Differences Between the 8th and 9th Editions of the TNM Staging System in Predicting Mortality in Non-Small Cell Lung Cancer Patients Staged with EBUS
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Patient Selection, and Data Collection
2.2. Statistical Analysis
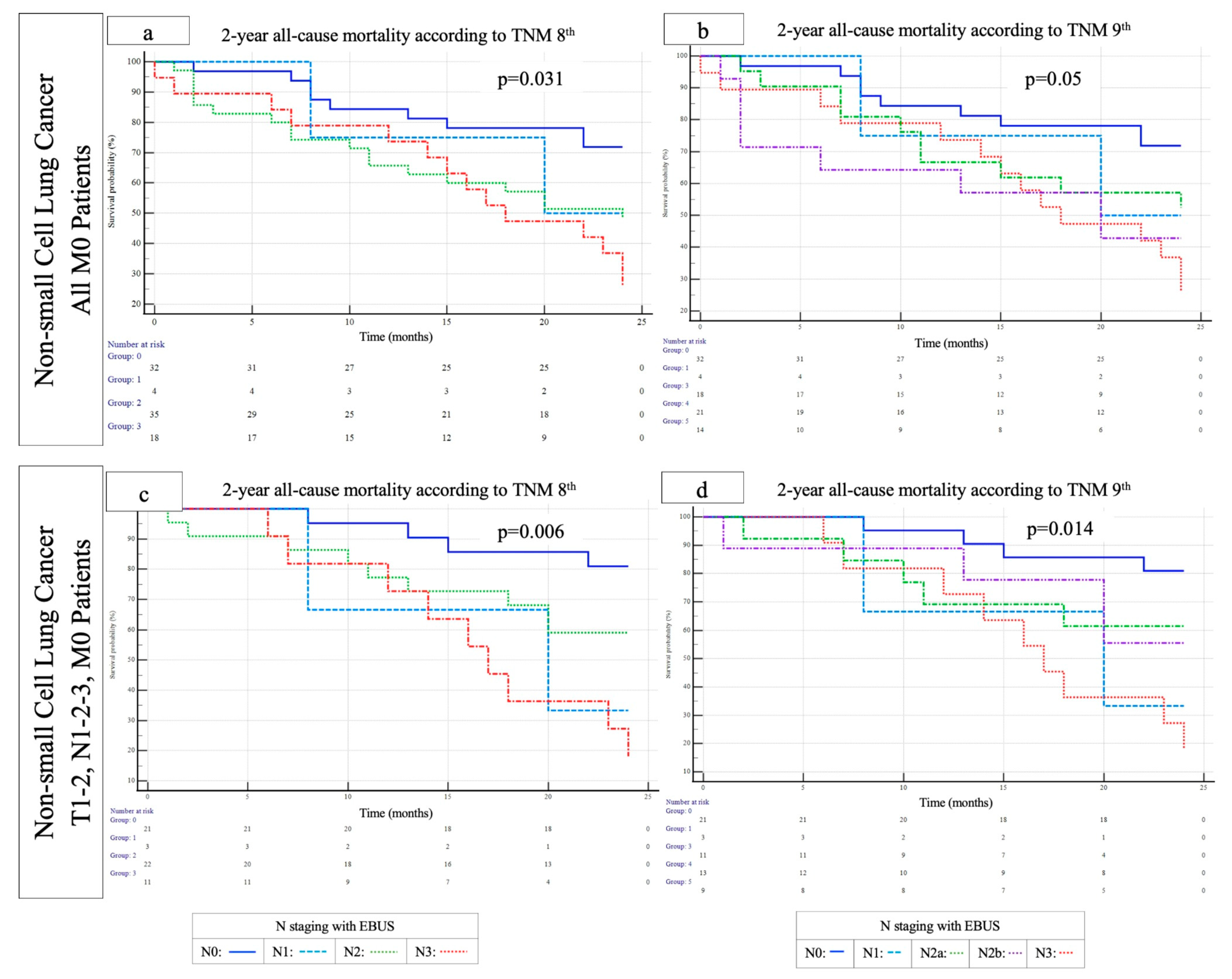
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EBUS | Endobronchial ultrasound |
| EBUS–TBNA | Endobronchial ultrasound–transbronchial needle aspiration |
| ERS | European Respiratory Society |
| ESGE | European Society of Gastrointestinal Endoscopy |
| ESTS | European Society of Thoracic Surgery |
| EUS | Endoscopic (esophageal) ultrasound using a gastrointestinal scope |
| EUS-B | Endoscopic (esophageal) ultrasound using an EBUS scope |
| EUS-FNA | Endoscopic (esophageal) ultrasound with fine needle aspiration, with the use of a gastrointestinal scope |
| HR | Hazard ratio |
| NSCLC | Non-small-cell lung cancer |
| PET/CT | Positron emission tomography/computed tomography |
| ROSE | Rapid on-site evaluation |
| TNM | Tumor-node-metastasis |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Mountain, C.F. A new international staging system for lung cancer. Chest 1986, 89 (Suppl. 4), 225S–233S. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Nishimura, K.K.; Giroux, D.J.; Chansky, K.; Hoering, A.; Rusch, V.; Rami-Porta, R.; Members of the IASLC Staging and Prognostic Factors Committee and of the Advisory Boards, and Participating Institutions. IASLC Lung Cancer Staging Project: The New Database to Inform Revisions in the Ninth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2023, 18, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Chansky, K.; Crowley, J.; Goldstraw, P.; Rusch, V.W.; Vansteenkiste, J.F.; Watanabe, H.; Wu, Y.L.; Zielinski, M.; Ball, D.; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 1675–1684. [Google Scholar] [CrossRef]
- Yun, J.K.; Lee, G.D.; Choi, S.; Kim, H.R.; Kim, Y.H.; Kim, D.K.; Park, S.I. Comparison between lymph node station- and zone-based classification for the future revision of node descriptors proposed by the International Association for the Study of Lung Cancer in surgically resected patients with non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2019, 56, 849–857. [Google Scholar] [CrossRef]
- Park, B.J.; Kim, T.H.; Shin, S.; Kim, H.K.; Choi, Y.S.; Kim, J.; Zo, J.I.; Shim, Y.M.; Cho, J.H. Recommended Change in the N Descriptor Proposed by the International Association for the Study of Lung Cancer: A Validation Study. J. Thorac. Oncol. 2019, 14, 1962–1969. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, S.M.; Choe, J.; Choi, S.; Do, K.H.; Seo, J.B. Prognostic performance of the N category in the 9th edition of lung cancer staging. Eur Radiol. 2024, 35, 3788–3799. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Nishimura, K.K.; Giroux, D.J.; Detterbeck, F.; Cardillo, G.; Edwards, J.G.; Fong, K.M.; Giuliani, M.; Huang, J.; Kernstine KHSr Marom, E.M.; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groups in the Forthcoming (Ninth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2024, 19, 1007–1027. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. 5), e211S–e250S. [Google Scholar] [CrossRef]
- Vilmann, P.; Clementsen, P.F.; Colella, S.; Siemsen, M.; De Leyn, P.; Dumonceau, J.M.; Herth, F.J.; Larghi, A.; Vazquez-Sequeiros, E.; Hassan, C.; et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015, 47, 545–559. [Google Scholar] [CrossRef]
- Vilmann, P.; Clementsen, P.F. Combined EUS and EBUS are complementary methods in lung cancer staging: Do not forget the esophagus. Endosc. Int. Open 2015, 3, E300–E301. [Google Scholar] [CrossRef] [PubMed]
- Crombag, L.M.M.; Dooms, C.; Stigt, J.A.; Tournoy, K.G.; Schuurbiers, O.C.J.; Ninaber, M.K.; Buikhuisen, W.A.; Hashemi, S.M.S.; Bonta, P.I.; Korevaar, D.A.; et al. Systematic and combined endosonographic staging of lung cancer (SCORE study). Eur. Respir. J. 2019, 53, 1800800. [Google Scholar] [CrossRef] [PubMed]
- Verdial, F.C.; Berfield, K.S.; Wood, D.E.; Mulligan, M.S.; Roth, J.A.; Francis, D.O.; Farjah, F. Safety and Costs of Endobronchial Ultrasound-Guided Nodal Aspiration and Mediastinoscopy. Chest 2020, 157, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Rusch, V.W.; Asamura, H.; Watanabe, H.; Giroux, D.J.; Rami-Porta, R.; Goldstraw, P.; Members of IASLC Staging Committee. The IASLC lung cancer staging project: A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2009, 4, 568–577. [Google Scholar] [CrossRef]
- Tesfaw, L.M.; Dessie, Z.G.; Mekonnen Fenta, H. Lung cancer mortality and associated predictors: Systematic review using 32 scientific research findings. Front. Oncol. 2023, 13, 1308897. [Google Scholar] [CrossRef]
- Malvezzi, M.; Santucci, C.; Boffetta, P.; Collatuzzo, G.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2023 with focus on lung cancer. Ann. Oncol. 2023, 34, 410–419. [Google Scholar] [CrossRef]
- Hwangbo, B.; Park, E.Y.; Yang, B.; Lee, G.K.; Kim, T.S.; Kim, H.Y.; Kim, M.S.; Lee, J.M. Long-term Survival According to N Stage Diagnosed by Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Non-small Cell Lung Cancer. Chest 2022, 161, 1382–1392. [Google Scholar] [CrossRef]
- Slavova-Azmanova, N.S.; Lizama, C.; Johnson, C.E.; Ludewick, H.P.; Lsester, L.; Karunarathne, S.; Phillips, M. Impact of the introduction of EBUS on time to management decision, complications, and invasive modalities used to diagnose and stage lung cancer: A pragmatic pre-post study. BMC Cancer 2016, 16, 44. [Google Scholar] [CrossRef]
- Bousema, J.E.; Dijkgraaf, M.G.W.; van der Heijden, E.H.F.M.; Verhagen, A.F.T.M.; Annema, J.T.; van den Broek, F.J.C.; MEDIASTrial study group. Endosonography With or Without Confirmatory Mediastinoscopy for Resectable Lung Cancer: A Randomized Clinical Trial. J. Clin. Oncol. 2023, 41, 3805–3815. [Google Scholar] [CrossRef]
- Figueiredo, V.R.; Cardoso, P.F.G.; Jacomelli, M.; Santos, L.M.; Minata, M.; Terra, R.M. EBUS-TBNA versus surgical mediastinoscopy for mediastinal lymph node staging in potentially operable non-small cell lung cancer: A systematic review and meta-analysis. J. Bras. Pneumol. 2020, 46, e20190221, (In English and Portuguese). [Google Scholar] [CrossRef]
- Sehgal, I.S.; Dhooria, S.; Aggarwal, A.N.; Behera, D.; Agarwal, R. Endosonography Versus Mediastinoscopy in Mediastinal Staging of Lung Cancer: Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2016, 102, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.L.; Loveland, P.M.; Gorelik, A.; Irving, L.; Steinfort, D.P. Preoperative Staging by EBUS in cN0/N1 Lung Cancer: Systematic Review and Meta-Analysis. J. Bronchol. Interv. Pulmonol. 2019, 26, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Steinfort, D.P.; Kothari, G.; Wallace, N.; Hardcastle, N.; Rangamuwa, K.; Dieleman, E.M.T.; Lee, P.; Li, P.; Simpson, J.A.; Yo, S.; et al. Systematic endoscopic staging of mediastinum to guide radiotherapy planning in patients with locally advanced non-small-cell lung cancer (SEISMIC): An international, multicentre, single-arm, clinical trial. Lancet Respir. Med. 2024, 12, 467–475. [Google Scholar] [CrossRef] [PubMed]
- De Leyn, P.; Dooms, C.; Kuzdzal, J.; Lardinois, D.; Passlick, B.; Rami-Porta, R.; Turna, A.; Van Schil, P.; Venuta, F.; Waller, D.; et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2014, 45, 787–798. [Google Scholar] [CrossRef]
- Huang, J.; Osarogiagbon, R.U.; Giroux, D.J.; Nishimura, K.K.; Bille, A.; Cardillo, G.; Detterbeck, F.; Kernstine, K.; Kim, H.K.; Lievens, Y.; et al. The International Association for the Study of Lung Cancer Staging Project for Lung Cancer: Proposals for the Revision of the N Descriptors in the Forthcoming Ninth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2024, 19, 766–785. [Google Scholar] [CrossRef]
- Son, J.W.; Lee, J.; Jeon, J.H.; Cho, S.; Jung, W.; Shih, B.C.; Kim, K.; Jheon, S. Validation of IASLC 9th edition TNM classification for lung cancer: Focus on N descriptor. BMC Cancer 2024, 24, 1460. [Google Scholar] [CrossRef]
- Yoo, C.; Yoon, S.; Lee, D.H.; Park, S.I.; Kim, D.K.; Kim, Y.H.; Kim, H.R.; Choi, S.H.; Kim, W.S.; Choi, C.M.; et al. Prognostic Significance of the Number of Metastatic pN2 Lymph Nodes in Stage IIIA-N2 Non-Small-Cell Lung Cancer After Curative Resection. Clin. Lung Cancer 2015, 16, e203–e212. [Google Scholar] [CrossRef]
- Saji, H.; Tsuboi, M.; Shimada, Y.; Kato, Y.; Yoshida, K.; Nomura, M.; Matsubayashi, J.; Nagao, T.; Kakihana, M.; Usuda, J.; et al. A proposal for combination of total number and anatomical location of involved lymph nodes for nodal classification in non-small cell lung cancer. Chest 2013, 143, 1618–1625. [Google Scholar] [CrossRef]
- Korevaar, D.A.; Crombag, L.M.; Cohen, J.F.; Spijker, R.; Bossuyt, P.M.; Annema, J.T. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: A systematic review and meta-analysis. Lancet Respir. Med. 2016, 4, 960–968. [Google Scholar] [CrossRef]
- Wu, T.; Cai, J.; Li, Y.; Xie, R.; Chen, K. Validation for revision of the stage IIIA(T1N2) in the forthcoming ninth edition of the TNM classification for lung cancer. BMC Cancer 2025, 25, 446. [Google Scholar] [CrossRef]
- Rami-Porta, R. The TNM classification of lung cancer-a historic perspective. J. Thorac. Dis. 2024, 16, 8053–8067. [Google Scholar] [CrossRef]
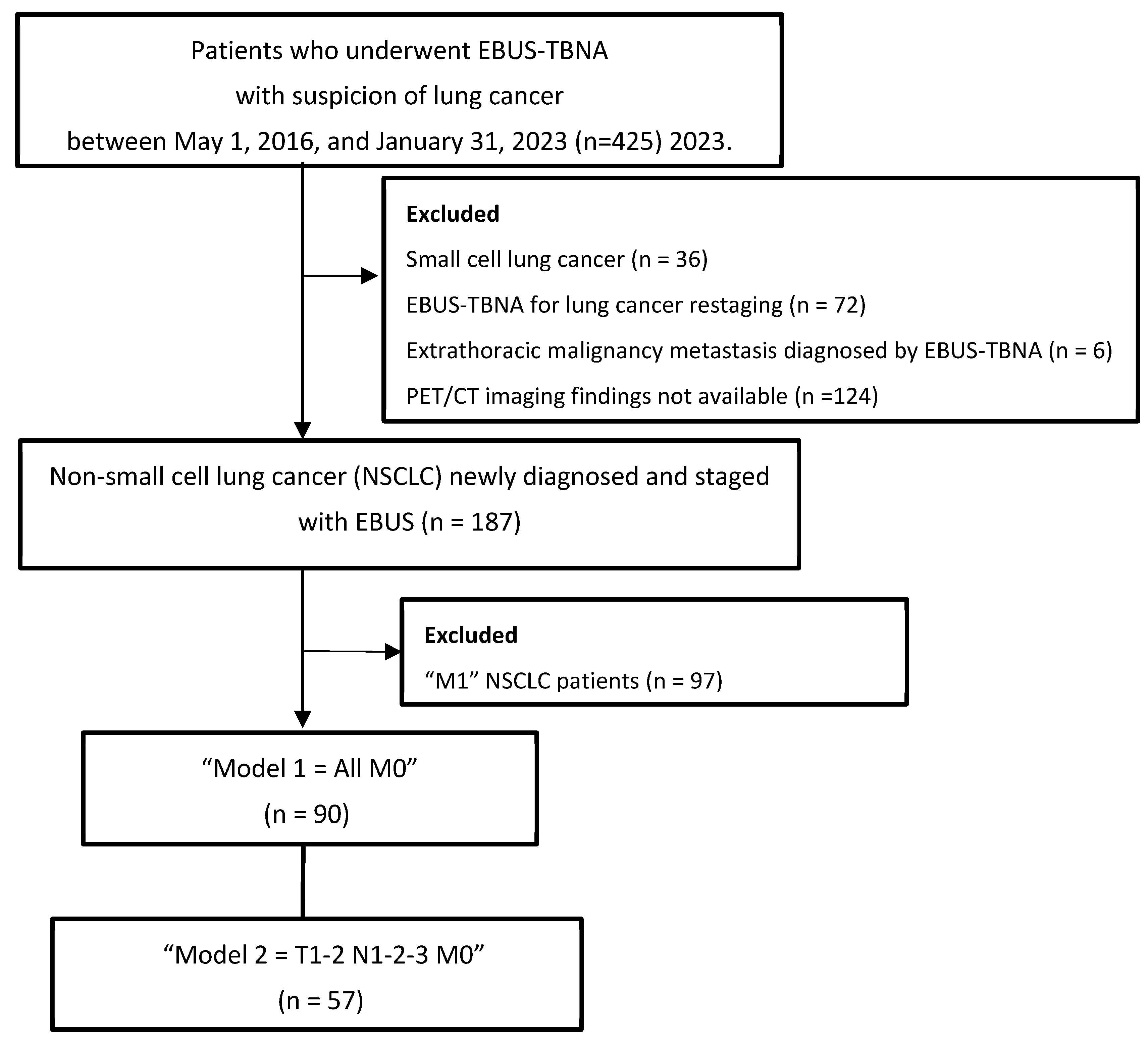
| All M0 NSCLC Patients (n = 90) | Model 1 = All M0 NSCLC Patients (n = 90) | Model 2 = T1–2 N1–2–3 M0 NSCLC Patients (n = 57) | |||||
|---|---|---|---|---|---|---|---|
| Deceased (n = 65) | Alive (n = 25) | p-Value | Deceased (n = 40) | Alive (n = 17) | p-Value | ||
| Age, years | 64.0 ± 9.6 | 65.2 ± 10.0 | 60.1 ± 7.6 | 0.024 | 64.8 ± 10.3 | 59.3 ± 7.9 | 0.06 |
| Gender, male, n (%) | 76 (84.4) | 56 (86.2) | 20 (80) | 0.522 | 32 (80) | 13 (76.5) | 0.737 |
| Smoking habits | |||||||
| Current smoker | 49 (54.4) | 36 (55.4) | 13 (52.0) | 0.949 | 20 (50) | 9 (52.9) | 0.912 |
| Ex-smoker | 28 (31.1) | 20 (30.8) | 8 (32.0) | 14 (35) | 5 (29.4) | ||
| Never smoked | 13 (14.4) | 9 (13.8) | 4 (16.0) | 6 (15) | 3 (17.6) | ||
| Smoking history, pack-year med (min–max) | 40 (8–144) | 40 (8–144) | 33 (10–70) | 0.041 | 40 (8–144) | 40 (15–70) | 0.689 |
| Presence of comorbidities, n (%) | 81 (90) | 61 (93.8) | 20 (80) | 0.109 | 27 (67.5) | 13 (76.5) | 0.752 |
| Comorbidity, n (%) | |||||||
| Hypertension | 30 (33.3) | 21 (32.3) | 9 (36.0) | 0.805 | 13 (32.5) | 7 (41.2) | 0.557 |
| COPD | 22 (24.4) | 18 (27.7) | 4 (16.0) | 0.288 | 10 (25) | 3 (17.6) | 0.734 |
| Diabetes mellitus | 17 (18.9) | 13 (20.0) | 4 (16.0) | 0.771 | 7 (17.5) | 4 (23.5) | 0.598 |
| Coronary artery disease | 15 (16.7) | 12 (18.5) | 3 (12) | 0.545 | 5 (12.5) | 2 (11.8) | 0.938 |
| Extrathoracic malignancy | 6 (6.7) | 5 (7.7) | 1 (4.0) | 0.529 | 4 (10) | 1 (5.9) | 0.615 |
| Cerebrovascular disease | 3 (3.3) | 3 (4.6) | 0 | 0.557 | 2 (5) | 0 | 0.348 |
| Asthma | 4 (4.4) | 4 (6.2) | 0 | 0.573 | 3 (7.5) | 0 | 0.542 |
| Interstitial lung disease | 2 (2.2) | 1 (1.5) | 1 (4) | 0.481 | 1 (2.5) | 1 (5.9) | 0.511 |
| Congestive heart disease | 1 (1.1) | 1 (1.5) | 0 | 0.533 | 1 (2.5) | 0 | 0.511 |
| Arrhythmia | 1 (1.1) | 1 (1.5) | 0 | 0.533 | 1 (2.5) | 0 | 0.511 |
| Chronic renal failure | 1 (1.1) | 1 (1.5) | 0 | 0.533 | 1 (2.5) | 0 | 0.511 |
| Autoimmune disease | 1 (1.1) | 1 (1.5) | 0 | 0.533 | 1 (2.5) | 0 | 0.511 |
| Tuberculosis history | 5 (5.6) | 4 (6.2) | 1 (4.0) | 0.689 | 3 (7.5) | 0 | 0.547 |
| Lung cancer pathological classification n (%) | |||||||
| Adenocarcinoma | 52 (57.8) | 36 (55.4) | 16 (64.0) | 27 (67.5) | 10 (58.8) | ||
| Squamous-cell carcinoma | 35 (38.9) | 28 (43.1) | 7 (28.0) | 0.170 | 12 (30) | 5 (29.4) | 0.353 |
| Undifferentiated non-small-cell carcinoma | 3 (3.3) | 1 (1.5) | 2 (8.0) | 1 (2.5) | 2 (11.8) | ||
| PET/CT tumor localization | |||||||
| Right upper lobe | 32 (35.6) | 26 (40.0) | 6 (24.0) | 0.295 | 18 (45) | 4 (23.5) | 0.112 |
| Right middle lobe | 14 (15.6) | 7 (10.8) | 7 (28.0) | 2 (5) | 4 (23.5) | ||
| Right lower lobe | 10 (11.1) | 7 (10.8) | 3 (12.0) | 5 (12.5) | 3 (17.6) | ||
| Left upper lobe | 29 (32.2) | 21 (32.3) | 8 (32.0) | 11 (27.5) | 6 (35.3) | ||
| Left lower lobe | 5 (5.6) | 4 (6.2) | 1 (4.0) | 4 (10) | 0 | ||
| Mass long axis (mm) med (min–max) | 30 (12–90) | 30 (12–90) | 30 (15–70) | 0.220 | 25 (13–48) | 23 (15–44) | 0.426 |
| Mass short axis (mm) med (min–max) | 25 (10–73) | 25 (10–73) | 25 (12–60) | 0.151 | 23 (10–42) | 20 (12–40) | 0.238 |
| Mass SUV med (min–max) | 10.0 (1.81–38.88) | 10 (1.81–38.88) | 10.9 (3.60–36.30) | 0.405 | 9.80 (3.40–38.88) | 7.68 (3.60–15.0) | 0.005 |
| PET staging, 8th TNM edition | |||||||
| 1A | 1 (1.1) | 1 (1.5) | 0 | 0.172 | 1 (2.5) | 0 | 0.684 |
| 1B | 4 (4.4) | 3 (4.6) | 1 (4.0) | 2 (5) | 1 (5.9) | ||
| 2B | 6 (6.7) | 5 (7.7) | 1 (4.0) | 4 (10) | 1 (5.9) | ||
| 3A | 34 (37.8) | 20 (30.8) | 14 (56.0) | 18 (45) | 11 (64.7) | ||
| 3B | 35 (38.9) | 26 (40.0) | 9 (36.0) | 15 (37.5) | 4 (23.5) | ||
| 3C | 10 (11.1) | 10 (15.4) | 0 | - | - | ||
| PET staging, 9th TNM edition | |||||||
| 1A | 1 (1.1) | 1 (1.5) | 0 | 0.110 | 1 (2.5) | 0 | 0.344 |
| 1B | 4 (4.4) | 3 (4.6) | 1 (4.0) | 2 (5) | 1 (5.9) | ||
| 2A | 4 (4.4) | 4 (6.2) | 0 | 4 (10) | 0 | ||
| 2B | 12 (13.3) | 6 (9.2) | 6 (24.0) | 5 (12.5) | 6 (35.3) | ||
| 3A | 23 (25.6) | 14 (21.5) | 9 (36.0) | 10 (25) | 4 (23.5) | ||
| 3B | 36 (40.0) | 27 (41.5) | 9 (36.0) | 18 (45) | 6 (35.3) | ||
| 3C | 10 (11.1) | 10 (15.4) | 0 | - | - | ||
| Number of malignant lymph nodes sampled with EBUS | |||||||
| 2R (n = 3) | 2 (66.7) | 1 (100) | 1 (50) | 0.386 | 1 (100) | 1 (50) | 0.386 |
| 4R (n = 66) | 32 (48.5) | 24 (50.0) | 8 (44.4) | 0.911 | 13 (44.8) | 6 (50) | 0.926 |
| 4L (n = 35) | 17 (48.6) | 15 (55.6) | 2 (25.0) | 0.316 | 10 (55.6) | 2 (33.3) | 0.315 |
| 7 (n = 82) | 29 (35.4) | 23 (39.7) | 6 (25.0) | 0.006 | 16 (44.4) | 4 (25) | 0.021 |
| 10R (n = 34) | 10 (29.4) | 9 (39.1) | 1 (9.1) | 0.146 | 7 (43.8) | 1 (14.3) | 0.373 |
| 11R (n = 49) | 15 (30.6) | 9 (27.3) | 6 (37.5) | 0.752 | 3 (13) | 4 (36.4) | 0.208 |
| 11L (n = 62) | 14 (22.6) | 12 (26.7) | 2 (11.8) | 0.228 | 7 (24.1) | 2 (15.4) | 0.570 |
| Eighth TNM edition “N” stage, with EBUS | |||||||
| N0 | 32 (35.6) | 20 (30.8) | 12 (48.0) | 0.398 | 13 (32.5) | 8 (47.1) | 0.686 |
| N1 | 4 (4.4) | 3 (4.6) | 1 (4.0) | 2 (5) | 1 (5.9) | ||
| N2 | 35 (38.9) | 26 (40.0) | 9 (36.0) | 16 (40) | 6 (35.3) | ||
| N3 | 19 (21.1) | 16 (24.6) | 3 (12.0) | 9 (22.5) | 2 (11.8) | ||
| Ninth TNM edition “N” stage, with EBUS | |||||||
| N0 | 32 (35.6) | 20 (30.8) | 12 (48.0) | 0.549 | 13 (32.5) | 8 (47.1) | 0.781 |
| N1 | 4 (4.4) | 3 (4.6) | 1 (4.0) | 2 (5) | 1 (5.9) | ||
| N2a | 21 (21.0) | 16 (24.6) | 5 (20.0) | 10 (25.0) | 3 (17.6) | ||
| N2b | 14 (15.6) | 10 (15.4) | 4 (16.0) | 6 (15.0) | 3 (17.6) | ||
| N3 | 19 (21.1) | 16 (24.6) | 3 (12.0) | 9 (22.5) | 2 (11.8) | ||
| Follow-up period, months | 26 (0–100) | 18 (0–74) | 42 (23–100) | 0.003 | 20 (1–74) | 36 (24–100) | <0.001 |
| Model 1 NSCLC, All M0 Patients | Model 2 NSCLC, T1–2 N1–2–3 M0 Patients | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| N staging with EBUS according to the 8th edition of the TNM | ||||||
| (Ref. N0) | ||||||
| N1 | 1.94 | 0.42–9.01 | 0.395 | 4.65 | 0.85–25.50 | 0.076 |
| N2 | 2.26 | 1.01–5.05 | 0.045 | 2.59 | 0.79–8.41 | 0.113 |
| N3 | 3.31 | 1.43–7.67 | 0.005 | 6.37 | 1.94–20.83 | 0.002 |
| N staging with EBUS according to the 9th edition of the TNM | ||||||
| (Ref. N0) | ||||||
| N1 | 1.94 | 0.42–9.01 | 0.394 | 4.65 | 0.85–25.50 | 0.076 |
| N2a | 1.97 | 0.80–4.86 | 0.139 | 2.51 | 0.67–9.38 | 0.169 |
| N2b | 2.78 | 1.07–7.22 | 0.035 | 2.67 | 0.67–10.74 | 0.163 |
| N3 | 3.31 | 1.43–7.67 | 0.005 | 6.37 | 1.94–20.83 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirdöğen, E.; Terzi, O.E.; Aydın Güçlü, Ö.; Ursavaş, A.; Karadağ, M. Differences Between the 8th and 9th Editions of the TNM Staging System in Predicting Mortality in Non-Small Cell Lung Cancer Patients Staged with EBUS. Diagnostics 2025, 15, 1570. https://doi.org/10.3390/diagnostics15131570
Demirdöğen E, Terzi OE, Aydın Güçlü Ö, Ursavaş A, Karadağ M. Differences Between the 8th and 9th Editions of the TNM Staging System in Predicting Mortality in Non-Small Cell Lung Cancer Patients Staged with EBUS. Diagnostics. 2025; 15(13):1570. https://doi.org/10.3390/diagnostics15131570
Chicago/Turabian StyleDemirdöğen, Ezgi, Orkun Eray Terzi, Özge Aydın Güçlü, Ahmet Ursavaş, and Mehmet Karadağ. 2025. "Differences Between the 8th and 9th Editions of the TNM Staging System in Predicting Mortality in Non-Small Cell Lung Cancer Patients Staged with EBUS" Diagnostics 15, no. 13: 1570. https://doi.org/10.3390/diagnostics15131570
APA StyleDemirdöğen, E., Terzi, O. E., Aydın Güçlü, Ö., Ursavaş, A., & Karadağ, M. (2025). Differences Between the 8th and 9th Editions of the TNM Staging System in Predicting Mortality in Non-Small Cell Lung Cancer Patients Staged with EBUS. Diagnostics, 15(13), 1570. https://doi.org/10.3390/diagnostics15131570






