Precision Medicine in Lung Cancer Screening: A Paradigm Shift in Early Detection—Precision Screening for Lung Cancer
Abstract
1. Introduction
2. Understanding Precision Medicine
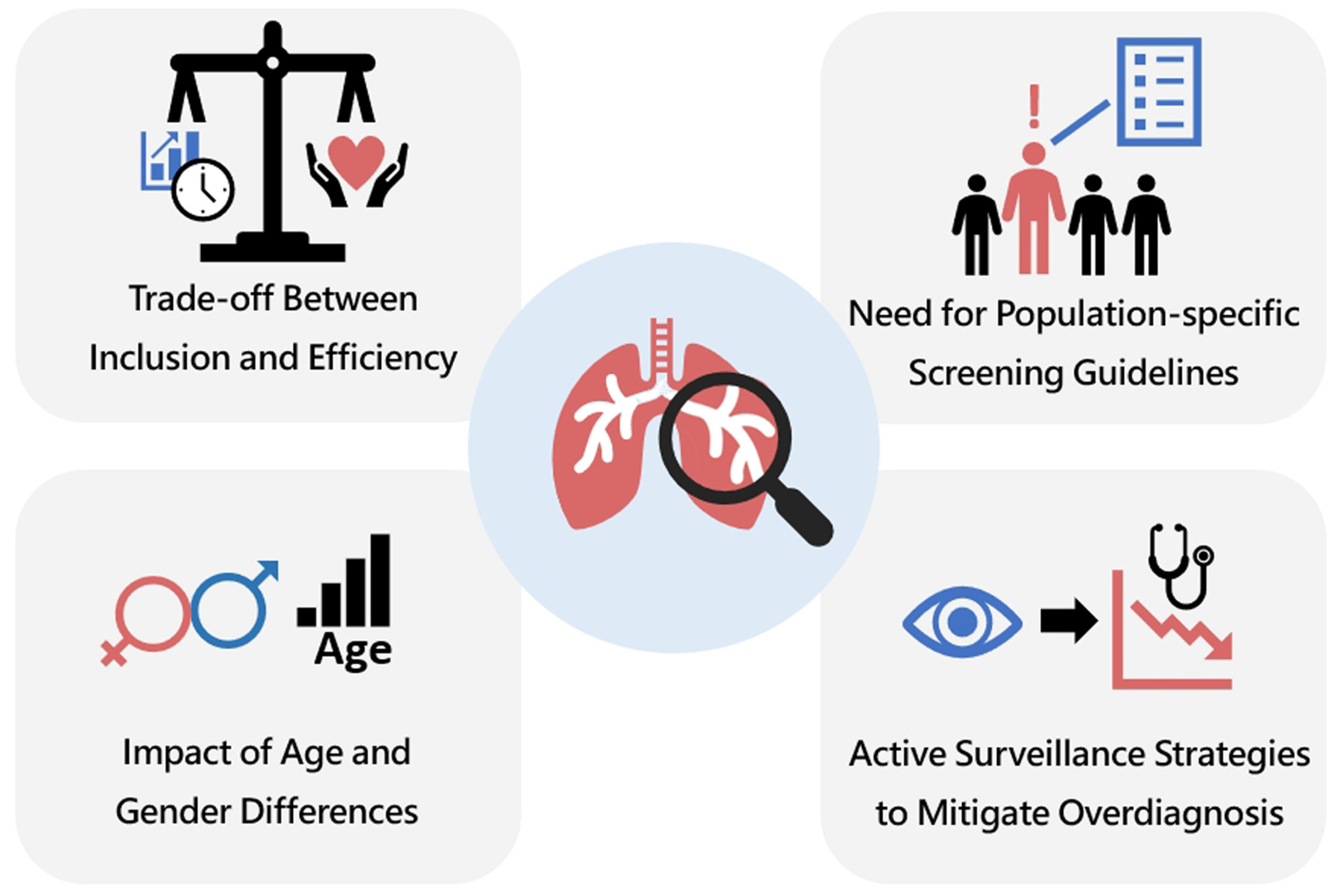
3. Traditional Lung Cancer Screening: Challenges Posed by Inconsistent Screening Guidelines
- (1)
- Trade-off Between Inclusion and Efficiency.
- (2)
- Impact of Age and Gender Differences.
- (3)
- Need for Population-Specific Screening Guidelines.
- (4)
- Active Surveillance Strategies to Mitigate Overdiagnosis.
4. Key Elements for Effective Precision Lung Cancer Screening
- (1)
- Risk Prediction Models.
- (2)
- Radiomics and AI.
- (3)
- Molecular and Biomarker-Based Screening.
- (4)
- Genomic Profiling and PRSs.
- (5)
- Government Policy and Human Behavioral and Environmental Data Integration
5. Implementing Precision Screening in Practice
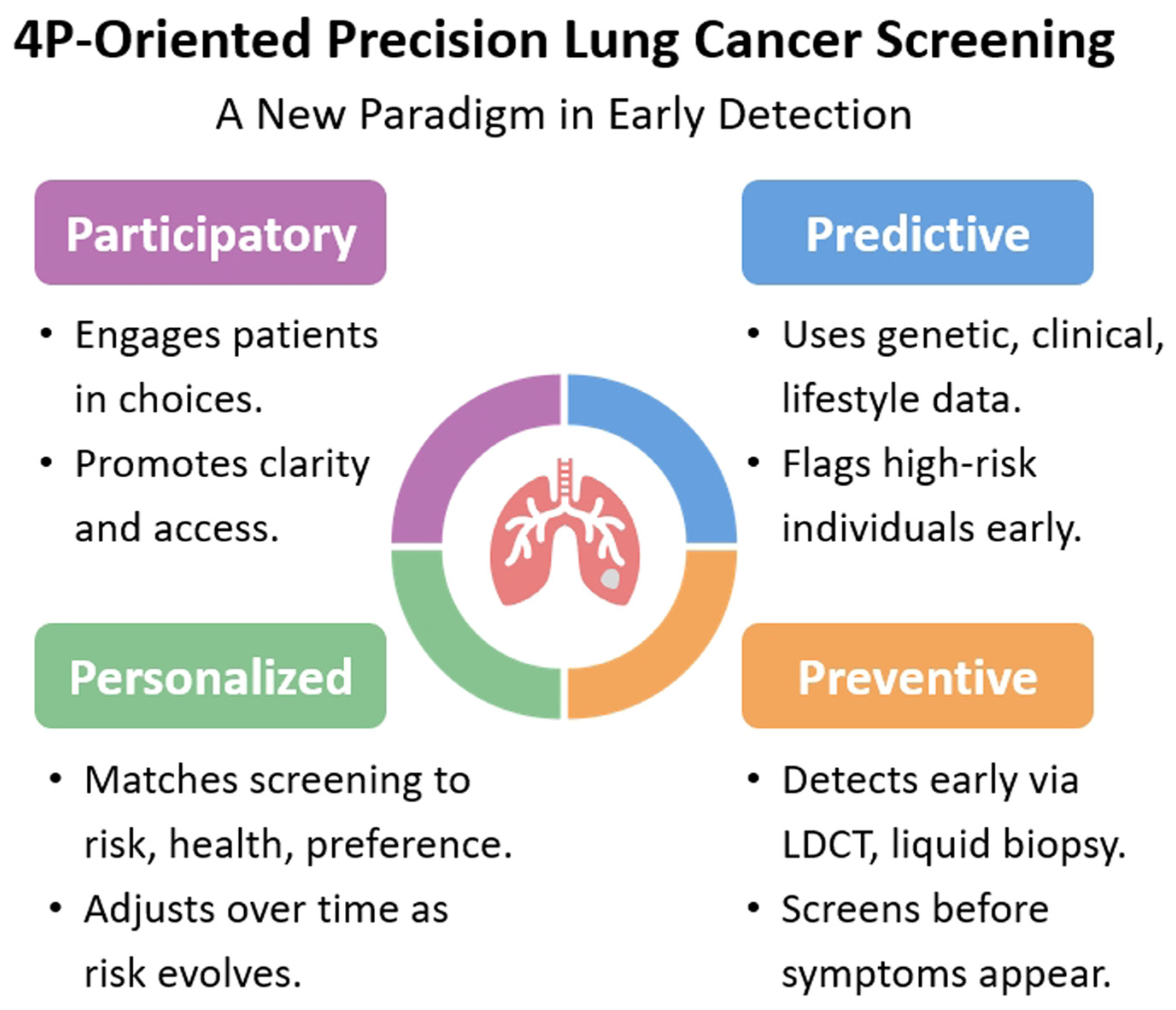
6. A New Paradigm in Early Detection: 4P-Oriented Precision Lung Cancer Screening
- (1)
- Data Infrastructure: EHRs, biobanks, and imaging repositories must be integrated and interoperable to support multifactorial risk modeling [109].
- (2)
- CDSTs: AI-driven dashboards can aid clinicians in identifying high-risk patients, recommending screening intervals, and managing incidental findings [86].
- (3)
- Patient Engagement: While lung cancer screening uptake rates remain relatively low in the United States, they tend to be higher in several Asian populations. This disparity may be attributed to differences in cultural norms, health literacy levels, and cancer-related perceptions, highlighting the need for culturally tailored patient engagement strategies [102,110,111,112,113]. SDM is crucial in precision screening. Patients should be educated about their personalized risk, potential benefits, and harms of screening to make informed choices.
- (4)
- Equity Considerations: Screening programs must ensure that precision tools do not inadvertently widen disparities. For example, algorithms should be trained on diverse populations to avoid bias and ensure equitable access [98].
- (5)
- Urgent Need in the Health Workforce: There is an urgent need to strengthen the health workforce for lung cancer screening. A multidisciplinary team—including radiologists, clinical physicians, and thoracic surgeons—is essential to ensure accurate diagnosis, timely treatment, and appropriate follow-up, especially as screening programs expand and early-stage lung cancers are increasingly detected in asymptomatic individuals [100]. In addition, nursing educators involved in fast-track screening pathways should also possess adequate health literacy on screening. An integrated, streamlined approach is essential to optimize both the overall screening process and health literacy education.
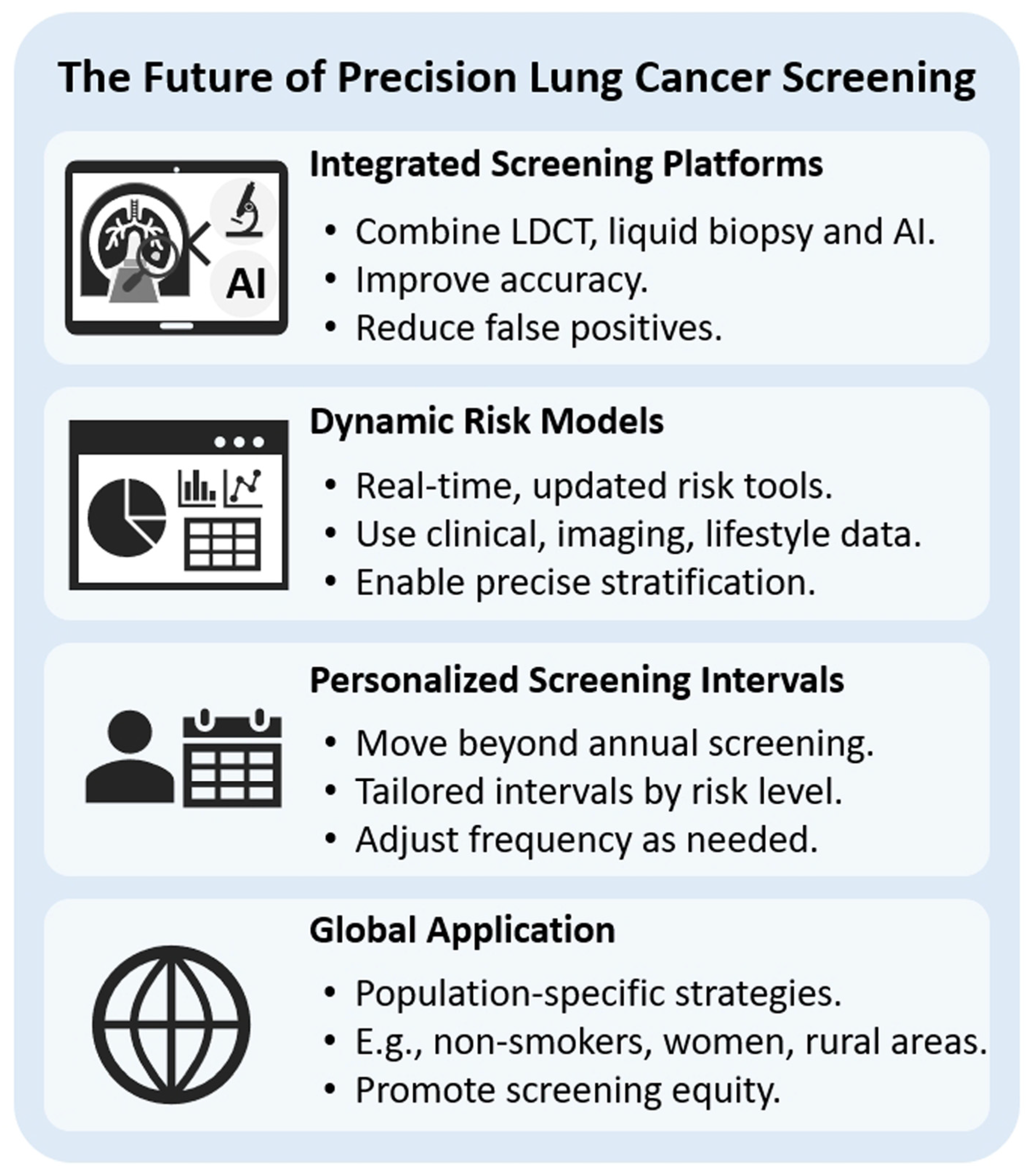
7. The Future of Precision Lung Cancer Screening
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LDCT | low-dose computed tomography |
| SSNs | subsolid nodules |
| USPSTF | United States Preventive Services Task Force |
| ER | efficiency ratio |
| LCS | lung cancer screening |
| CGSL | China guideline for the screening and early detection of lung cancer |
| NCCN | National Comprehensive Cancer Network |
| I-ELCAP | International Early Lung Cancer Action Program |
| AI | artificial intelligence |
| PRSs | polygenic risk scores |
| SNPs | single-nucleotide polymorphisms |
| MCED | multi-cancer early detection |
| SDM | shared decision-making |
| GGNs | ground-glass nodules |
| IPA | invasive pulmonary adenocarcinoma |
| S-RRL | serial longitudinal radiomics-based RRL model |
| CEA | carcinoembryonic antigen |
| ctDNA | circulating tumor DNA |
| MRD | minimal residual disease |
| EHRs | electronic health records |
| CDSTs | clinical decision support tools |
References
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Ledda, R.E.; Funk, G.C.; Sverzellati, N. The pros and cons of lung cancer screening. Eur. Radiol. 2025, 35, 267–275. [Google Scholar] [CrossRef]
- Bonney, A.; Malouf, R.; Marchal, C.; Manners, D.; Fong, K.M.; Marshall, H.M.; Irving, L.B.; Manser, R. Impact of low-dose computed tomography (LDCT) screening on lung cancer-related mortality. Cochrane Database Syst. Rev. 2022, 8, Cd013829. [Google Scholar] [CrossRef]
- Sadate, A.; Occean, B.V.; Beregi, J.P.; Hamard, A.; Addala, T.; de Forges, H.; Fabbro-Peray, P.; Frandon, J. Systematic review and meta-analysis on the impact of lung cancer screening by low-dose computed tomography. Eur. J. Cancer 2020, 134, 107–114. [Google Scholar] [CrossRef]
- Tseng, C.H.; Tsuang, B.J.; Chiang, C.J.; Ku, K.C.; Tseng, J.S.; Yang, T.Y.; Hsu, K.H.; Chen, K.C.; Yu, S.L.; Lee, W.C.; et al. The Relationship Between Air Pollution and Lung Cancer in Nonsmokers in Taiwan. J. Thorac. Oncol. 2019, 14, 784–792. [Google Scholar] [CrossRef]
- Ha, S.Y.; Choi, S.J.; Cho, J.H.; Choi, H.J.; Lee, J.; Jung, K.; Irwin, D.; Liu, X.; Lira, M.E.; Mao, M.; et al. Lung cancer in never-smoker Asian females is driven by oncogenic mutations, most often involving EGFR. Oncotarget 2015, 6, 5465–5474. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, C. Lung cancer in never smokers-the East Asian experience. Transl. Lung Cancer Res. 2018, 7, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Hung, Y.C.; Wu, Y.J.; Tang, E.K.; Wu, F.Z. Understanding East-West differences in subsolid nodules: Prevalence and overdiagnosis implications in lung cancer screening. Ann. Med. 2025, 57, 2478321. [Google Scholar] [CrossRef]
- Guan, X.; Qin, T.; Qi, T. Precision Medicine in Lung Cancer Theranostics: Paving the Way from Traditional Technology to Advance Era. Cancer Control. 2022, 29, 10732748221077351. [Google Scholar] [CrossRef]
- Denny, J.C.; Collins, F.S. Precision medicine in 2030-seven ways to transform healthcare. Cell 2021, 184, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Juang, Y.R.; Ang, L.; Seow, W.J. Predictive performance of risk prediction models for lung cancer incidence in Western and Asian countries: A systematic review and meta-analysis. Sci. Rep. 2025, 15, 4259. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.Z.; Chang, Y.C. Toward More Effective Lung Cancer Risk Stratification to Empower Screening Programs for the Asian Nonsmoking Population. J. Am. Coll. Radiol. 2023, 20, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Coupland, C.A.C.; Burchardt, J.; Baldwin, D.R.; Gleeson, F.V.; Hippisley-Cox, J. Predicting the future risk of lung cancer: Development, and internal and external validation of the CanPredict (lung) model in 19·67 million people and evaluation of model performance against seven other risk prediction models. Lancet Respir. Med. 2023, 11, 685–697. [Google Scholar] [CrossRef]
- Davis, M.B.; Martini, R. Precision oncology and genetic ancestry: The science behind population-based cancer disparities. Cancer Cell 2025, 43, 619–622. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wu, F.Z.; Yang, S.C.; Tang, E.K.; Liang, C.H. Radiomics in Early Lung Cancer Diagnosis: From Diagnosis to Clinical Decision Support and Education. Diagnostics 2022, 12, 1064. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Jett, J.R. The Intersection of Lung Cancer Screening, Radiomics, and Artificial Intelligence: Can One Scan Really Predict the Future Development of Lung Cancer? J. Clin. Oncol. 2023, 41, 2141–2143. [Google Scholar] [CrossRef]
- De Luca, G.R.; Diciotti, S.; Mascalchi, M. The Pivotal Role of Baseline LDCT for Lung Cancer Screening in the Era of Artificial Intelligence. Arch. Bronconeumol. 2024, 61, 359–367. [Google Scholar] [CrossRef]
- Lin, W.C.; Shie, R.H.; Yuan, T.H.; Tseng, C.H.; Chiang, C.J.; Lee, W.C.; Chan, C.C. A nationwide case-referent study on elevated risks of adenocarcinoma lung cancer by long-term PM(2.5) exposure in Taiwan since 1997. Environ. Res. 2024, 252 Pt 2, 118889. [Google Scholar] [CrossRef]
- Olsson, A.; Bouaoun, L.; Schüz, J.; Vermeulen, R.; Behrens, T.; Ge, C.; Kromhout, H.; Siemiatycki, J.; Gustavsson, P.; Boffetta, P.; et al. Lung Cancer Risks Associated with Occupational Exposure to Pairs of Five Lung Carcinogens: Results from a Pooled Analysis of Case-Control Studies (SYNERGY). Environ. Health Perspect. 2024, 132, 17005. [Google Scholar] [CrossRef]
- Ostrin, E.J.; Sidransky, D.; Spira, A.; Hanash, S.M. Biomarkers for Lung Cancer Screening and Detection. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2411–2415. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, R.; Yu, R.; Zhu, Q.; Li, C.; He, W.; Liu, J. Detection of VOCs in exhaled breath for lung cancer diagnosis. Microchem. J. 2024, 199, 110051. [Google Scholar] [CrossRef]
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P.; et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J. Thorac. Oncol. 2019, 14, 343–357. [Google Scholar] [CrossRef]
- Naithani, N.; Atal, A.T.; Tilak, T.; Vasudevan, B.; Misra, P.; Sinha, S. Precision medicine: Uses and challenges. Med. J. Armed Forces India 2021, 77, 258–265. [Google Scholar] [CrossRef]
- Boeri, M.; Zanghì, A.; Pastorino, U. New Horizons in Lung Cancer Screening: Eligibility Criteria, Risk Models, and Emerging Challenges. J. Thorac. Oncol. 2025, 20, 422–424. [Google Scholar] [CrossRef]
- Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Kubik, M.; et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 962–970. [Google Scholar] [CrossRef]
- Kerpel-Fronius, A.; Tammemägi, M.; Cavic, M.; Henschke, C.; Jiang, L.; Kazerooni, E.; Lee, C.T.; Ventura, L.; Yang, D.; Lam, S.; et al. Screening for Lung Cancer in Individuals Who Never Smoked: An International Association for the Study of Lung Cancer Early Detection and Screening Committee Report. J. Thorac. Oncol. 2022, 17, 56–66. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, L.; Wang, F.; Huang, Y.; Wang, J.; Zhao, S.; Qi, L.; Liu, L.; Liang, M.; Hou, D.; et al. Assessing the efficiency of eligibility criteria for low-dose computed tomography lung screening in China according to current guidelines. BMC Med. 2024, 22, 267. [Google Scholar] [CrossRef]
- Bergmann, L.L.; Hobbs, S.B. Beyond the AJR: The Impact of Screening Population in Lung Cancer Overdiagnosis. AJR Am. J. Roentgenol. 2023, 220, 148. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wen, C.P.; Wu, A.; Welch, H.G. Association of Computed Tomographic Screening Promotion with Lung Cancer Overdiagnosis Among Asian Women. JAMA Intern. Med. 2022, 182, 283–290. [Google Scholar] [CrossRef]
- Chen, H.-H.; Tang, E.-K.; Wu, Y.-J.; Wu, F.-Z. Impact of annual trend volume of low-dose computed tomography for lung cancer screening on overdiagnosis, overmanagement, and gender disparities. Cancer Imaging 2024, 24, 73. [Google Scholar] [CrossRef]
- Wood, D.E.; Kazerooni, E.A.; Baum, S.L.; Eapen, G.A.; Ettinger, D.S.; Hou, L.; Jackman, D.M.; Klippenstein, D.; Kumar, R.; Lackner, R.P.; et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 412–441. [Google Scholar] [CrossRef] [PubMed]
- Issanov, A.; Aravindakshan, A.; Puil, L.; Tammemägi, M.C.; Lam, S.; Dummer, T.J.B. Risk prediction models for lung cancer in people who have never smoked: A protocol of a systematic review. Diagn. Progn. Res. 2024, 8, 3. [Google Scholar] [CrossRef]
- Goo, J.M.; Jung, K.W.; Kim, H.Y.; Kim, Y. Potential Overdiagnosis with CT Lung Cancer Screening in Taiwanese Female: Status in South Korea. Korean J. Radiol. 2022, 23, 571–573. [Google Scholar] [CrossRef]
- Kim, S.Y.; Silvestri, G.A.; Kim, Y.W.; Kim, R.Y.; Um, S.W.; Im, Y.; Hwang, J.H.; Choi, S.H.; Eom, J.S.; Gu, K.M.; et al. Screening for Lung Cancer, Overdiagnosis, and Healthcare Utilization: A Nationwide Population-Based Study. J. Thorac. Oncol. 2025, 20, 577–588. [Google Scholar] [CrossRef]
- Liu, Y.C.; Liang, C.H.; Wu, Y.J.; Chen, C.S.; Tang, E.K.; Wu, F.Z. Managing Persistent Subsolid Nodules in Lung Cancer: Education, Decision Making, and Impact of Interval Growth Patterns. Diagnostics 2023, 13, 2674. [Google Scholar] [CrossRef] [PubMed]
- LoPiccolo, J.; Gusev, A.; Christiani, D.C.; Jänne, P.A. Lung cancer in patients who have never smoked—An emerging disease. Nat. Rev. Clin. Oncol. 2024, 21, 121–146. [Google Scholar] [CrossRef]
- Tang, E.K.; Wu, Y.J.; Chen, C.S.; Wu, F.Z. Prediction of the stage shift growth of early-stage lung adenocarcinomas by volume-doubling time. Quant. Imaging Med. Surg. 2024, 14, 3983–3996. [Google Scholar] [CrossRef]
- Payne, R.G.; Anker, C.J.; Sprague, B.L.; No, H.J.; Lin, S.H.; Lester-Coll, N.H. Active Surveillance for Early Stage Lung Cancer. Clin. Lung Cancer 2022, 23, 226–235. [Google Scholar] [CrossRef]
- Huang, Y.; Bao, T.; Zhang, T.; Ji, G.; Wang, Y.; Ling, Z.; Li, W. Machine Learning Study of SNPs in Noncoding Regions to Predict Non-Small Cell Lung Cancer Susceptibility. Clin. Oncol. R. Coll. Radiol. 2023, 35, 701–712. [Google Scholar] [CrossRef]
- Weissfeld, J.L.; Lin, Y.; Lin, H.M.; Kurland, B.F.; Wilson, D.O.; Fuhrman, C.R.; Pennathur, A.; Romkes, M.; Nukui, T.; Yuan, J.M.; et al. Lung Cancer Risk Prediction Using Common SNPs Located in GWAS-Identified Susceptibility Regions. J. Thorac. Oncol. 2015, 10, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Lv, J.; Zhu, M.; Wang, Y.; Qin, N.; Ma, H.; He, Y.Q.; Zhang, R.; Tan, W.; Fan, J.; et al. Identification of risk loci and a polygenic risk score for lung cancer: A large-scale prospective cohort study in Chinese populations. Lancet Respir. Med. 2019, 7, 881–891. [Google Scholar] [CrossRef]
- Wei, X.; Sun, D.; Gao, J.; Zhang, J.; Zhu, M.; Yu, C.; Ma, Z.; Fu, Y.; Ji, C.; Pei, P.; et al. Development and evaluation of a polygenic risk score for lung cancer in never-smoking women: A large-scale prospective Chinese cohort study. Int. J. Cancer 2024, 154, 807–815. [Google Scholar] [CrossRef]
- Haas, K.; Brillante, C.; Sharp, L.; Elzokaky, A.K.; Pasquinelli, M.; Feldman, L.; Kovitz, K.L.; Joo, M. Lung cancer screening: Assessment of health literacy and readability of online educational resources. BMC Public Health 2018, 18, 1356. [Google Scholar] [CrossRef]
- Zhao, M.; Xue, G.; He, B.; Deng, J.; Wang, T.; Zhong, Y.; Li, S.; Wang, Y.; He, Y.; Chen, T.; et al. Integrated multiomics signatures to optimize the accurate diagnosis of lung cancer. Nat. Commun. 2025, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Ye, Y.; Cheng, W.; Xu, L.; Huang, M.; Chen, Y.; Zhu, J.; Lu, X.; Yan, F. Multi-Omics Integrative Analysis of Lung Adenocarcinoma: An in silico Profiling for Precise Medicine. Front. Med. 2022, 9, 894338. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, R.; Gan, J.; Zheng, Q.; Wang, G.; Tao, W.; Yang, M.; Li, W.; Ji, G.; Li, W. Application of artificial intelligence in lung cancer screening: A real-world study in a Chinese physical examination population. Thorac. Cancer 2024, 15, 2061–2072. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wu, F.Z. AI-Enhanced CAD in Low-Dose CT: Balancing Accuracy, Efficiency, and Overdiagnosis in Lung Cancer Screening. Thorac. Cancer 2025, 16, e15499. [Google Scholar] [CrossRef]
- Lee, M.; Hwang, E.J.; Lee, J.H.; Nam, J.G.; Lim, W.H.; Park, H.; Park, C.M.; Choi, H.; Park, J.; Goo, J.M. Artificial Intelligence for Low-Dose CT Lung Cancer Screening: Comparison of Utilization Scenarios. AJR Am. J. Roentgenol. 2025. [Google Scholar] [CrossRef]
- Hüsing, A.; Kaaks, R. Risk prediction models versus simplified selection criteria to determine eligibility for lung cancer screening: An analysis of German federal-wide survey and incidence data. Eur. J. Epidemiol. 2020, 35, 899–912. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Schöttker, B.; Holleczek, B.; Brenner, H. Comparison of discrimination performance of 11 lung cancer risk models for predicting lung cancer in a prospective cohort of screening-age adults from Germany followed over 17 years. Lung Cancer 2022, 174, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bhamani, A.; Creamer, A.; Verghese, P.; Prendecki, R.; Horst, C.; Tisi, S.; Hall, H.; Khaw, C.R.; Mullin, M.; McCabe, J.; et al. Low-dose CT for lung cancer screening in a high-risk population (SUMMIT): A prospective, longitudinal cohort study. Lancet Oncol. 2025, 26, 609–619. [Google Scholar] [CrossRef]
- Feng, X.; Goodley, P.; Alcala, K.; Guida, F.; Kaaks, R.; Vermeulen, R.; Downward, G.S.; Bonet, C.; Colorado-Yohar, S.M.; Albanes, D.; et al. Evaluation of risk prediction models to select lung cancer screening participants in Europe: A prospective cohort consortium analysis. Lancet Digit. Health 2024, 6, e614–e624. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.C.; Liam, C.K.; Andarini, S.; Park, S.; Tan, D.S.W.; Singh, N.; Jang, S.H.; Vardhanabhuti, V.; Ramos, A.B.; Nakayama, T.; et al. Lung Cancer Screening in Asia: An Expert Consensus Report. J. Thorac. Oncol. 2023, 18, 1303–1322. [Google Scholar] [CrossRef]
- Triphuridet, N.; Henschke, C. Landscape on CT screening for lung cancer in Asia. Lung Cancer 2019, 10, 107–124. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; He, X.; Zhou, T.; Liu, Y.; Zhang, X.; Guo, Y.; Guo, J.; Bian, J. Optimizing Strategy for Lung Cancer Screening: From Risk Prediction to Clinical Decision Support. JCO Clin. Cancer Inform. 2025, 9, e2400291. [Google Scholar] [CrossRef]
- Alonso, S.G.; de la Torre Díez, I.; Zapiraín, B.G. Predictive, Personalized, Preventive and Participatory (4P) Medicine Applied to Telemedicine and eHealth in the Literature. J. Med. Syst. 2019, 43, 140. [Google Scholar] [CrossRef]
- El Ayachy, R.; Giraud, N.; Giraud, P.; Durdux, C.; Giraud, P.; Burgun, A.; Bibault, J.E. The Role of Radiomics in Lung Cancer: From Screening to Treatment and Follow-Up. Front. Oncol. 2021, 11, 603595. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.Z.; Wu, Y.J.; Chen, C.S.; Tang, E.K. Prediction of Interval Growth of Lung Adenocarcinomas Manifesting as Persistent Subsolid Nodules ≤3 cm Based on Radiomic Features. Acad. Radiol. 2023, 30, 2856–2869. [Google Scholar] [CrossRef]
- Lee, G.; Park, H.; Bak, S.H.; Lee, H.Y. Radiomics in Lung Cancer from Basic to Advanced: Current Status and Future Directions. Korean J. Radiol. 2020, 21, 159–171. [Google Scholar] [CrossRef]
- Choi, W.; Oh, J.H.; Riyahi, S.; Liu, C.J.; Jiang, F.; Chen, W.; White, C.; Rimner, A.; Mechalakos, J.G.; Deasy, J.O.; et al. Radiomics analysis of pulmonary nodules in low-dose CT for early detection of lung cancer. Med. Phys. 2018, 45, 1537–1549. [Google Scholar] [CrossRef]
- Chen, C.H.; Chang, C.K.; Tu, C.Y.; Liao, W.C.; Wu, B.R.; Chou, K.T.; Chiou, Y.R.; Yang, S.N.; Zhang, G.; Huang, T.C. Radiomic features analysis in computed tomography images of lung nodule classification. PLoS ONE 2018, 13, e0192002. [Google Scholar] [CrossRef]
- Jiang, Y.; Che, S.; Ma, S.; Liu, X.; Guo, Y.; Liu, A.; Li, G.; Li, Z. Radiomic signature based on CT imaging to distinguish invasive adenocarcinoma from minimally invasive adenocarcinoma in pure ground-glass nodules with pleural contact. Cancer Imaging 2021, 21, 1. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Zhang, Y.; Li, X.; Li, Q.; Ye, Z. Radiomic analysis of pulmonary ground-glass opacity nodules for distinction of preinvasive lesions, invasive pulmonary adenocarcinoma and minimally invasive adenocarcinoma based on quantitative texture analysis of CT. Chin. J. Cancer Res. 2018, 30, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhu, W.; Shen, Y.; Wang, J.; Xu, R.; Qutesh, C.; Song, L.; Gan, Y.; Pu, C.; Hu, H. Radiomic-Based Quantitative CT Analysis of Pure Ground-Glass Nodules to Predict the Invasiveness of Lung Adenocarcinoma. Front. Oncol. 2020, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Yang, Y.; Huang, Q.; Cui, F.; Lian, Y.; Zhang, S.; Yao, L.; Peng, W.; Li, X.; Pang, P.; et al. Use of a Radiomics Model to Predict Tumor Invasiveness of Pulmonary Adenocarcinomas Appearing as Pulmonary Ground-Glass Nodules. BioMed Res. Int. 2018, 2018, 6803971. [Google Scholar] [CrossRef]
- Yagi, T.; Yamazaki, M.; Ohashi, R.; Ogawa, R.; Ishikawa, H.; Yoshimura, N.; Tsuchida, M.; Ajioka, Y.; Aoyama, H. HRCT texture analysis for pure or part-solid ground-glass nodules: Distinguishability of adenocarcinoma in situ or minimally invasive adenocarcinoma from invasive adenocarcinoma. Jpn. J. Radiol. 2018, 36, 113–121. [Google Scholar] [CrossRef]
- Yang, B.; Guo, L.; Lu, G.; Shan, W.; Duan, L.; Duan, S. Radiomic signature: A non-invasive biomarker for discriminating invasive and non-invasive cases of lung adenocarcinoma. Cancer Manag. Res. 2019, 11, 7825–7834. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Y.; Yang, Z.; Sun, Y.; Li, C.; Jin, L.; Gao, P.; He, W.; Wang, P.; Shi, H.; et al. Development and validation of a radiomics nomogram for identifying invasiveness of pulmonary adenocarcinomas appearing as subcentimeter ground-glass opacity nodules. Eur. J. Radiol. 2019, 112, 161–168. [Google Scholar] [CrossRef]
- Wu, Y.J.; Liu, Y.C.; Liao, C.Y.; Tang, E.K.; Wu, F.Z. A comparative study to evaluate CT-based semantic and radiomic features in preoperative diagnosis of invasive pulmonary adenocarcinomas manifesting as subsolid nodules. Sci. Rep. 2021, 11, 66. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Ying, L.; Lee, E.; Chan, H.P.; Chughtai, A.; Hadjiiski, L.M.; Kazerooni, E.A. Leveraging Serial Low-Dose CT Scans in Radiomics-Based Reinforcement Learning to Improve Early Diagnosis of Lung Cancer at Baseline Screening. Radiol. Cardiothorac. Imaging 2024, 6, e230196. [Google Scholar] [CrossRef] [PubMed]
- Demircioğlu, A. Reproducibility and interpretability in radiomics: A critical assessment. Diagn. Interv. Radiol. 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marmor, H.N.; Zorn, J.T.; Deppen, S.A.; Massion, P.P.; Grogan, E.L. Biomarkers in Lung Cancer Screening: A Narrative Review. Curr. Chall. Thorac. Surg. 2023, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Bestvina, C.M.; Garassino, M.C.; Neal, J.W.; Wakelee, H.A.; Diehn, M.; Vokes, E.E. Early-Stage Lung Cancer: Using Circulating Tumor DNA to Get Personal. J. Clin. Oncol. 2023, 41, 4093–4096. [Google Scholar] [CrossRef]
- Tang, L.; Li, R.; Wen, H.; Zhou, Q.; Xu, C. Circulating tumor DNA (ctDNA)-based minimal residual disease in non-small cell lung cancer. Chin. Med J. Pulm. Crit. Care Med. 2023, 1, 207–214. [Google Scholar] [CrossRef]
- Revelo, A.E.; Martin, A.; Velasquez, R.; Kulandaisamy, P.C.; Bustamante, J.; Keshishyan, S.; Otterson, G. Liquid biopsy for lung cancers: An update on recent developments. Ann. Transl. Med. 2019, 7, 349. [Google Scholar] [CrossRef]
- Chan, H.T.; Chin, Y.M.; Nakamura, Y.; Low, S.K. Clonal Hematopoiesis in Liquid Biopsy: From Biological Noise to Valuable Clinical Implications. Cancers 2020, 12, 2277. [Google Scholar] [CrossRef]
- Di Capua, D.; Bracken-Clarke, D.; Ronan, K.; Baird, A.M.; Finn, S. The Liquid Biopsy for Lung Cancer: State of the Art, Limitations and Future Developments. Cancers 2021, 13, 3923. [Google Scholar] [CrossRef]
- Lai, G.G.Y.; Tan, D.S.W. Lung cancer screening in never smokers. Curr. Opin. Oncol. 2025, 37, 95–104. [Google Scholar] [CrossRef]
- Rolfo, C.; Russo, A.; Malapelle, U. The next frontier of early lung cancer and minimal residual disease detection: Is multiomics the solution? EBioMedicine 2023, 92, 104605. [Google Scholar] [CrossRef]
- Nguyen, V.T.C.; Vo, D.H.; Tran, T.T.; Tran, T.T.; Nguyen, T.H.H.; Vo, T.D.H.; Van, T.T.V.; Vu, T.L.; Lam, M.Q.; Nguyen, G.T.H.; et al. Cost-effective shallow genome-wide sequencing for profiling plasma cfDNA signatures to enhance lung cancer detection. Future Oncol. 2025, 21, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Chen, I.C.; Chen, Y.M.; Hsiao, T.H.; Tseng, J.S.; Huang, Y.H.; Hsu, K.H.; Lin, H.; Yang, T.Y.; Shao, Y.J. Using a Polygenic Risk Score to Improve the Risk Prediction of Non-Small Cell Lung Cancer in Taiwan. JCO Precis. Oncol. 2024, 8, e2400236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Ding, Y.; Chen, T.; Mucci, L.; Albanes, D.; Landi, M.T.; Caporaso, N.E.; Lam, S.; Tardon, A.; et al. Impact of individual level uncertainty of lung cancer polygenic risk score (PRS) on risk stratification. Genome Med. 2024, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.P.; Katki, H.A.; Tanner, N.T.; Triplette, M.; Sakoda, L.C.; Wiener, R.S.; Cardarelli, R.; Carter-Harris, L.; Crothers, K.; Fathi, J.T.; et al. Addressing Disparities in Lung Cancer Screening Eligibility and Healthcare Access. An Official American Thoracic Society Statement. Am. J. Respir. Crit. Care Med. 2020, 202, e95–e112. [Google Scholar] [CrossRef]
- Lin, Y.A.; Hong, Y.T.; Lin, X.J.; Lin, J.L.; Xiao, H.M.; Huang, F.F. Barriers and facilitators to uptake of lung cancer screening: A mixed methods systematic review. Lung Cancer 2022, 172, 9–18. [Google Scholar] [CrossRef]
- Schapira, M.M.; Hubbard, R.A.; Whittle, J.; Vachani, A.; Kaminstein, D.; Chhatre, S.; Rodriguez, K.L.; Bastian, L.A.; Kravetz, J.D.; Asan, O.; et al. Lung Cancer Screening Decision Aid Designed for a Primary Care Setting: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2330452. [Google Scholar] [CrossRef]
- Li, C.; Cheng, B.; Li, J.; Xiong, S.; Fu, W.; Jiang, Y.; Zhou, C.; Zhong, R.; Li, F.; Zhang, Q.; et al. Non-Risk-Based Lung Cancer Screening with Low-Dose Computed Tomography. JAMA 2025, e254017. [Google Scholar] [CrossRef]
- Wang, C.; Dong, X.; Tan, F.; Wu, Z.; Huang, Y.; Zheng, Y.; Luo, Z.; Xu, Y.; Zhao, L.; Li, J.; et al. Risk-Adapted Starting Age of Personalized Lung Cancer Screening: A Population-Based, Prospective Cohort Study in China. Chest 2024, 165, 1538–1554. [Google Scholar] [CrossRef]
- Hardavella, G.; Frille, A.; Chalela, R.; Sreter, K.B.; Petersen, R.H.; Novoa, N.; de Koning, H.J. How will lung cancer screening and lung nodule management change the diagnostic and surgical lung cancer landscape? Eur. Respir. Rev. 2024, 33, 230232. [Google Scholar] [CrossRef]
- Bradley, S.H.; Shinkins, B.; Kennedy, M.P. What is the balance of benefits and harms for lung cancer screening with low-dose computed tomography? J. R Soc. Med. 2021, 114, 164–170. [Google Scholar] [CrossRef]
- Silva, M.; Prokop, M.; Jacobs, C.; Capretti, G.; Sverzellati, N.; Ciompi, F.; van Ginneken, B.; Schaefer-Prokop, C.M.; Galeone, C.; Marchianò, A.; et al. Long-Term Active Surveillance of Screening Detected Subsolid Nodules Is a Safe Strategy to Reduce Overtreatment. J. Thorac. Oncol. 2018, 13, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Qi, L.; Xia, C.; Liu, J.; Chen, J.; Cui, S.; Xue, L.; Cheng, S.; Jiang, X.; Wang, J. Pulmonary Subsolid Nodules: Upfront Surgery or Watchful Waiting? Chest 2025, 167, 1764–1777. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Kong, Y.; Huang, L.; Luo, H.; Zhu, X. Big data-driven precision medicine: Starting the custom-made era of iatrology. Biomed. Pharmacother. 2020, 129, 110445. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, G.S.; Phillips, K.A. Precision Medicine: From Science To Value. Health Aff. 2018, 37, 694–701. [Google Scholar] [CrossRef]
- Aldea, M.; Friboulet, L.; Apcher, S.; Jaulin, F.; Mosele, F.; Sourisseau, T.; Soria, J.C.; Nikolaev, S.; André, F. Precision medicine in the era of multi-omics: Can the data tsunami guide rational treatment decision? ESMO Open 2023, 8, 101642. [Google Scholar] [CrossRef]
- Hsiao, W.W.; Lin, J.C.; Fan, C.T.; Chen, S.S. Precision health in Taiwan: A data-driven diagnostic platform for the future of disease prevention. Comput. Struct. Biotechnol. J. 2022, 20, 1593–1602. [Google Scholar] [CrossRef]
- Alharbi, A.B.; Berrou, I.; Umaru, N.; Al Hamid, A.; Shebl, N.A. Understanding factors influencing the implementation of medicine risk communications by healthcare professionals in clinical practice: A systematic review using the Theoretical Domains Framework. Res. Soc. Adm. Pharm. 2024, 20, 86–98. [Google Scholar] [CrossRef]
- Haddad, D.N.; Sandler, K.L.; Henderson, L.M.; Rivera, M.P.; Aldrich, M.C. Disparities in Lung Cancer Screening: A Review. Ann. Am. Thorac. Soc. 2020, 17, 399–405. [Google Scholar] [CrossRef]
- Lei, F.; Chen, W.T.; Brecht, M.L.; Zhang, Z.F.; Hu, Y.; Xu, T.; Wang, S.; Lee, E. Cross-Cultural Adaptation of Lung Cancer Screening Health Belief Scale in Chinese Americans: A Methodological Study. J. Nurs. Meas. 2023, 31, 489–501. [Google Scholar] [CrossRef]
- Hung, Y.C.; Tang, E.K.; Wu, Y.J.; Chang, C.J.; Wu, F.Z. Impact of low-dose computed tomography for lung cancer screening on lung cancer surgical volume: The urgent need in health workforce education and training. Medicine 2021, 100, e26901. [Google Scholar] [CrossRef]
- Moscatelli, M.; Manconi, A.; Pessina, M.; Fellegara, G.; Rampoldi, S.; Milanesi, L.; Casasco, A.; Gnocchi, M. An infrastructure for precision medicine through analysis of big data. BMC Bioinform. 2018, 19 (Suppl. S10), 351. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.; Wilsdon, T.; Sarwar, I.; Roediger, A.; Yuan, M. Why is the screening rate in lung cancer still low? A seven-country analysis of the factors affecting adoption. Front. Public Health 2023, 11, 1264342. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Doria-Rose, V.P.; Silvestri, G.A.; Yabroff, K.R. Evaluating Lung Cancer Screening Uptake, Outcomes, and Costs in the United States: Challenges with Existing Data and Recommendations for Improvement. J. Natl. Cancer Inst. 2019, 111, 342–349. [Google Scholar] [CrossRef]
- Lapen, K.; Chino, F.; Noble, A.; Jin, J.O.; Levit, L.A.; Kirkwood, K.; Schenkel, C.; Subbiah, I.M. Key Strategies to Promote Professional Wellness and Reduce Burnout in Oncology Clinicians. JCO Oncol. Pract. 2025, 1-7Op2400199. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Z.; Zhao, X. Dynamic predictive maintenance strategy for system remaining useful life prediction via deep learning ensemble method. Reliab. Eng. Syst. Saf. 2024, 245, 110012. [Google Scholar] [CrossRef]
- Manser, R.L. Lung cancer screening: Screening frequency and lung cancer risk. Transl. Cancer Res. 2016, 5, S1227–S1232. [Google Scholar] [CrossRef]
- Hood, L.; Friend, S.H. Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat. Rev. Clin. Oncol. 2011, 8, 184–187. [Google Scholar] [CrossRef]
- Perachino, M.; Ortiz, C.; Carmona, J.; Saura, C.; Vivancos, A. Expanding screening through the use of liquid biopsy for early cancer detection. Commun. Med. 2025, 5, 167. [Google Scholar] [CrossRef]
- Steinberg, M.B.; Young, W.J.; Miller Lo, E.J.; Bover-Manderski, M.T.; Jordan, H.M.; Hafiz, Z.; Kota, K.J.; Mukherjee, R.; Garthe, N.E.; Sonnenberg, F.A.; et al. Electronic Health Record Prompt to Improve Lung Cancer Screening in Primary Care. Am. J. Prev. Med. 2023, 65, 892–895. [Google Scholar] [CrossRef]
- Kim, R.Y.; Rendle, K.A.; Mitra, N.; Neslund-Dudas, C.; Greenlee, R.T.; Honda, S.A.; Schapira, M.M.; Simoff, M.J.; Jeon, J.; Meza, R.; et al. Adherence to Annual Lung Cancer Screening and Rates of Cancer Diagnosis. JAMA Netw. Open 2025, 8, e250942. [Google Scholar] [CrossRef]
- Cavers, D.; Nelson, M.; Rostron, J.; Robb, K.A.; Brown, L.R.; Campbell, C.; Akram, A.R.; Dickie, G.; Mackean, M.; van Beek, E.J.R.; et al. Understanding patient barriers and facilitators to uptake of lung screening using low dose computed tomography: A mixed methods scoping review of the current literature. Respir. Res. 2022, 23, 374. [Google Scholar] [CrossRef] [PubMed]
- Rolle, L.D.; Olazagasti, C.; Lopes, G.; Rodriguez, E.; Crane, T.E. USPSTF Lung Cancer Screening Guidelines and Disparities in Screening Adherence. JAMA Oncol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Bhamani, A.; Naidu, S.B.; Patrick, T.; Anandan, L.; Desai, K.; Bojang, F.; Verghese, P.; Robinson, P.; Patel, S.; Thakrar, R.; et al. Improving uptake of lung cancer screening: An observational study on the impact of timed appointments and reminders. Thorax 2025, 80, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, N.; Sosa, A.E.; Rosell, R. Annual or biennial lung cancer CT screening? J. Thorac. Dis. 2016, 8, 2424–2426. [Google Scholar] [CrossRef]
- Field, J.K.; Duffy, S.W. Lung cancer CT screening: Is annual screening necessary? Lancet Oncol. 2016, 17, 543–544. [Google Scholar] [CrossRef]
- Pinsky, P.F. Lung cancer screening with low-dose CT: A world-wide view. Transl. Lung Cancer Res. 2018, 7, 234–242. [Google Scholar] [CrossRef]
- Hardavella, G.; Frille, A.; Sreter, K.B.; Atrafi, F.; Yousaf-Khan, U.; Beyaz, F.; Kyriakou, F.; Bellou, E.; Mullin, M.L.; Janes, S.M. Lung cancer screening: Where do we stand? Breathe 2024, 20, 230190. [Google Scholar] [CrossRef]

| Metric | CGSL | NCCN | USPSTF | I-ELCAP |
|---|---|---|---|---|
| Eligibility rate 1 | 13.92% | 6.97% | 6.81% | 53.46% |
| Efficiency ratio (ER) 2 | 1.46% | 1.64% | 1.51% | 1.13% |
| Inclusion rate 3 | 19.0% | 9.5% | 9.3% | 73.0% |
| Proportion of detected lung cancers 4 | 29.2% | 16.4% | 14.8% | 86.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-H.; Wu, Y.-J.; Wu, F.-Z. Precision Medicine in Lung Cancer Screening: A Paradigm Shift in Early Detection—Precision Screening for Lung Cancer. Diagnostics 2025, 15, 1562. https://doi.org/10.3390/diagnostics15121562
Chen H-H, Wu Y-J, Wu F-Z. Precision Medicine in Lung Cancer Screening: A Paradigm Shift in Early Detection—Precision Screening for Lung Cancer. Diagnostics. 2025; 15(12):1562. https://doi.org/10.3390/diagnostics15121562
Chicago/Turabian StyleChen, Hsin-Hung, Yun-Ju Wu, and Fu-Zong Wu. 2025. "Precision Medicine in Lung Cancer Screening: A Paradigm Shift in Early Detection—Precision Screening for Lung Cancer" Diagnostics 15, no. 12: 1562. https://doi.org/10.3390/diagnostics15121562
APA StyleChen, H.-H., Wu, Y.-J., & Wu, F.-Z. (2025). Precision Medicine in Lung Cancer Screening: A Paradigm Shift in Early Detection—Precision Screening for Lung Cancer. Diagnostics, 15(12), 1562. https://doi.org/10.3390/diagnostics15121562







