Structural and Hormonal Changes in Reproductive-Age Women Post-COVID-19: A Cross-Sectional Ultrasound and Biochemical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
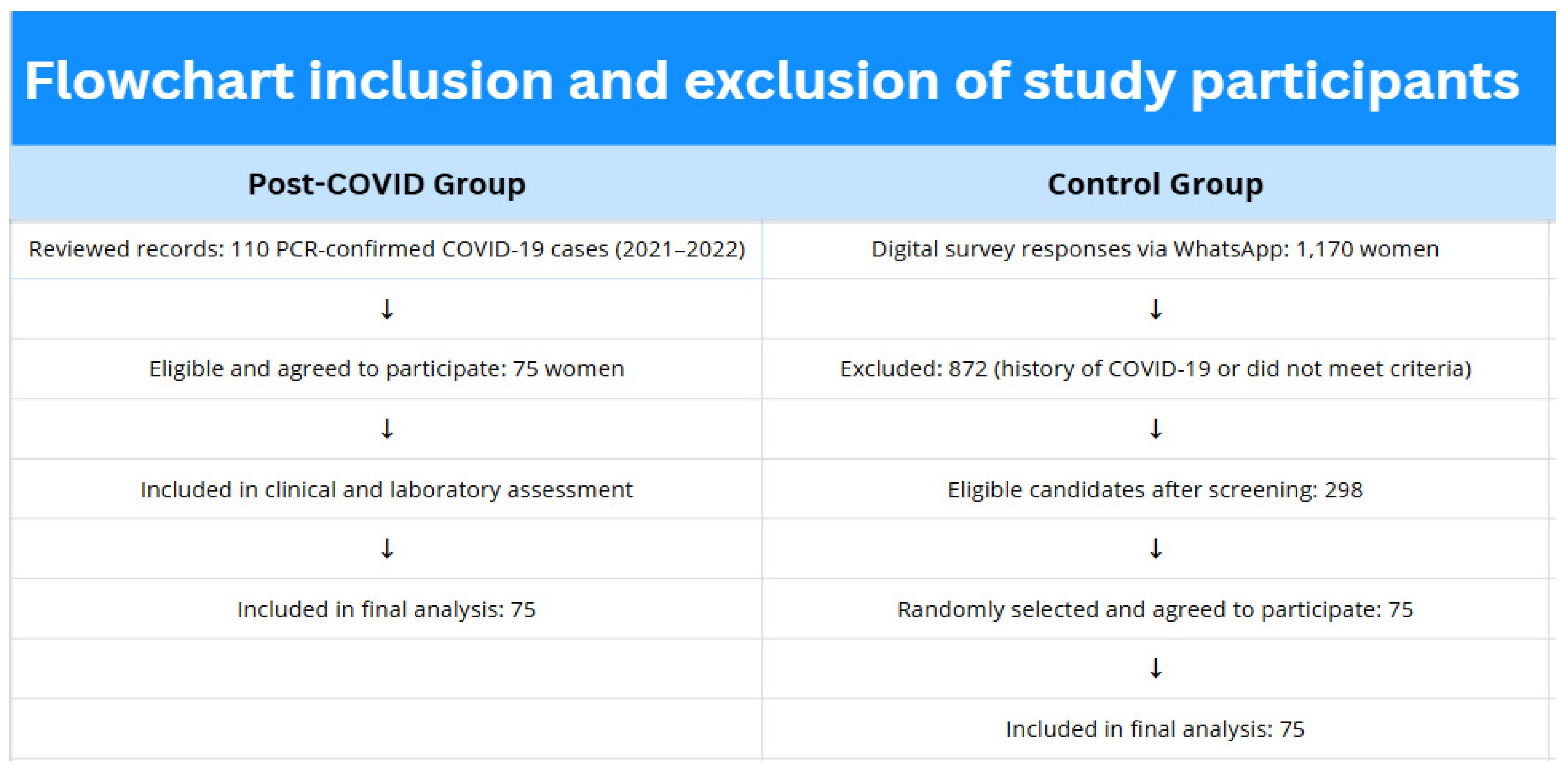
2.2. Participant Recruitment
2.3. Ethical Approval
2.4. Ultrasound Examinations
2.5. Laboratory Assessments
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Menstrual Irregularities
3.3. Ultrasound Examination of the Pelvic Organs
3.4. Breast Ultrasound
3.5. Biochemical and Hormonal Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronavirus Disease (COVID-19) Pandemic. World Health Organization. 2023. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 14 June 2025).
- Nägele, M.P.; Haubner, B.; Tanner, F.C.; Ruschitzka, F.; Flammer, A.J. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis 2020, 314, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.W.; Ilyas, I.; Weng, J.P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef]
- Nazir, M.; Asghar, S.; Rathore, M.A.; Shahzad, A.; Shahid, A.; Ashraf Khan, A.; Malik, A.; Fakhar, T.; Kausar, H.; Malik, J. Menstrual abnormalities after COVID-19 vaccines: A systematic review. Vacunas 2022, 23, S77–S87. [Google Scholar] [CrossRef]
- Moiynbayeva, S.; Auezova, A.; Kamaliev, M.; Erkenova, S. Long-term effects of Long COVID on the female reproductive system: A literature review. Reprod. Med. 2023, 3, 35–39. (In Russian) [Google Scholar] [CrossRef]
- Kurmanova, G.; Ayazbekov, A.; Kurmanova, A.; Rakhimbayeva, M.; Trimova, G.; Kulembayeva, A. PostCOVID-19 Impact on Perinatal Outcomes. Diagnostics 2025, 15, 57. [Google Scholar] [CrossRef]
- Karibayeva, I.; Moiynbayeva, S.; Akhmetov, V.; Yerkenova, S.; Shaikova, K.; Moshkalova, G.; Mussayeva, D.; Tarakova, B. Interrupted time series analysis of the impact of the COVID-19 pandemic and compulsory social health insurance system on fertility rates: A study of live births in Kazakhstan, 2019–2023. Front. Public Health 2024, 12, 1454420. [Google Scholar] [CrossRef]
- Vo, T.N.; Nguyen, P.N. Severe disease progression of postmolar gestational neoplasm in a Vietnamese young female patient after treatment refusal: Insights from a case report and literature review. Exp. Oncol. 2024, 46, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Phelan, N.; Behan, L.A.; Owens, L. The Impact of the COVID-19 Pandemic on Women’s Reproductive Health. Front. Endocrinol. 2021, 12, 642755. [Google Scholar] [CrossRef]
- Li, K.; Chen, G.; Hou, H.; Liao, Q.; Chen, J.; Bai, H.; Lee, S.; Wang, C.; Li, H.; Cheng, L.; et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod. Biomed. Online 2021, 42, 260–267. [Google Scholar] [CrossRef]
- Ranjbar, F.; Haghani, S.; Aghashahi, M.; Gharacheh, M. Changes in menstrual cycles among Iranian women during the COVID-19 pandemic: A cross-sectional study. Int. J. Reprod. Biomed. 2024, 22, 283–294. [Google Scholar] [CrossRef]
- D’Ippolito, S.; Turchiano, F.; Vitagliano, A.; Scutiero, G.; Lanzone, A.; Scambia, G.; Greco, P. Is There a Role for SARS-CoV-2/COVID-19 on the Female Reproductive System? Front. Physiol. 2022, 13, 845156. [Google Scholar] [CrossRef]
- Yerkenova, S.; Lokshin, V.; Saduakassova, S.; Zhabchenko, I.; Damulina, D.; Abshekenova, A.; Imasheva, B. Preconception care to improve pregnancy outcomes in COVID-19 survival women: A systematic review. Res. J. Pharm. Technol. 2023, 16, 5485–5491. [Google Scholar] [CrossRef]
- Pollack, B.; von Saltza, E.; McCorkell, L.; Santos, L.; Hultman, A.; Cohen, A.K.; Soares, L. Female reproductive health impacts of Long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: A literature review. Front. Rehabil. Sci. 2023, 4, 1122673. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, D.; Dolgushin, G.; Ivanets, T.; Vtorushina, V.; Dolgushina, N. The impact of COVID-19 on the ovarian reserve in women. Akusherstvo Ginekol. Obstet. Gynecol. 2022, 10, 123–128. (In Russian) [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Moazzami, B.; Chaichian, S.; Samie, S.; Zolbin, M.M.; Jesmi, F.; Akhlaghdoust, M.; Pishkuhi, M.A.; Mirshafiei, Z.S.; Khalilzadeh, F.; Safari, D. Does endometriosis increase susceptibility to COVID-19 infections? A case–control study in women of reproductive age. BMC Women’s Health 2021, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.Y.; Li, C.; Xu, W.; Liu, L.; Wei, M.L.; Fei, H.Y.; Li, J.; Zhou, F.; Zhang, S.Y. Factors associated with the incidence of ectopic pregnancy in women undergoing assisted reproductive treatment. Chin. Med. J. 2020, 133, 2054–2060. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Ata, B.; Vermeulen, N.; Mocanu, E.; Gianaroli, L.; Lundin, K.; Rautakallio-Hokkanen, S.; Tapanainen, J.S.; Veiga, A. SARS-CoV-2, fertility and assisted reproduction. Hum. Reprod. Update 2023, 29, 177–196. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062, Erratum in: Lancet 2020, 395, 1038. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.N.; Nguyen, V.T. Additional value of Doppler ultrasound to B-mode ultrasound in assessing for uterine intracavitary pathologies among perimenopausal and postmenopausal bleeding women: A multicentre prospective observational study in Vietnam. J. Ultrasound 2023, 26, 459–469. [Google Scholar] [CrossRef]
- WHO. Reproductive Health Indicators. 2022. Available online: https://www.who.int/data/nutrition/nlis/info/reproductive-health (accessed on 14 June 2025).
- Moiynbayeva, S.; Akhmetov, V.; Narymbayeva, N.; Shaikova, K.; Makhanbetkulova, D.; Bapayeva, M.; Abdirova, T.; Popova, T.; Karibayeva, I. Health policy implications for cardiovascular disease, type 2 diabetes mellitus, and stroke in Central Asia: A decadal forecast of their impact on women of reproductive age. Front. Public Health 2024, 12, 1456187. [Google Scholar] [CrossRef]
- Baimakhanova, B.; Sadanov, A.; Trenozhnikova, L.; Balgimbaeva, A.; Baimakhanova, G.; Orasymbet, S.; Tleubekova, D.; Amangeldi, A.; Turlybaeva, Z.; Nurgaliyeva, Z.; et al. Understanding the Burden and Management of Urinary Tract Infections in Women. Diseases 2025, 13, 59. [Google Scholar] [CrossRef]
- Kozhekenova, N.; Moiynbayeva, S.; Jeremic, D.; Dinic, M.; Semenov, P.; Nurgaliyeva, Z.; Tolekova, S.; Miller, A.; Smasheva, A.; Milicevic, M.S. The burden of COVID-19 in primary care of Almaty, Kazakhstan, 2021–2022. Sci. Rep. 2025, 15, 5186. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.S.; Malathi, T.; Gowda, M.A.S. A Cross-Sectional Study on Post-COVID-19 Menstrual Abnormalities in Women of Reproductive Age Group at a Tertiary Care Hospital. Obstet. Gynecol. Int. 2025, 2025, 1771858. [Google Scholar] [CrossRef]
- Madaan, S.; Talwar, D.; Jaiswal, A.; Kumar, S.; Acharya, N.; Acharya, S.; Dewani, D. PostCOVID-19 Menstrual Abnormalities and Infertility. J. Educ. Health Promot. 2022, 11, 170. [Google Scholar] [CrossRef]

| Characteristic | Post-COVID-19 Group (n = 75) | Control Group (n = 75) | p-Value |
|---|---|---|---|
| Age, years (mean ± SD) | 33.6 ± 5.2 | 32.9 ± 5.7 | 0.482 |
| BMI, kg/m2 (mean ± SD) | 24.8 ± 3.9 | 24.1 ± 3.5 | 0.293 |
| Urban residence, n (%) | 72 (96%) | 74 (98.7%) | 0.618 |
| Married, n (%) | 58 (77.3%) | 60 (80.0%) | 0.693 |
| Nulliparous, n (%) | 18 (24.0%) | 21 (28.0%) | 0.568 |
| History of irregular menstruation, n (%) | 27 (36.0%) | 15 (20.0%) | 0.035 * |
| History of gynecologic diseases, n (%) | 19 (25.3%) | 12 (16.0%) | 0.164 |
| Smoking, n (%) | 6 (8.0%) | 4 (5.3%) | 0.515 |
| COVID-19 vaccination, n (%) | 47 (62.7%) | 0 (excluded by design) | - |
| Indicators | Post-COVID-19 (%) | Control (%) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Presence of structural changes (%) | 53.5 | 12.0 | 8.56 (3.85–19.06) | <0.001 a |
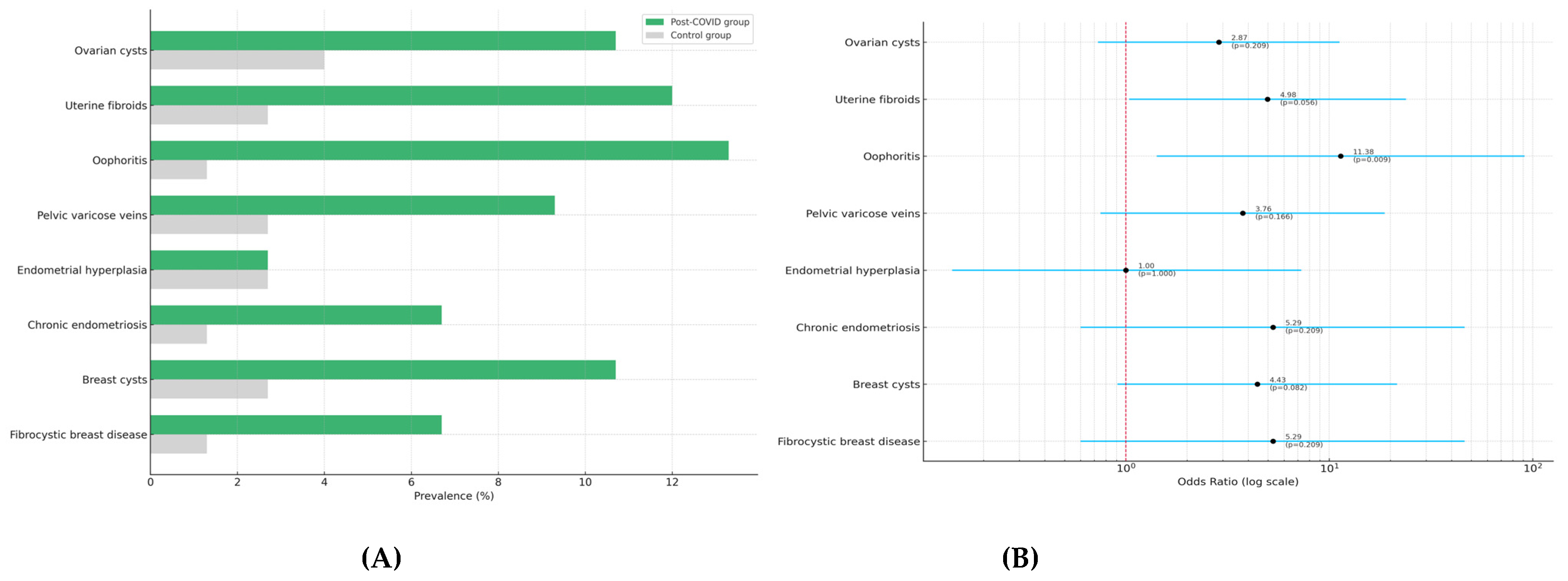
| Ovarian cysts (%) | 10.7 | 4.0 | 2.87 (0.73–11.25) | 0.209 |
| Uterine fibroids (%) | 12.0 | 2.7 | 4.98 (1.04–23.88) | 0.056 |
| Oophoritis (%) | 13.3 | 1.3 | 11.38 (1.42–91.36) | 0.009 b |
| Pelvic varicose veins (%) Endometriosis (%) | 9.3 6.7 | 2.7 1.3 | 3.76 (0.75–18.72) 5.29 (0.60–46.38) | 0.166 0.209 |
| Indicators | Post-COVID-19 (%) | Control (%) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Ovarian cysts | 10.7 | 4.0 | 2.87 (0.73–11.25) | 0.209 |
| Uterine fibroids | 12.0 | 2.7 | 4.98 (1.04–23.88) | 0.056 a |
| Oophoritis | 13.3 | 1.3 | 11.38 (1.42–91.36) | 0.009 b |
| Pelvic varicose veins | 9.3 | 2.7 | 3.76 (0.75–18.72) | 0.166 |
| Endometrial hyperplasia | 2.7 | 2.7 | 1.00 (0.14–7.29) | 1.000 |
| Chronic endometriosis | 6.7 | 1.3 | 5.29 (0.60–46.38) | 0.209 |
| Breast cysts | 10.7 | 2.7 | 4.43 (0.91–21.53) | 0.082 |
| Fibrocystic breast disease | 6.7 | 1.3 | 5.29 (0.60–46.38) | 0.209 |
| Marker | Post-COVID-19 Group (Median ± IQR) | Control Group (Median ± IQR) | p-Value |
|---|---|---|---|
| Fibrinogen (g/L) | 3.05 ± 2.5–3.5 | 2.80 ± 2.3–3.1 | <0.05 |
| TSH (mIU/L) | 3.30 ± 3.0–3.5 | 3.70 ± 3.5–4.0 | >0.05 |
| Ferritin (μg/L) | 420.0 ± 350–490 | 311.0 ± 280–340 | <0.05 |
| Estradiol (pg/mL) | 57.0 ± 50–65 | 11.2 ± 10–12 | <0.05 |
| Prolactin (ng/mL) | 15.0 ± 12–18 | 15.0 ± 12–18 | >0.05 |
| Progesterone (ng/mL) | 8.0 ± 6–10 | 10.0 ± 8–12 | >0.05 |
| AMH (ng/mL) | 2.5 ± 2.0–3.0 | 3.0 ± 2.5–3.5 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerkenova, S.; Lokshin, V.; Kurmanova, A.; Moiynbayeva, S.; Alikeyeva, G.; Kalibekova, G.; Abdirova, T.; Zhantenova, Z.; Shaikova, K.; Mireeva, A.; et al. Structural and Hormonal Changes in Reproductive-Age Women Post-COVID-19: A Cross-Sectional Ultrasound and Biochemical Study. Diagnostics 2025, 15, 1536. https://doi.org/10.3390/diagnostics15121536
Yerkenova S, Lokshin V, Kurmanova A, Moiynbayeva S, Alikeyeva G, Kalibekova G, Abdirova T, Zhantenova Z, Shaikova K, Mireeva A, et al. Structural and Hormonal Changes in Reproductive-Age Women Post-COVID-19: A Cross-Sectional Ultrasound and Biochemical Study. Diagnostics. 2025; 15(12):1536. https://doi.org/10.3390/diagnostics15121536
Chicago/Turabian StyleYerkenova, Sandugash, Vyacheslav Lokshin, Almagul Kurmanova, Sharapat Moiynbayeva, Galiya Alikeyeva, Gulnara Kalibekova, Tamara Abdirova, Zamira Zhantenova, Kuralay Shaikova, Alla Mireeva, and et al. 2025. "Structural and Hormonal Changes in Reproductive-Age Women Post-COVID-19: A Cross-Sectional Ultrasound and Biochemical Study" Diagnostics 15, no. 12: 1536. https://doi.org/10.3390/diagnostics15121536
APA StyleYerkenova, S., Lokshin, V., Kurmanova, A., Moiynbayeva, S., Alikeyeva, G., Kalibekova, G., Abdirova, T., Zhantenova, Z., Shaikova, K., Mireeva, A., & Turgumbayeva, A. (2025). Structural and Hormonal Changes in Reproductive-Age Women Post-COVID-19: A Cross-Sectional Ultrasound and Biochemical Study. Diagnostics, 15(12), 1536. https://doi.org/10.3390/diagnostics15121536








