno, W.; Yoneda, M.; Nozaki, Y.; Fujita, K.; Kirikoshi, H.; Saito, S. Novel ultrasound-based Acoustic Radiation Force Elastography in patients with nonalcoholic fatty liver disease (NAFLD). Hepatology 2009, 50 (Suppl. S4), 790A. [Google Scholar]
- Yoneda, M.; Suzuki, K.; Kato, S.; Fujita, K.; Nozaki, Y.; Hosono, K.; Saito, S.; Nakajima, A. Nonalcoholic Fatty Liver Disease: US-based Acoustic Radiation Force Impulse Elastography. Radiology 2010, 256, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Braticevici, C.F.; Sporea, I.; Panaitescu, E.; Tribus, L. Value of Acoustic Radiation Force Impulse Imaging Elastography for Non-invasive Evaluation of Patients with Nonalcoholic Fatty Liver Disease. Ultrasound Med. Biol. 2013, 39, 1942–1950. [Google Scholar] [CrossRef]
- Lee, D.H.; Cho, E.J.; Bae, J.S.; Lee, J.Y.; Yu, S.J.; Kim, H.; Lee, K.B.; Han, J.K.; Choi, B.I. Accuracy of Two-Dimensional Shear Wave Elastography and Attenuation Imaging for Evaluation of Patients with Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2021, 19, 797–805.e7. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Fowler, K.J.; Boehringer, A.S.; Montes, V.; Schlein, A.N.; Covarrubias, Y.; Wolfson, T.; Hong, C.W.; Valasek, M.A.; Andre, M.P.; et al. Comparative diagnostic performance of ultrasound shear wave elastography and magnetic resonance elastography for classifying fibrosis stage in adults with biopsy-proven nonalcoholic fatty liver disease. Eur. Radiol. 2021, 32, 2457–2469. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Tublin, M.E.; Yu, L.; Chopra, K.B.; Lippello, A.; Behari, J. Comparison of 2D Shear Wave Elastography, Transient Elastography, and MR Elastography for the Diagnosis of Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Am. J. Roentgenol. 2020, 214, W20–W26. [Google Scholar] [CrossRef]
- Ogino, Y.; Wakui, N.; Nagai, H.; Matsuda, T. Comparison of strain elastography and shear wave elastography in diagnosis of fibrosis in nonalcoholic fatty liver disease. J. Med. Ultrason. 2023, 50, 187–195. [Google Scholar] [CrossRef]
- Zhou, J.; Yan, F.; Xu, J.; Lu, Q.; Zhu, X.; Gao, B.; Zhang, H.; Yang, R.; Luo, Y. Diagnosis of steatohepatitis and fibrosis in biopsy-proven nonalcoholic fatty liver diseases: Including two-dimension real-time shear wave elastography and noninvasive fibrotic biomarker scores. Quant. Imaging Med. Surg. 2022, 12, 1800–1814. [Google Scholar] [CrossRef]
- Ozturk, A.; Mohammadi, R.; Pierce, T.T.; Kamarthi, S.; Dhyani, M.; Grajo, J.R.; Corey, K.E.; Chung, R.T.; Bhan, A.K.; Chhatwal, J.; et al. Diagnostic Accuracy of Shear Wave Elastography as a Non-invasive Biomarker of High-Risk Non-alcoholic Steatohepatitis in Patients with Non-alcoholic Fatty Liver Disease. Ultrasound Med. Biol. 2020, 46, 972–980. [Google Scholar] [CrossRef]
- Sharpton, S.R.; Tamaki, N.; Bettencourt, R.; Madamba, E.; Jung, J.; Liu, A.; Behling, C.; Valasek, M.A.; Loomba, R. Diagnostic accuracy of two-dimensional shear wave elastography and transient elastography in nonalcoholic fatty liver disease. Ther. Adv. Gastroenterol. 2021, 14, 17562848211050436. [Google Scholar] [CrossRef]
- The Liver Meeting: Boston, Massachusetts Nov 10-14, 2023. Hepatology 2023, 78, S1–S2154. [CrossRef]
- Imajo, K.; Honda, Y.; Kobayashi, T.; Nagai, K.; Ozaki, A.; Iwaki, M.; Kessoku, T.; Ogawa, Y.; Takahashi, H.; Saigusa, Y.; et al. Direct Comparison of US and MR Elastography for Staging Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2022, 20, 908–917.e11. [Google Scholar] [CrossRef]
- Herrmann, E.; De Lédinghen, V.; Cassinotto, C.; Chu, W.C.W.; Leung, V.Y.-F.; Ferraioli, G.; Filice, C.; Castera, L.; Vilgrain, V.; Ronot, M.; et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology 2018, 67, 260–272. [Google Scholar] [CrossRef]
- Mendoza, Y.P.; Rodrigues, S.G.; Delgado, M.G.; Murgia, G.; Lange, N.F.; Schropp, J.; Montani, M.; Dufour, J.; Berzigotti, A. Inflammatory activity affects the accuracy of liver stiffness measurement by transient elastography but not by two-dimensional shear wave elastography in non-alcoholic fatty liver disease. Liver Int. 2021, 42, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Jamialahmadi, T.; Nematy, M.; Jangjoo, A.; Goshayeshi, L.; Rezvani, R.; Ghaffarzadegan, K.; Nooghabi, M.J.; Shalchian, P.; Zangui, M.; Javid, Z.; et al. Measurement of Liver Stiffness with 2D-Shear Wave Elastography (2D-SWE) in Bariatric Surgery Candidates Reveals Acceptable Diagnostic Yield Compared to Liver Biopsy. Obes. Surg. 2019, 29, 2585–2592. [Google Scholar] [CrossRef] [PubMed]
- Petzold, G.; Bremer, S.C.; Knoop, R.F.; Amanzada, A.; Raddatz, D.; Ellenrieder, V.; Ströbel, P.; Kunsch, S.; Neesse, A. Noninvasive assessment of liver fibrosis in a real-world cohort of patients with known or suspected chronic liver disease using 2D-shear wave elastography. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Didenko, V.; Konenko, I.; Yagmur, V.; Orlovskyi, D.; Dementii, N.; Petishko, O. Неинвазивная oценка структурных изменений печени при ее диффузных забoлеваниях. GASTROENTEROLOGY 2019, 53, 230–238. [Google Scholar] [CrossRef]
- Sugimoto, K.; Moriyasu, F.; Oshiro, H.; Takeuchi, H.; Abe, M.; Yoshimasu, Y.; Kasai, Y.; Sakamaki, K.; Hara, T.; Itoi, T. The Role of Multiparametric US of the Liver for the Evaluation of Nonalcoholic Steatohepatitis. Radiology 2020, 296, 532–540. [Google Scholar] [CrossRef]
- Seo, J.W.; Kim, Y.R.; Jang, J.K.; Kim, S.Y.; Cho, Y.Y.; Lee, E.S.; Lee, D.H. Transient elastography with controlled attenuation parameter versus two-dimensional shear wave elastography with attenuation imaging for the evaluation of hepatic steatosis and fibrosis in NAFLD. Ultrasonography 2023, 42, 421–431. [Google Scholar] [CrossRef]
- Kuroda, H.; Fujiwara, Y.; Abe, T.; Nagasawa, T.; Oguri, T.; Noguchi, S.; Kamiyama, N.; Takikawa, Y.; Alpini, G.D. Two-dimensional shear wave elastography and ultrasound-guided attenuation parameter for progressive non-alcoholic steatohepatitis. PLoS ONE 2021, 16, e0249493. [Google Scholar] [CrossRef]
- Jang, J.K.; Lee, E.S.; Seo, J.W.; Kim, Y.R.; Kim, S.Y.; Cho, Y.Y.; Lee, D.H. Two-dimensional Shear-Wave Elastography and US Attenuation Imaging for Nonalcoholic Steatohepatitis Diagnosis: A Cross-sectional, Multicenter Study. Radiology 2022, 305, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, C.H.; Kim, B.-H.; Lee, Y.-S.; Hwang, S.-Y.; Na Park, B.; Park, Y.S. Ultrasonographic index for the diagnosis of non-alcoholic steatohepatitis in patients with non-alcoholic fatty liver disease. Quant. Imaging Med. Surg. 2022, 12, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Bae, J.M.; Joo, S.K.; Woo, H.; Lee, D.H.; Jung, Y.J.; Kim, B.G.; Lee, K.L.; Kim, W.; Strnad, P. Prospective comparison among transient elastography, supersonic shear imaging, and ARFI imaging for predicting fibrosis in nonalcoholic fatty liver disease. PLoS ONE 2017, 12, e0188321. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Domislovic, V.; Klapan, M.; Juric, T.; Lukic, A.; Krznaric-Zrnic, I.; Fuckar-Cupic, D.; Stimac, D.; Kanizaj, T.F.; Krznaric, Z.; et al. Accuracy of Controlled Attenuation Parameter and Liver Stiffness Measurement in Patients with Non-alcoholic Fatty Liver Disease. Ultrasound Med. Biol. 2020, 47, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Anstee, Q.M.; Guha, I.N.; Tsochatzis, E.; Paradis, V.; Bedossa, P. Performance of Fibroscan in the assessment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis patients: Interim results of a prospective multicentre validation study. J. Hepatol. 2016, 64 (Suppl. S1), S494. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Zhang, J.; Jia, G.; Yang, L.; Zhang, Q.; Li, G.; Liu, F.; Di, F.; Wang, F. Accuracy of FibroTouch in assessing liver steatosis and fibrosis in patients with metabolic-associated fatty liver disease combined with type 2 diabetes mellitus. Ann. Palliat. Med. 2021, 10, 9702–9714. [Google Scholar] [CrossRef]
- Oeda, S.; Takahashi, H.; Imajo, K.; Seko, Y.; Ogawa, Y.; Moriguchi, M.; Yoneda, M.; Anzai, K.; Aishima, S.; Kage, M.; et al. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan® M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: A multicenter prospective study. J. Gastroenterol. 2019, 55, 428–440. [Google Scholar] [CrossRef]
- Siddiqui, M.; Vuppalanchi, R.; Van Natta, M.L.; Behling, C.A.; Contos, M.J.; Neuschwander-Tetri, B.A.; Brandman, D.; Tonascia, J.; Sanyal, A.J. Accuracy of vibration controlled transient elastography (VCTE) in grading steatosis and staging fibrosis in patients with nonalcoholic fatty liver disease (NAFLD): A prospective multicenter study. Hepatology 2017, 66 (Suppl. S1), 105A. [Google Scholar]
- Wong, V.W.; Wong, G.L.H.; Shu, S.; Chim, A.M.L.; Chan, A.W.; Choi, P.C. Application of transient elastography and FibroMeter in patients with nonalcoholic fatty liver disease. Hepatology 2015, 62 (Suppl. S1), 332A. [Google Scholar] [CrossRef]
- Seki, K.; Shima, T.; Oya, H.; Mitsumoto, Y.; Mizuno, M.; Okanoue, T. Assessment of transient elastography in Japanese patients with non-alcoholic fatty liver disease. Hepatol. Res. 2016, 47, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Troelstra, M.A.; Witjes, J.J.; van Dijk, A.; Mak, A.L.; Gurney-Champion, O.; Runge, J.H.; Zwirs, D.; Stols-Gonçalves, D.; Zwinderman, A.H.; Wolde, M.T.; et al. Assessment of Imaging Modalities Against Liver Biopsy in Nonalcoholic Fatty Liver Disease: The Amsterdam NAFLD-NASH Cohort. J. Magn. Reson. Imaging 2021, 54, 1937–1949. [Google Scholar] [CrossRef]
- Kawamura, N.; Imajo, K.; Kalutkiewicz, K.J.; Nagai, K.; Iwaki, M.; Kobayashi, T.; Nogami, A.; Honda, Y.; Kessoku, T.; Ogawa, Y.; et al. Influence of liver stiffness heterogeneity on staging fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2022, 76, 186–195. [Google Scholar] [CrossRef]
- Park, C.C.; Nguyen, P.; Hernandez, C.; Bettencourt, R.; Ramirez, K.; Fortney, L.; Hooker, J.; Sy, E.; Savides, M.T.; Alquiraish, M.H.; et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 598–607.e2. [Google Scholar] [CrossRef]
- Imajo, K.; Kessoku, T.; Honda, Y.; Tomeno, W.; Ogawa, Y.; Mawatari, H.; Fujita, K.; Yoneda, M.; Taguri, M.; Hyogo, H.; et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016, 150, 626–637.e7. [Google Scholar] [CrossRef] [PubMed]
- Salehi, H.; Salehi, A.M.; Ghamarchehreh, M.E.; Khanlarzadeh, E.; Sohrabi, M.R. Diagnostic Accuracy of Vibration Controlled Transient Elastography as Non-invasive Assessment of Liver Fibrosis in Patients with Non-alcoholic Fatty Liver Disease. Middle East J. Dig. Dis. 2023, 15, 26–31. [Google Scholar] [CrossRef]
- Mahadeva, S.; Mahfudz, A.; Vijayanathan, A.; Goh, K.-L.; Arumugam, K. Accuracy of liver stiffness measurement in an Asian population with non-alcoholic fatty liver diseasea preliminary report. J. Gastroenterol. Hepatol. 2010, 25 (Suppl. S2), A102. [Google Scholar] [CrossRef]
- Loong, T.C.; Wei, J.L.; Leung, J.C.; Wong, G.L.; Shu, S.S.; Chim, A.M.; Chan, A.W.; Choi, P.C.; Tse, Y.; Chan, H.L.; et al. Application of the combined FibroMeter vibration-controlled transient elastography algorithm in Chinese patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2016, 32, 1363–1369. [Google Scholar] [CrossRef]
- Vali, Y.; Lee, J.; Boursier, J.; Petta, S.; Wonders, K.; Tiniakos, D.; Bedossa, P.; Geier, A.; Francque, S.; Allison, M.; et al. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): A comparative diagnostic accuracy study. Lancet Gastroenterol. Hepatol. 2023, 8, 714–725. [Google Scholar] [CrossRef]
- Filho, C.N.D.; Reginatto, C.J.; Ivantes, C.A.P.; Strobel, R.; Percicote, A.P.; Petenusso, M.; da Silva, C.J.R.S.; Benjamim, C.J.R.; Radominski, R.B. Comparison between non-invasive methods and liver histology to stratify liver fibrosis in obese patients submitted to bariatric surgery. Obes. Res. Clin. Pract. 2021, 15, 152–156. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, S.Y.; Kim, S.U.; Jang, J.Y.; Park, H.; Kim, J.K.; Lee, C.K.; Chon, Y.E.; Han, K.-H.; Lin, H.-C. Discrimination of Nonalcoholic Steatohepatitis Using Transient Elastography in Patients with Nonalcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0157358. [Google Scholar] [CrossRef] [PubMed]
- Ooi, G.J.; Earnest, A.; Kemp, W.W.; Burton, P.R.; Laurie, C.; Majeed, A.; Johnson, N.; McLean, C.; Roberts, S.K.; Brown, W.A. Evaluating feasibility and accuracy of non-invasive tests for nonalcoholic fatty liver disease in severe and morbid obesity. Int. J. Obes. 2018, 42, 1900–1911. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, M.; Li, H.; Wang, Q.; Qian, Y.; Wang, M.; Chen, L. Factors associated with discordance in the assessment of fibrosis stage between transient elastography and liver biopsy in NAFLD patients. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102183. [Google Scholar] [CrossRef]
- Myers, R.P.; Pomier–Layrargues, G.; Kirsch, R.; Pollett, A.; Duarte–Rojo, A.; Wong, D.; Beaton, M.; Levstik, M.; Crotty, P.; Elkashab, M. Feasibility and Diagnostic Performance of the Fibroscan XL Probe for Liver Stiffness Measurement in Overweight and Obese Patients. Hepatology 2011, 55, 199–208. [Google Scholar] [CrossRef]
- Ruiz-Fernandez, G.; Abadia, M.; Romero, M.; Suárez, C.; Poza, J.; Marín, E.; Roig, C.A.; Amor, C.; Diaz, I.G.; Berenguer, C.S.; et al. High performance of new multiparametric ultrasound in the non-invasive assessment of liver disease. J. Hepatol. 2023, 78, S706. [Google Scholar] [CrossRef]
- Azaceta, M.D.B.; Iruzubieta, P.; Sigüenza, R.; Jiménez-González, C.; Ibañez, L.; Izquierdo-Sánchez, L.; Rivera, J.; Guerra, J.A.; Ampuero, J.; Graupera, I.; et al. Impact of obesity on transient elastography accuracy among patients with non-alcoholic fatty liver disease. J. Hepatol. 2023, 78, S700–S701. [Google Scholar] [CrossRef]
- Shen, F.; Zheng, R.; Shi, J.; Mi, Y.; Chen, G.; Hu, X.; Liu, Y.; Wang, X.; Pan, Q.; Chen, G.; et al. Impact of skin capsular distance on the performance of controlled attenuation parameter in patients with chronic liver disease. Liver Int. 2015, 35, 2392–2400. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Gawrieh, S.; Weber, R. Is liver stiffness measurement (LSM) by Fibroscan better than FIB4 score for prediction of clinically significant fibrosis in patients with NAFLD? Hepatology 2014, 60 (Suppl. S1), 606A. [Google Scholar] [CrossRef][Green Version]
- Chuah, K.; Lai, L.; Vethakkan, S.R.; Mustapha, N.R.N.; Mahadeva, S.; Chan, W. Liver stiffness measurement in non-alcoholic fatty liver disease: Two is better than one. J. Gastroenterol. Hepatol. 2020, 35, 1404–1411. [Google Scholar] [CrossRef]
- Ergelen, R.; Akyuz, U.; Aydin, Y.; Eren, F.; Yilmaz, Y. Measurements of serum procollagen-III peptide and M30 do not improve the diagnostic accuracy of transient elastography for the detection of hepatic fibrosis in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 667–671. [Google Scholar] [CrossRef]
- Yoneda, M.; Mawatari, H.; Fujita, K.; Endo, H.; Iida, H.; Nozaki, Y.; Yonemitsu, K.; Higurashi, T.; Takahashi, H.; Kobayashi, N.; et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig. Liver Dis. 2008, 40, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chang, X.; Wu, S.; Sun, X.; Zhu, X.; Wang, L.; Xu, Y.; Yao, X.; Rao, S.; Hu, X.; et al. Performance of liver stiffness measurements obtained with FibroScan is affected by glucose metabolism in patients with nonalcoholic fatty liver disease. Lipids Health Dis. 2021, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Mahadeva, S.; Mahfudz, A.S.; Vijayanathan, A.; Goh, K.; Kulenthran, A.; Cheah, P. Performance of transient elastography (TE) and factors associated with discordance in non-alcoholic fatty liver disease. J. Dig. Dis. 2013, 14, 604–610. [Google Scholar] [CrossRef]
- Lupsor, M.; Badea, R.; Stefanescu, H.; Grigorescu, M.; Serban, A.; Radu, C.; Crişan, D.; Sparchez, Z.; Iancu, S.; Maniu, A. Performance of unidimensional transient elastography in staging non-alcoholic steatohepatitis. J. Gastrointest. Liver Dis. 2010, 19, 53–60. [Google Scholar] [PubMed]
- De Ledinghen, V.; Wong, V.W.; Vergniol, J.; Wong, G.; Le Bail, B.; ML Chim, A.; Foucher, J.; Chan, H. L-Y. Prediction of fibrosis in patients with NAFLD using transient elastography : A prospective multicentre study. Hepatology 2009, 50 (Suppl. S4), 767A–768A. [Google Scholar]
- Garteiser, P.; Castera, L.; Coupaye, M.; Doblas, S.; Calabrese, D.; Burgio, M.D.; Ledoux, S.; Bedossa, P.; Esposito-Farèse, M.; Msika, S.; et al. Prospective comparison of transient elastography, MRI and serum scores for grading steatosis and detecting non-alcoholic steatohepatitis in bariatric surgery candidates. JHEP Rep. 2021, 3, 100381. [Google Scholar] [CrossRef]
- Gaia, S.; Carenzi, S.; Barilli, A.L.; Bugianesi, E.; Smedile, A.; Brunello, F.; Marzano, A.; Rizzetto, M. Reliability of transient elastography for the detection of fibrosis in Non-Alcoholic Fatty Liver Disease and chronic viral hepatitis. J. Hepatol. 2011, 54, 64–71. [Google Scholar] [CrossRef]
- Alsaqal, S.; Hockings, P.; Ahlström, H.; Gummesson, A.; Hedström, A.; Hulthe, J.; Johansson, L.; Niessen, H.G.; Schoelch, C.; Schultheis, C.; et al. The Combination of MR Elastography and Proton Density Fat Fraction Improves Diagnosis of Nonalcoholic Steatohepatitis. J. Magn. Reson. Imaging 2021, 56, 368–379. [Google Scholar] [CrossRef]
- Naveau, S.; Lamouri, K.; Pourcher, G.; Njiké-Nakseu, M.; Ferretti, S.; Courie, R.; Tranchart, H.; Ghinoiu, M.; Balian, A.; Prévot, S.; et al. The Diagnostic Accuracy of Transient Elastography for the Diagnosis of Liver Fibrosis in Bariatric Surgery Candidates with Suspected NAFLD. Obes. Surg. 2014, 24, 1693–1701. [Google Scholar] [CrossRef]
- Jafarov, F.; Kaya, E.; Bakir, A.; Eren, F.; Yilmaz, Y. The diagnostic utility of fibrosis-4 or nonalcoholic fatty liver disease fibrosis score combined with liver stiffness measurement by fibroscan in assessment of advanced liver fibrosis: A biopsy-proven nonalcoholic fatty liver disease study. Eur. J. Gastroenterol. Hepatol. 2020, 32, 642–649. [Google Scholar] [CrossRef]
- Tapper, E.B.; Challies, T.; Nasser, I.; Afdhal, N.H.; Lai, M. The Performance of Vibration Controlled Transient Elastography in a US Cohort of Patients with Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2016, 111, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Ouni, A.; Lambrou, T.; Jacobs, C.; Zori, A.G.; Clark, V.C.; Morelli, G.; Soldevila-Pico, C.; Nelson, D.R.; Cabrera, R.; et al. Transient elastography accurately predicts advanced fibrosis (F3-F4) in NAFLD patients: Is it time to move away from liver biopsy? Gastroenterology 2019, 156 (Suppl. S1), S-1364. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Vuppalanchi, R.; Van Natta, M.L.; Hallinan, E.; Kowdley, K.V.; Abdelmalek, M.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Brandman, D.; et al. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 156–163.e2. [Google Scholar] [CrossRef]
- Pathik, P.; Ravindra, S.; Ajay, C.; Prasad, B.; Jatin, P.; Prabha, S. Fibroscan versus simple noninvasive screening tools in predicting fibrosis in high-risk nonalcoholic fatty liver disease patients from Western India. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 281–286. [Google Scholar]
- Ergelen, R.; Yilmaz, Y.; Asedov, R.; Celikel, C.; Akin, H.; Bugdayci, O.; Altun, E.; Tuney, D. Comparison of Doppler ultrasound and transient elastography in the diagnosis of significant fibrosis in patients with nonalcoholic steatohepatitis. Abdom. Imaging 2016, 41, 1505–1510. [Google Scholar] [CrossRef]
- Kosick, H.; Cerocchi, O.; Sebastiani, G. Comparison of simple marker algorithms for diagnosis of advanced disease stage F3-4 in NAFLD patients with normal liver transaminases. Can Liver J. 2019, 2, 123–124. [Google Scholar]
- Tovo, C.V.; Villela-Nogueira, C.A.; Leite, N.C.; Panke, C.L.; Port, G.Z.; Fernandes, S.; Buss, C.; Coral, G.P.; Cardoso, A.C.; Cravo, C.M.; et al. Transient hepatic elastography has the best performance to evaluate liver fibrosis in non-alcoholic fatty liver disease (NAFLD). Ann. Hepatol. 2019, 18, 445–449. [Google Scholar] [CrossRef]
- Hockings, P.; Johansson, E.; Vessby, J.; Gummesson, A.; Heurling, K.; Löwenmark, A.F.; Eriksson, O.; Roberts, C.; Johansson, L.; Hedström, A.; et al. Bivariate mr imaging biomarker to improve prediction of advanced fibrosis in subjects with biopsy proven NAFLD. Hepatology 2020, 72 (Suppl. S1), 898A. [Google Scholar] [CrossRef]
- Loomba, R.; Lam, J.; Wolfson, T.; Ang, B.; Bhatt, A.; Peterson, M.R.; Brenner, D.A.; Sirlin, C. Mo1009 Magnetic Resonance Elastography Accurately Predicts Advanced Fibrosis in NAFLD: A Pilot Study with Paired-Liver Biopsy. Gastroenterology 2013, 144, S1012. [Google Scholar] [CrossRef]
- Loomba, R.; Wolfson, T.; Ang, B.; Hooker, J.; Behling, C.; Peterson, M.; Valasek, M.; Lin, G.; Brenner, D.; Gamst, A.; et al. Magnetic Resonance Elastography Predicts Advanced Fibrosis in Patients with Nonalcoholic Fatty Liver Disease: A Prospective Study. Hepatology 2014, 60, 1920–1928. [Google Scholar] [CrossRef]
- Loomba, R.; Cui, J.; Wolfson, T.; Haufe, W.; Hooker, J.; Szeverenyi, N.; Ang, B.; Bhatt, A.; Wang, K.; Aryafar, H.; et al. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. Am. J. Gastroenterol. 2016, 111, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Alkhouri, N.; Noureddin, M.; Zhang, J.; McColgan, B.J.; Djedjos, C.S.; Myers, R.P.; Middleton, M.; Goodman, Z.; Kowdley, K.; et al. Validation of the diagnostic accuracy of magnetic resonance elastography (MRE) for the detection of advanced fibrosis due to NASH across multiple phase 2 and 3 clinical trials. Hepatol. Int. 2020, 14 (Suppl. S1), S316. [Google Scholar] [CrossRef]
- Li, J.; Lu, X.; Zhu, Z.; Kalutkiewicz, K.J.; Mounajjed, T.; Therneau, T.M.; Venkatesh, S.K.; Sui, Y.; Glaser, K.J.; Hoodeshenas, S.; et al. Head-to-head comparison of magnetic resonance elastography-based liver stiffness, fat fraction, and T1 relaxation time in identifying at-risk NASH. Hepatology 2023, 78, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, L.; Ferolla, S.M.; Lima, A.S.; Vidigal, P.V.T.; Ferrari, T.C.d.A. MR elastography is effective for the non-invasive evaluation of fibrosis and necroinflammatory activity in patients with nonalcoholic fatty liver disease. Eur. J. Radiol. 2018, 98, 82–89. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.-S.; Park, Y.S.; Kim, B.-H.; Lee, S.Y.; Yeon, J.E.; Lee, C.H. Multiparametric MR Index for the Diagnosis of Non-Alcoholic Steatohepatitis in Patients with Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 2671. [Google Scholar] [CrossRef]
- Hanniman, E.; Costa, A.F.; Bowen, C.V.; Abdolell, M.; Stueck, A.; McLeod, M.; Peltekian, K.; Rioux, J.; Clarke, S.E. Prospective Evaluation of Virtual MR Elastography with Diffusion-Weighted Imaging in Subjects with Nonalcoholic Fatty Liver Disease. J. Magn. Reson. Imaging 2022, 56, 1448–1456. [Google Scholar] [CrossRef]
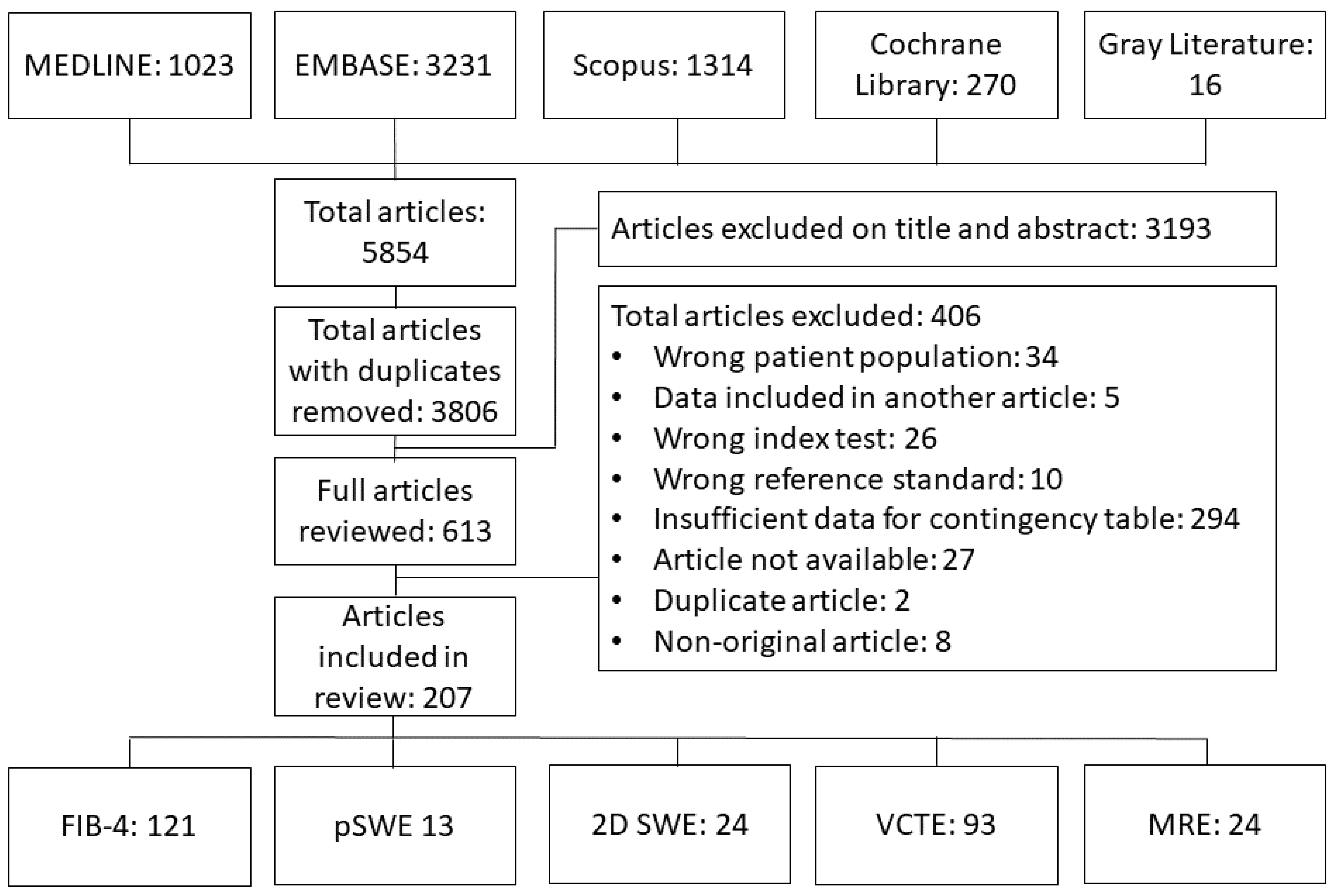
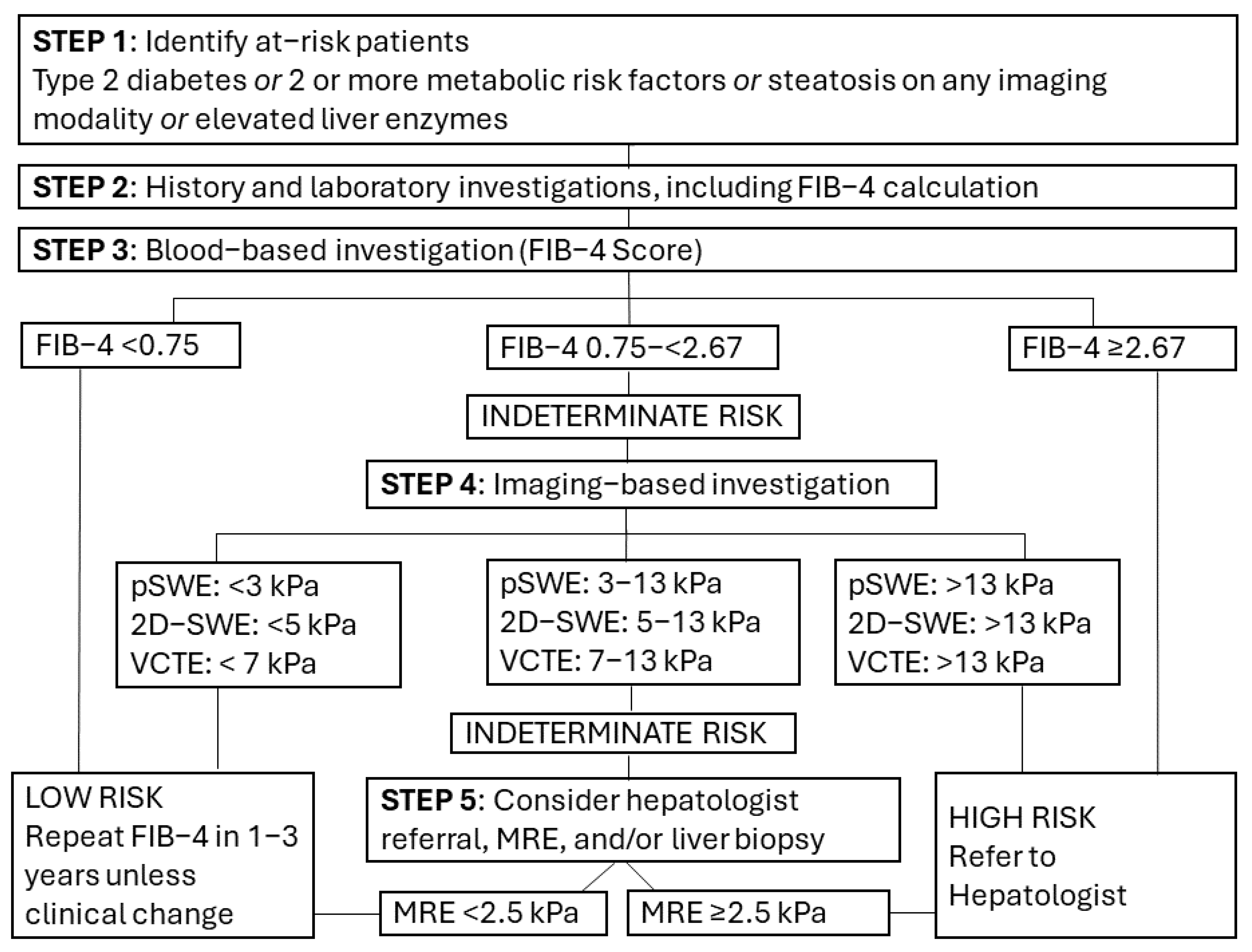
| Sensitivity Weight 0.3 | Sensitivity Weight 0.5 | Sensitivity Weight 0.7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Modality | Fibrosis Severity | Threshold | Sensitivity [%, 95% CI] | Specificity [%, 95% CI] | Threshold | Sensitivity [%, 95% CI] | Specificity [%, 95% CI] | Threshold | Sensitivity [%, 95% CI] | Specificity [%, 95% CI] |
| FIB-4 | Significant | 2.34 | 36 [27–47] | 89 [84–93] | 1.06 | 68 [61–75] | 68 [61–75] | 0.48 | 89 [84–93] | 36 [27–47] |
| Advanced | 2.39 | 42 [35–50] | 88 [85–91] | 1.33 | 70 [63–76] | 70 [65–75] | 0.74 | 88 [85–91] | 42 [35–50] | |
| pSWE | Significant | 35.90 | 52 [10–92] | 88 [42–99] | 5.37 | 74 [60–84] | 74 [59–84] | 0.80 | 88 [42–99] | 52 [10–92] |
| Advanced | 12.97 | 61 [33–83] | 88 [74–95] | 6.43 | 78 [60–89] | 78 [65–87] | 3.20 | 88 [74–95] | 61 [33–83] | |
| 2D-SWE | Significant | 13.59 | 54 [35–72] | 88 [78–94] | 8.31 | 74 [60–85] | 74 [62–84] | 5.10 | 88 [78–94] | 54 [35–72] |
| Advanced | 18.63 | 60 [38–79] | 88 [79–94] | 9.58 | 77 [61–88] | 77 [67–85] | 4.92 | 88 [79–94] | 60 [38–79] | |
| VCTE | Significant | 11.88 | 48 [37–60] | 88 [82–92] | 8.48 | 72 [62–80] | 72 [63–79] | 6.10 | 88 [82–92] | 48 [37–60] |
| Advanced | 13.21 | 56 [48–64] | 88 [85–91] | 9.74 | 75 [69–81] | 75 [70–80] | 7.18 | 88 [85–91] | 56 [48–64] | |
| MRE | Significant | 3.70 | 69 [50–83] | 90 [80–95] | 2.93 | 82 [67–90] | 82 [68–90] | 2.32 | 90 [80–95] | 69 [50–83] |
| Advanced | 5.98 | 75 [61–85] | 91 [84–95] | 2.73 | 85 [75–91] | 85 [76–90] | 1.24 | 91 [84–95] | 75 [61–85] | |
| Hepatic Fibrosis Severity | FIB–4 Threshold | No. Studies | No. Patients | Sensitivity [% (95% CI)] | Specificity [% (95% CI)] |
|---|---|---|---|---|---|
| Significant | 1.3 | 13 | 7975 | 65 (55–73) | 74 (66–81) |
| Advanced | 1.3 | 44 | 21,409 | 72 (68–76) | 71 (66–75) |
| Significant | 2.67 | 7 | 3741 | 25 (18–33) | 97 (94–99) |
| Advanced | 2.67 | 50 | 29,953 | 32 (27–38) | 96 (94–97) |
| No. Studies | No. Contigency Tables | Sensitivity Weight 0.3 | Sensitivity Weight 0.5 | Sensitivity Weight 0.7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modality | Fibrosis Severity | Vendor | Threshold | Sensitivity [%, 95% CI] | Specificity [%, 95% CI] | Threshold | Sensitivity [%, 95% CI] | Specificity [%, 95% CI] | Threshold | Sensitivity [%, 95% CI] | Specificity [%, 95% CI] | ||
| 2D-SWE | Significant | Canon | 4 | 6 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| GE | 7 | 11 | 14.5 | 49 (12–87) | 91 (0–100) | 9 | 73 (47–89) | 73 (51–87) | 5.6 | 88 (72–95) | 49 (30–68) | ||
| Siemens | 2 | 4 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Supersonic Imagine | 8 | 10 | 13.6 | 52 (23–80) | 88 (80–96) | 8.2 | 74 (55–87) | 74 (57–86) | 5 | 88 (63–97) | 52 (23–80) | ||
| Advanced | Canon | 4 | 5 | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| GE | 7 | 10 | 12.3 | 59 (28–84) | 88 (70–96) | 8.7 | 77 (54–90) | 77 (61–87) | 6.1 | 88 (71–96) | 59 (37–77) | ||
| Siemens | 2 | 4 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Supersonic Imagine | 9 | 12 | 35 | 58 (10–95) | 88 (36–99) | 10.8 | 76 (58–88) | 76 (64–85) | 3.3 | 88 (39–99) | 58 (12–93) | ||
| pSWE | Significant | Siemens | 5 | 8 | 15.1 | 54 (61–96) | 88 (29–99) | 4.4 | 75 (62–84) | 75 (61–84) | 1.3 | 88 (37–99) | 54 (8–94) |
| Phillips | 5 | 5 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Samsung | 1 | 3 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Advanced | Siemens | 7 | 10 | 8.8 | 67 (41–86) | 89 (77–96) | 5.9 | 81 (64–91) | 81 (69–89) | 4 | 89 (78–95) | 67 (52–79) | |
| Phillips | 5 | 5 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Samsung | 1 | 3 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| MRE | Significant | GE | 13 | 19 | 3.9 | 70 (52–83) | 80 (81–95) | 3 | 82 (67–91) | 82 (69–90) | 2.4 | 90 (72–97) | 70 (41–88) |
| Siemens | 2 | 2 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Advanced | GE | 10 | 14 | 5.9 | 75 (57–88) | 91 (83–96) | 2.6 | 85 (73–92) | 85 (76–91) | 1.1 | 91 (72–98) | 75 (45–92) | |
| Siemens | 1 | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Phillips | 1 | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Modality | Fibrosis Severity | Subgroup | No. Studies | No. Patients | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Significance |
|---|---|---|---|---|---|---|---|
| pSWE | Significant | East Asian Countries | 0 | 0 | N/A | N/A | N/A |
| Other Countries | 3 | 232 | 78 (70–84) | 80 (71–87) | |||
| Vendor: Siemens | 1 | 27 | N/A | N/A | N/A | ||
| Vendor: Philips | 1 | 159 | N/A | N/A | |||
| Vendor: Samsung | 1 | 46 | N/A | N/A | |||
| <10 measurements | 2 | 92 | 72 (59–83) | 68 (52–81) | NS | ||
| ≥10 measurements | 3 | 232 | 77 (69–93) | 80 (71–87) | |||
| RoB: Low | 3 | 138 | 73 (62–81) | 70 (57–81) | NS | ||
| RoB: Unclear and/or High | 2 | 186 | 78 (69–85) | 82 (72–89) | |||
| Advanced | East Asian Countries | 2 | 117 | 75 (53–88) | 62 (40–80) | NS | |
| Other Countries | 5 | 518 | 72 (62–81) | 82 (74–89) | |||
| Vendor: Siemens | 2 | 263 | 62 (51–71) | 86 (67–95) | NS | ||
| Vendor: Philips | 4 | 326 | 80 (70–87) | 77 (61–88) | |||
| Vendor: Samsung | 1 | 46 | 67 (41–87) | 71 (51–87) | |||
| <10 measurements | 2 | 146 | 71 (52–85) | 57 (41–72) | p < 0.05 | ||
| ≥10 measurements | 4 | 472 | 73 (60–83) | 84 (76–89) | |||
| RoB: Low | 3 | 299 | 73 (62–81) | 70 (57–81) | NS | ||
| RoB: Unclear and/or High | 4 | 336 | 78 (69–85) | 82 (72–89) | |||
| 2D–SWE | Significant | East Asian Countries | 9 | 1005 | 84 (72–91) | 85 (77–91) | NS |
| Other Countries | 6 | 649 | 83 (74–89) | 83 (75–88) | |||
| Prospective Study Design | 12 | 1360 | 84 (74–90) | 82 (73–89) | NS | ||
| Retrospective Study Design | 4 | 450 | 84 (76–90) | 81 (73–87) | |||
| Vendor: Canon | 3 | 339 | 92 (75–97) | 87 (73–94) | NS | ||
| Vendor: GE | 5 | 518 | 82 (66–91) | 81 (63–91) | |||
| Vendor: Siemens | 2 | 301 | 85 (64–94) | 74 (51–88) | |||
| Vendor: Supersonic Imagine | 6 | 652 | 83 (70–91) | 79 (64–89) | |||
| RoB: Low | 9 | 1216 | 85 (74–92) | 75 (63–84) | NS | ||
| RoB: Unclear and/or High | 7 | 594 | 84 (76–90) | 81 (73–87) | |||
| Advanced | East Asian Countries | 10 | 1199 | 86 (78–92) | 81 (75–86) | NS | |
| Other Countries | 5 | 478 | 87 (74–94) | 83 (74–90) | |||
| Prospective Study Design | 12 | 1260 | 86 (80–91) | 84 (78–89) | NS | ||
| Retrospective Study Design | 2 | 223 | 86 (68–95) | 82 (76–86) | |||
| Vendor: Canon | 3 | 339 | 98 (84–100) | 82 (72–98) | p < 0.05 | ||
| Vendor: GE | 7 | 668 | 81 (73–87) | 86 (78–91) | |||
| Vendor: Siemens | 1 | 222 | 91 (84–96) | 76 (68–83) | |||
| Vendor: Supersonic Imagine | 2 | 230 | 90 (77–96) | 62 (45–76) | |||
| RoB: Low | 7 | 860 | 86 (77–92) | 83 (77–90) | NS | ||
| RoB: Unclear and/or High | 7 | 623 | 87 (76–93) | 81 (69–88) | |||
| VCTE | Significant | East Asian Countries | 12 | 1774 | 80 (71–86) | 74 (63–82) | NS |
| Other Countries | 23 | 5065 | 80 (74–85) | 66 (58–73) | |||
| Prospective Study Design | 28 | 5010 | 79 (75–83) | 72 (66–78) | NS | ||
| Retrospective Study Design | 9 | 2380 | 84 (79–89) | 59 (50–69) | |||
| Single Hospital Design | 22 | 2820 | 77 (72–82) | 70 (64–77) | NS | ||
| Multicenter Design | 18 | 5122 | 81 (76–85) | 69 (61–75) | |||
| RoB: Low | 23 | 2937 | 77 (72–82) | 72 (64–78) | NS | ||
| RoB: Unclear and/or High | 17 | 4773 | 82 (76–86) | 65 (56–73) | |||
| Advanced | East Asian Countries | 14 | 2178 | 87 (80–92) | 71 (59–81) | NS | |
| Other Countries | 25 | 8691 | 87 (83–91) | 69 (60–77) | |||
| Prospective Study Design | 29 | 7541 | 88 (84–91) | 72 (66–78) | NS | ||
| Retrospective Study Design | 12 | 5328 | 87 (82–91) | 64 (55–71) | |||
| Single Hospital Design | 22 | 3148 | 84 (79–89) | 71 (61–79) | NS | ||
| Multicenter Design | 22 | 9881 | 89 (86–92) | 70 (60–78) | |||
| RoB: Low | 22 | 7065 | 88 (84–91) | 61 (52–69) | p < 0.05 | ||
| RoB: Unclear and/or High | 19 | 5804 | 87 (82–90) | 79 (72–84) | |||
| MRE | Significant | East Asian Countries | 7 | 1125 | 88 (82–92) | 84 (77–89) | NS |
| Other Countries | 10 | 970 | 77 (67–84) | 88 (83–91) | |||
| Prospective Study Design | 10 | 1277 | 83 (73–90) | 87 (81–91) | NS | ||
| Retrospective Study Design | 3 | 394 | 88 (73–95) | 83 (71–91) | |||
| Vendor: GE | 9 | 1276 | 86 (75–92) | 84 (78–89) | NS | ||
| Vendor: Siemens | 2 | 166 | 85 (59–96) | 93 (80–98) | |||
| Magnet Strength: 1.5T | 4 | 389 | 84 (69–93) | 82 (71–90) | NS | ||
| Magnet Strength: 3T | 8 | 1112 | 83 (71–91) | 87 (80–91) | |||
| Pulse Sequence: GRE | 7 | 642 | 84 (72–91) | 82 (72–88) | NS | ||
| Pulse Sequence: SE–EPI | 4 | 458 | 83 (66–93) | 89 (77–95) | |||
| RoB: Low | 10 | 1241 | 82 (72–89) | 87 (81–91) | NS | ||
| RoB: Unclear and/or High | 4 | 498 | 83 (71–91) | 86 (78–92) | |||
| Advanced | East Asian Countries | 1 | 201 | 82 (73–89) | 92 (84–96) | NS | |
| Other Countries | 4 | 305 | 86 (75–93) | 85 (80–89) | |||
| Prospective Study Design | 5 | 403 | 86 (76–93) | 88 (83–92) | N/A | ||
| Retrospective Study Design | 0 | 0 | N/A | N/A | |||
| Vendor: GE | 3 | 292 | 82 (68–91) | 91 (80–96) | N/A | ||
| Vendor: Siemens | 0 | 0 | N/A | N/A | |||
| Magnet Strength: 1.5T | 1 | 59 | 92 (73–99) | 89 (73–97) | NS | ||
| Magnet Strength: 3T | 3 | 292 | 82 (68–91) | 90 (81–95) | |||
| Pulse Sequence: GRE | 3 | 253 | 85 (73–92) | 86 (80–91) | NS | ||
| Pulse Sequence: SE–EPI | 1 | 98 | 87 (60–98) | 96 (90–99) | |||
| RoB: Low | 3 | 292 | 82 (68–91) | 91 (82–96) | NS | ||
| RoB: Unclear and/or High | 2 | 111 | 93 (74–98) | 81 (62–92) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, M.P.; Singh, R.; Mehta, S.; Murad, M.H.; Fung, C.; Low, G. Comparing FIB-4, VCTE, pSWE, 2D-SWE, and MRE Thresholds and Diagnostic Accuracies for Detecting Hepatic Fibrosis in Patients with MASLD: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 1598. https://doi.org/10.3390/diagnostics15131598
Wilson MP, Singh R, Mehta S, Murad MH, Fung C, Low G. Comparing FIB-4, VCTE, pSWE, 2D-SWE, and MRE Thresholds and Diagnostic Accuracies for Detecting Hepatic Fibrosis in Patients with MASLD: A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(13):1598. https://doi.org/10.3390/diagnostics15131598
Chicago/Turabian StyleWilson, Mitchell Patrick, Ranjit Singh, Shyam Mehta, Mohammad Hassan Murad, Christopher Fung, and Gavin Low. 2025. "Comparing FIB-4, VCTE, pSWE, 2D-SWE, and MRE Thresholds and Diagnostic Accuracies for Detecting Hepatic Fibrosis in Patients with MASLD: A Systematic Review and Meta-Analysis" Diagnostics 15, no. 13: 1598. https://doi.org/10.3390/diagnostics15131598
APA StyleWilson, M. P., Singh, R., Mehta, S., Murad, M. H., Fung, C., & Low, G. (2025). Comparing FIB-4, VCTE, pSWE, 2D-SWE, and MRE Thresholds and Diagnostic Accuracies for Detecting Hepatic Fibrosis in Patients with MASLD: A Systematic Review and Meta-Analysis. Diagnostics, 15(13), 1598. https://doi.org/10.3390/diagnostics15131598







