Enhancing Hippocampal Subfield Visualization Through Deep Learning Reconstructed MRI Scans
Abstract
1. Introduction
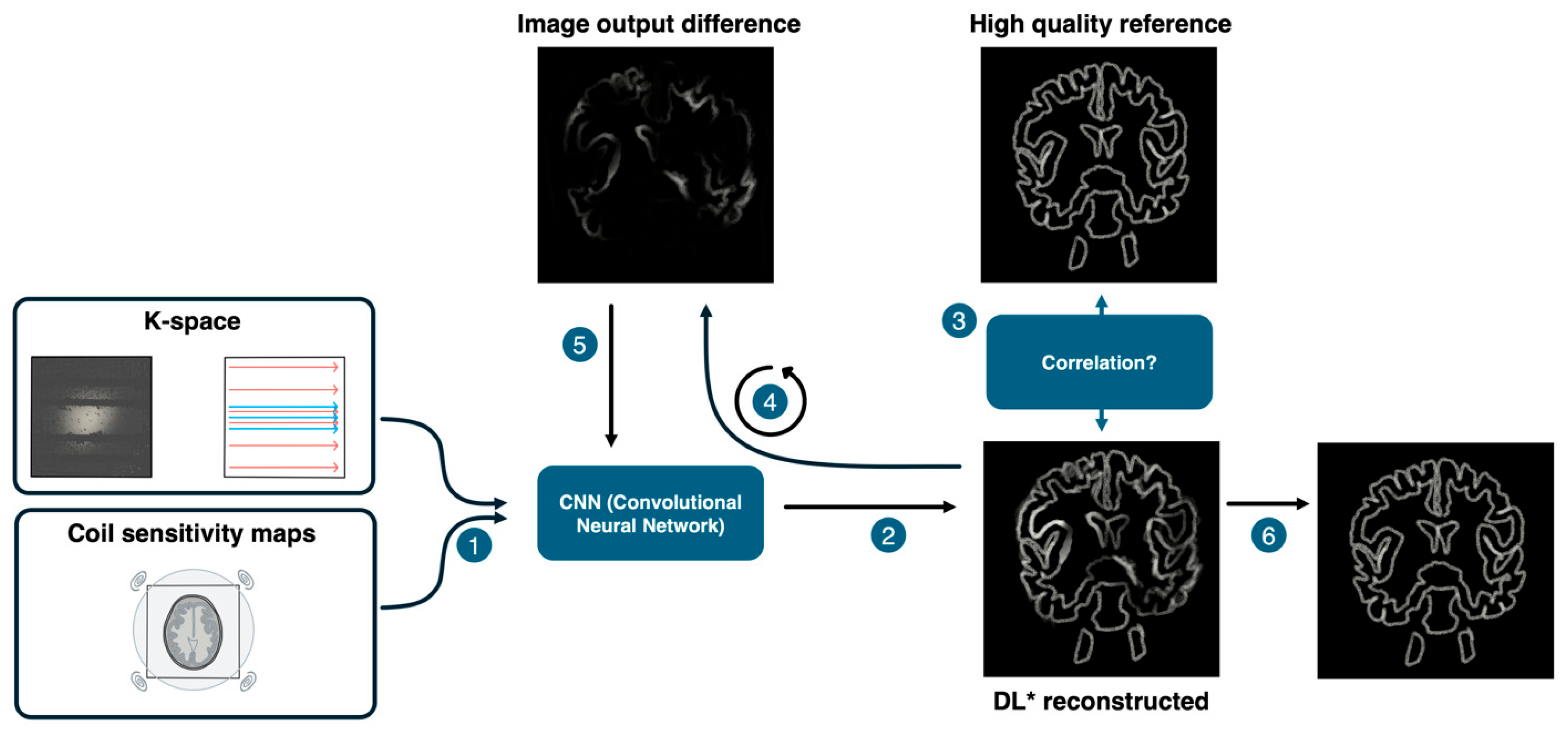
2. Materials & Methods
2.1. Study Design
2.2. Patient Characteristics
2.3. MRI Acquisition Parameters
2.4. Image Evaluation Using FreeSurfer
2.5. Statistical Analysis
2.6. Setting a 95% CI for Hippocampal Pathology Detection
3. Results
4. Discussion
4.1. Comparing T2 TSE DRB with T2 TSE
4.2. Testing the 95% CI for Hippocampal Pathology Detection
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernasconi:, A.; Cendes, F.; Theodore, W.H.; Gill, R.S.; Koepp, M.J.; Hogan, R.E.; Jackson, G.D.; Federico, P.; Labate, A.; Vaudano, A.E.V.; et al. Recommendations for the use of structural magnetic resonance imaging in the care of patients with epilepsy: A consensus report from the International League Against Epilepsy Neuroimaging Task Force. Epilepsia 2019, 60, 1054–1068. [Google Scholar] [CrossRef] [PubMed]
- Hakami, T.; McIntosh, A.; Todaro, M.; Lui, E.; Yerra, R.; Tan, K.M.; French, C.; Li, S.; Desmond, P.; Matkovic, Z.; et al. MRI-identified pathology in adults with new-onset seizures. Neurology 2013, 81, 920–927. [Google Scholar] [CrossRef]
- Von Oertzen, J.; Urbach, H.; Jungbluth, S.; Kurthen, M.; Reuber, M.; Fernández, G.; Elger, C.E. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J. Neurol. Neurosurg. Psychiatry 2002, 73, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Sijbers, J.; Scheunders, P.; Bonnet, N.; Van Dyck, D.; Raman, E. Quantification and improvement of the signal-to-noise ratio in a magnetic resonance image acquisition procedure. Magn. Reson. Imaging 1996, 14, 1157–1163. [Google Scholar] [CrossRef]
- Herrmann, J.; Koerzdoerfer, G.; Nickel, D.; Mostapha, M.; Nadar, M.; Gassenmaier, S.; Kuestner, T.; Othman, A.E. Feasibility and Implementation of a Deep Learning MR Reconstruction for TSE Sequences in Musculoskeletal Imaging. Diagnostics 2021, 11, 1484. [Google Scholar] [CrossRef] [PubMed]
- Schlemper, J.; Caballero, J.; Hajnal, J.V.; Price, A.N.; Rueckert, D. A Deep Cascade of Convolutional Neural Networks for Dynamic MR Image Reconstruction. IEEE Trans. Med. Imaging 2018, 37, 491–503. [Google Scholar] [CrossRef]
- Hammernik, K.; Klatzer, T.; Kobler, E.; Recht, M.P.; Sodickson, D.K.; Pock, T.; Knoll, F. Learning a variational network for reconstruction of accelerated MRI data. Magn. Reson. Med. 2018, 79, 3055–3071. [Google Scholar] [CrossRef]
- So, E.L.; Lee, R.W. Epilepsy surgery in MRI-negative epilepsies. Curr. Opin. Neurol. 2014, 27, 206–212. [Google Scholar] [CrossRef]
- Kim, H.; Bernhardt, B.C.; Kulaga-Yoskovitz, J.; Caldairou, B.; Bernasconi, A.; Bernasconi, N. Multivariate hippocampal subfield analysis of local MRI intensity and volume: Application to temporal lobe epilepsy. Med. Image Comput. Comput. Assist. Interv. 2014, 17 Pt 2, 170–178. [Google Scholar] [CrossRef]
- Bernasconi, A.; Antel, S.B.; Collins, D.L.; Bernasconi, N.; Olivier, A.; Dubeau, F.; Pike, G.B.; Andermann, F.; Arnold, D.L. Texture analysis and morphological processing of magnetic resonance imaging assist detection of focal cortical dysplasia in extra-temporal partial epilepsy. Ann. Neurol. 2001, 49, 770–775. [Google Scholar] [CrossRef]
- Winston, G.P.; Vos, S.B.; Burdett, J.L.; Cardoso, M.J.; Ourselin, S.; Duncan, J.S. Automated T2 relaxometry of the hippocampus for temporal lobe epilepsy. Epilepsia 2017, 58, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, A.; Bernasconi, N.; Caramanos, Z.; Reutens, D.C.; Andermann, F.; Dubeau, F.; Tampieri, D.; Pike, B.G.; Arnold, D.L. T2 relaxometry can lateralize mesial temporal lobe epilepsy in patients with normal MRI. Neuroimage 2000, 12, 739–746. [Google Scholar] [CrossRef]
- Suh, P.S.; Park, J.E.; Roh, Y.H.; Kim, S.; Jung, M.; Koo, Y.S.; Lee, S.-A.; Choi, Y.; Kim, H.S. Improving Diagnostic Performance of MRI for Temporal Lobe Epilepsy With Deep Learning-Based Image Reconstruction in Patients With Suspected Focal Epilepsy. Korean J. Radiol. 2024, 25, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Manmatharayan, A.; Kogan, M.; Matias, C.; Syed, M.; Shelley, I.; Chinni, A.; Kang, K.; Talekar, K.; Faro, S.H.; Mohamed, F.B.; et al. Automated subfield volumetric analysis of amygdala, hippocampus, and thalamic nuclei in mesial temporal lobe epilepsy. World Neurosurg. X 2023, 19, 100212. [Google Scholar] [CrossRef]
- Available online: https://www.siemens-healthineers.com/at/magnetic-resonance-imaging/3t-mri-scanner/magnetom-vida (accessed on 20 July 2024).
- FreeSurfer. Available online: https://surfer.nmr.mgh.harvard.edu/fswiki/FreeSurferWiki (accessed on 20 July 2024).
- Iglesias, J.E.; Augustinack, J.C.; Nguyen, K.; Player, C.M.; Player, A.; Wright, M.; Roy, N.; Frosch, M.P.; McKee, A.C.; Wald, L.L.; et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage 2015, 115, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Corporation, M. Microsoft Excel, Version 16.83; Microsoft Corporation: Redmond, WA, USA, 2018. [Google Scholar]
- Bhatia, S.; Bookheimer, S.Y.; Gaillard, W.D.; Theodore, W.H. Measurement of whole temporal lobe and hippocampus for MR volumetry: Normative data. Neurology 1993, 43, 2006–2010. [Google Scholar] [CrossRef]
- Nobis, L.; Manohar, S.G.; Smith, S.M.; Alfaro-Almagro, F.; Jenkinson, M.; Mackay, C.E.; Husain, M. Hippocampal volume across age: Nomograms derived from over 19,700 people in UK Biobank. Neuroimage Clin. 2019, 23, 101904. [Google Scholar] [CrossRef]
- Li, Y.J.; Ga, S.N.; Huo, Y.; Li, S.Y.; Gao, X.G. Characteristics of hippocampal volumes in healthy Chinese from MRI. Neurol. Res. 2007, 29, 803–806. [Google Scholar] [CrossRef]
- Blaimer, M.; Breuer, F.; Mueller, M.; Heidemann, R.M.; Griswold, M.A.; Jakob, P.M. SMASH, SENSE, PILS, GRAPPA: How to choose the optimal method. Top. Magn. Reson. Imaging 2004, 15, 223–236. [Google Scholar] [CrossRef]
- Glockner, J.F.; Hu, H.H.; Stanley, D.W.; Angelos, L.; King, K. Parallel MR imaging: A user’s guide. Radiographics 2005, 25, 1279–1297. [Google Scholar] [CrossRef]
- Zahneisen, B.; Poser, B.A.; Ernst, T.; Stenger, A.V. Simultaneous Multi-Slice fMRI using spiral trajectories. Neuroimage 2014, 92, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.C. Compressed sensing MRI: A review from signal processing perspective. BMC Biomed. Eng. 2019, 1, 8. [Google Scholar] [CrossRef]
- Lin, D.J.; Johnson, P.M.; Knoll, F.; Lui, Y.W. Artificial Intelligence for MR Image Reconstruction: An Overview for Clinicians. J. Magn. Reson. Imaging 2021, 53, 1015–1028. [Google Scholar] [CrossRef]
- Iuga, A.I.; Rauen, P.S.; Siedek, F.; Große-Hokamp, N.; Sonnabend, K.; Maintz, D.; Lennartz, S.; Bratke, G. A deep learning-based reconstruction approach for accelerated magnetic resonance image of the knee with compressed sense: Evaluation in healthy volunteers. Br. J. Radiol. 2023, 96, 20220074. [Google Scholar] [CrossRef]
- Bash, S.; Wang, L.; Airriess, C.; Zaharchuk, G.; Gong, E.; Shankaranarayanan, A.; Tanenbaum, L.N. Deep Learning Enables 60% Accelerated Volumetric Brain MRI While Preserving Quantitative Performance: A Prospective, Multicenter, Multireader Trial. AJNR Am. J. Neuroradiol. 2021, 42, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Estler, A.; Hauser, T.K.; Mengel, A.; Brunnée, M.; Zerweck, L.; Richter, V.; Zuena, M.; Schuhholz, M.; Ernemann, U.; Gohla, G. Deep Learning Accelerated Image Reconstruction of Fluid-Attenuated Inversion Recovery Sequence in Brain Imaging: Reduction of Acquisition Time and Improvement of Image Quality. Acad. Radiol. 2024, 31, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tao, H.; Li, X.; Hu, Y.; Liu, C.; Zhou, B.; Cai, J.; Nickel, D.; Fu, C.; Xiong, B.; et al. Prospective Comparison of Standard and Deep Learning-reconstructed Turbo Spin-Echo MRI of the Shoulder. Radiology 2024, 310, e231405. [Google Scholar] [CrossRef]
- Blümcke, I.; Thom, M.; Aronica, E.; Armstrong, D.D.; Bartolomei, F.; Bernasconi, A.; Bernasconi, N.; Bien, C.G.; Cendes, F.; Coras, R.; et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: A Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 2013, 54, 1315–1329. [Google Scholar] [CrossRef]
- Günbey, C.; Söylemezoğlu, F.; Bilginer, B.; Karlı Oğuz, K.; Akalan, N.; Topçu, M.; Turanlı, G.; Yalnızoğlu, D. International consensus classification of hippocampal sclerosis and etiologic diversity in children with temporal lobectomy. Epilepsy Behav. 2020, 112, 107380. [Google Scholar] [CrossRef]
- Brown, E.M.; Pierce, M.E.; Clark, D.C.; Fischl, B.R.; Iglesias, J.E.; Milberg, W.P.; McGlinchey, R.E.; Salat, D.H. Test-retest reliability of FreeSurfer automated hippocampal subfield segmentation within and across scanners. Neuroimage 2020, 210, 116563. [Google Scholar] [CrossRef]
- Alves, I.S.; Coutinho, A.M.N.; Vieira, A.P.F.; Rocha, B.P.; Passos, U.L.; Gonçalves, V.T.; Silva, P.D.S.; Zhan, M.X.; Pinho, P.C.; Delgado, D.S.; et al. Imaging Aspects of the Hippocampus. Radiographics 2022, 42, 822–840. [Google Scholar] [CrossRef] [PubMed]
- Salmenperä, T.; Könönen, M.; Roberts, N.; Vanninen, R.; Pitkänen, A.; Kälviäinen, R. Hippocampal damage in newly diagnosed focal epilepsy: A prospective MRI study. Neurology 2005, 64, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Lin, Y.; Rosen, D.; Du, J.; He, L.; Wang, Y. Identifying Morphological Patterns of Hippocampal Atrophy in Patients With Mesial Temporal Lobe Epilepsy and Alzheimer Disease. Front. Neurol. 2020, 11, 21. [Google Scholar] [CrossRef]
- Pulsipher, D.T.; Seidenberg, M.; Morton, J.J.; Geary, E.; Parrish, J.; Hermann, B. MRI volume loss of subcortical structures in unilateral temporal lobe epilepsy. Epilepsy Behav. 2007, 11, 442–449. [Google Scholar] [CrossRef]
- Bien, C.G.; Szinay, M.; Wagner, J.; Clusmann, H.; Becker, A.J.; Urbach, H. Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging-negative epilepsies. Arch. Neurol. 2009, 66, 1491–1499. [Google Scholar] [CrossRef]
- Severino, M.; Geraldo, A.F.; Utz, N.; Tortora, D.; Pogledic, I.; Klonowski, W.; Triulzi, F.; Arrigoni, F.; Mankad, K.; Mancini, G.M.S.; et al. Definitions and classification of malformations of cortical development: Practical guidelines. Brain 2020, 143, 2874–2894. [Google Scholar] [CrossRef]
- Oegema, R.; Barkovich, A.J.; Mancini, G.M.S.; Guerrini, R.; Dobyns, W.B. Subcortical heterotopic gray matter brain malformations: Classification study of 107 individuals. Neurology 2019, 93, e1360–e1373. [Google Scholar] [CrossRef]
- Liu, R.S.; Lemieux, L.; Bell, G.S.; Sisodiya, S.M.; Bartlett, P.A.; Shorvon, S.D.; Sander, J.W.A.S.; Duncan, J.S. The structural consequences of newly diagnosed seizures. Ann. Neurol. 2002, 52, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Van Paesschen, W.; Duncan, J.S.; Stevens, J.M.; Connelly, A. Longitudinal quantitative hippocampal magnetic resonance imaging study of adults with newly diagnosed partial seizures: One-year follow-up results. Epilepsia 1998, 39, 633–639. [Google Scholar] [CrossRef]
- Thom, M. Review: Hippocampal sclerosis in epilepsy: A neuropathology review. Neuropathol. Appl. Neurobiol. 2014, 40, 520–543. [Google Scholar] [CrossRef]
- Moghaddam, H.S.; Aarabi, M.H.; Mehvari-Habibabadi, J.; Sharifpour, R.; Mohajer, B.; Mohammadi-Mobarakeh, N.; Hashemi-Fesharaki, S.S.; Elisevich, K.; Nazem-Zadeh, M.-R. Distinct patterns of hippocampal subfield volume loss in left and right mesial temporal lobe epilepsy. Neurol. Sci. 2021, 42, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
 parasubiculum,
parasubiculum,  HATA,
HATA,  fimbria,
fimbria,  hippacampal_fissure,
hippacampal_fissure,  HP_tail,
HP_tail,  presubiculum-head,
presubiculum-head,  presubiculum-body,
presubiculum-body,  subiculum-head,
subiculum-head,  subiculum-body,
subiculum-body,  CA1-head,
CA1-head,  CA1-body,
CA1-body,  CA3-head,
CA3-head,  CA3-body,
CA3-body,  CA4-head,
CA4-head,  CA4-body,
CA4-body,  GC-ML-DG-head,
GC-ML-DG-head,  GC-ML-DG-body,
GC-ML-DG-body,  molecular_layer_HP-head,
molecular_layer_HP-head,  moleculcular_layer_HP-body.
moleculcular_layer_HP-body.
 parasubiculum,
parasubiculum,  HATA,
HATA,  fimbria,
fimbria,  hippacampal_fissure,
hippacampal_fissure,  HP_tail,
HP_tail,  presubiculum-head,
presubiculum-head,  presubiculum-body,
presubiculum-body,  subiculum-head,
subiculum-head,  subiculum-body,
subiculum-body,  CA1-head,
CA1-head,  CA1-body,
CA1-body,  CA3-head,
CA3-head,  CA3-body,
CA3-body,  CA4-head,
CA4-head,  CA4-body,
CA4-body,  GC-ML-DG-head,
GC-ML-DG-head,  GC-ML-DG-body,
GC-ML-DG-body,  molecular_layer_HP-head,
molecular_layer_HP-head,  moleculcular_layer_HP-body.
moleculcular_layer_HP-body.
 parasubiculum,
parasubiculum,  HATA,
HATA,  fimbria,
fimbria,  hippacampal_fissure,
hippacampal_fissure,  HP_tail,
HP_tail,  presubiculum-head,
presubiculum-head,  presubiculum-body,
presubiculum-body,  subiculum-head,
subiculum-head,  subiculum-body,
subiculum-body,  CA1-head,
CA1-head,  CA1-body,
CA1-body,  CA3-head,
CA3-head,  CA3-body,
CA3-body,  CA4-head,
CA4-head,  CA4-body,
CA4-body,  GC-ML-DG-head,
GC-ML-DG-head,  GC-ML-DG-body,
GC-ML-DG-body,  molecular_layer_HP-head,
molecular_layer_HP-head,  moleculcular_layer_HP-body.
moleculcular_layer_HP-body.
 parasubiculum,
parasubiculum,  HATA,
HATA,  fimbria,
fimbria,  hippacampal_fissure,
hippacampal_fissure,  HP_tail,
HP_tail,  presubiculum-head,
presubiculum-head,  presubiculum-body,
presubiculum-body,  subiculum-head,
subiculum-head,  subiculum-body,
subiculum-body,  CA1-head,
CA1-head,  CA1-body,
CA1-body,  CA3-head,
CA3-head,  CA3-body,
CA3-body,  CA4-head,
CA4-head,  CA4-body,
CA4-body,  GC-ML-DG-head,
GC-ML-DG-head,  GC-ML-DG-body,
GC-ML-DG-body,  molecular_layer_HP-head,
molecular_layer_HP-head,  moleculcular_layer_HP-body.
moleculcular_layer_HP-body.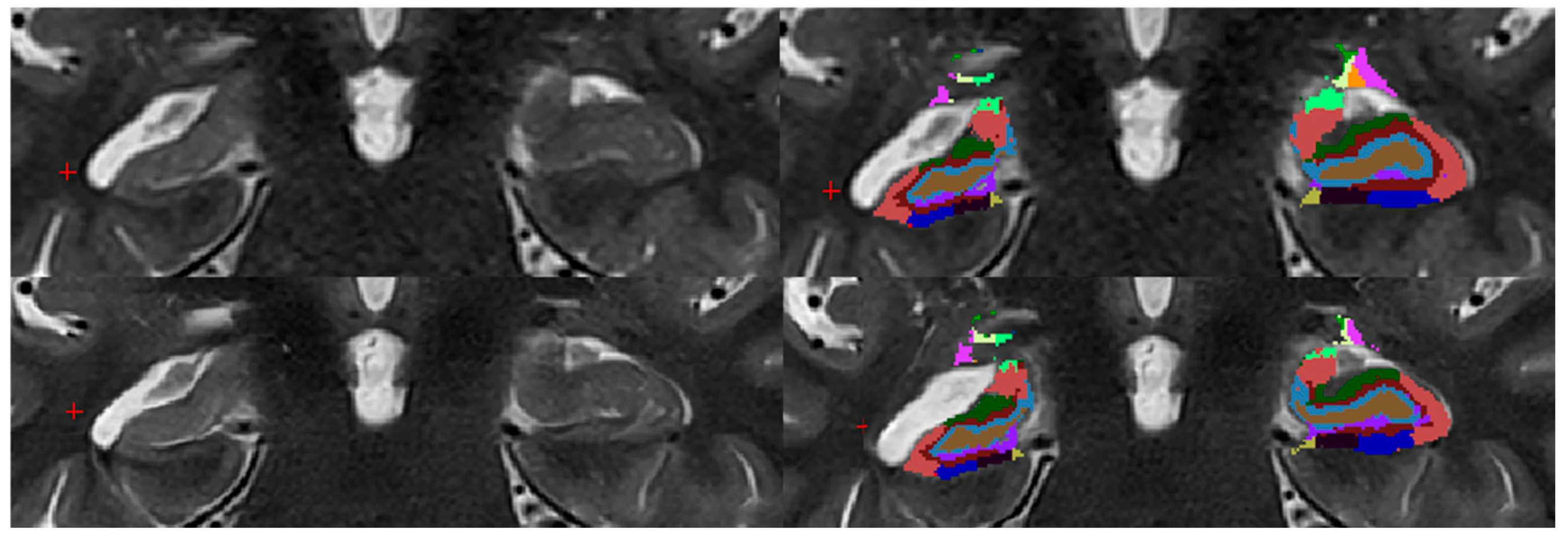

| Pathology | Subjects | Gender | Age | |
|---|---|---|---|---|
| Male | Female | |||
| Hippocampal sclerosis | 1 (3%) | 0 (0%) | 1 (100%) | 57 ± 0 |
| FCD a | 2 (6%) | 2 (100%) | 0 (0%) | 42 ± 28 |
| Edema b | 2 (6%) | 2 (100%) | 0 (0%) | 29 ± 9 |
| Epilepsy (without visible pathology) | 11 (31%) | 6 (55%) | 5 (45%) | 41 ± 13 |
| Healthy | 20 (56%) | 11 (55%) | 9 (45%) | 37 ± 15 |
| Overall | 36 (100%) | 21 (58%) | 15 (42%) | 39 ± 14 |
| Sequence | ||||
|---|---|---|---|---|
| T1 3D MPRAGE | T2 2D TSE | T2 2D TSE DRB | ||
| MRT Settings | Slice orientation | sagittal | coronal | coronal |
| Slices | 192 | 35 | 35 | |
| Acceleration | GRAPPA d R = 2 | GRAPPA d R = 2 | GRAPPA d R = 4 | |
| Reconstruction | GRAPPA d | GRAPPA d | DRB e | |
| Slice thickness (mm) | 0.90 | 2.00 | 2.00 | |
| TR a (ms) | 2300 | 4100.00 | 4100.00 | |
| TE b (ms) | 2.32 | 76.00 | 76.00 | |
| Flip Angle (deg.) | 8 | 150.00 | 150.00 | |
| Deep Resolve | OFF | OFF | ON | |
| Concatenations | 1 | 2 | 2 | |
| Voxel size (mm) | 0.9 × 0.9 × 0.9 | 0.2 × 0.2 × 2 | 0.2 × 0.2 × 2 | |
| Averages | 1 | 3 | 3 | |
| Distance factor (%) | 50 | 0 | 0 | |
| FOV c Read (mm) | 230 | 170 | 170 | |
| FOV c Phase (%) | 100 | 100 | 100 | |
| Phase Resolution (%) | 100 | 85 | 85 | |
| Trajectory | Cartesian | Cartesian | Cartesian | |
| Bandwidth (Hz/Px) | 200 | 200 | 200 | |
| Echo Spacing (ms) | 7.06 | 10.9 | 10.9 | |
| Dimensions | 3D | 2D | 2D | |
| RF Pulse Type | Normal | Normal | Normal | |
| Gradient Mode | Normal | Fast | Fast | |
| Flow Compensation | None | Read | Read | |
| Turbo Factor | 240 | 19 | 19 | |
| Phase Oversampling (%) | 0 | 0 | 0 | |
| Slice Oversampling (%) | 25 | / | / | |
| Base Resolution | 256 | 384 | 384 | |
| Slice Resolution (%) | 100 | / | / | |
| Reference Lines | 24 | 44 | 44 | |
| Coil Selection | automatic | automatic | automatic | |
| Coil Combination | adaptive | adaptive | adaptive | |
| Echo Trains per Slice | / | 6 | 6 | |
| Time (min.) | 5:21 | 3:51 | 2:37 | |
| Pathology | n | Accelerated (T2 TSE DRB *)—Motion Artifacts | Standardized (T2 TSE +)—Motion Artifacts |
|---|---|---|---|
| Hippocampal Sclerosis | 1 | 0 | 1 |
| Focal Cortical Dysplasia (FCD) | 2 | 0 | 0 |
| Edema | 2 | 0 | 1 |
| Epilepsy (without visible pathology) | 11 | 0 | 0 |
| Healthy Controls | 20 | 1 | 2 |
| Total | 36 | 1 | 4 |
| Region | T2 TSE ‡ DRB † Sequence | T2 TSE ‡ Sequence | ||
|---|---|---|---|---|
| MEANhealthy (mm3) | SDhealthy * (mm3) | MEANhealthy (mm3) | SDhealthy * (mm3) | |
| Parasubiculum | −7.6 | 13.8 | −7.2 | 13.8 |
| Presubiculum-Head | −6.3 | 16.3 | −5.9 | 13.1 |
| Subiculum-Head | −2.5 | 18.9 | −2.6 | 18.2 |
| CA1-Head | 11.0 | 36.8 | 9.3 | 40.5 |
| CA2/3-Head | 9.4 | 17.7 | 7.8 | 17.3 |
| CA4-Head | 5.2 | 14.0 | 7.4 | 13.4 |
| GC-ML-DG-head | 6.3 | 18.6 | 9.0 | 18.6 |
| molecular_layer_HP-head | −2.1 | 25.0 | −4.7 | 23.8 |
| HATA | 0.7 | 8.1 | 0.5 | 8.8 |
| Presubiculum-body | −19.0 | 22.5 | −18.8 | 20.0 |
| Subiculum-body | −13.5 | 18.0 | −15.6 | 14.2 |
| CA1-Body | 9.1 | 23.2 | 4.5 | 22.6 |
| CA2/3-body | 7.4 | 16.5 | 5.5 | 17.7 |
| CA4-body | −0.8 | 11.8 | −0.2 | 12.3 |
| GC-ML-DG-body | −1.1 | 14.9 | −0.7 | 16.0 |
| molecular_layer_HP-body | 7.4 | 25.0 | 11.3 | 23.9 |
| fimbria | 0.1 | 17.0 | 0.1 | 19.5 |
| Hippocampal_tail | 0.7 | 57.0 | 0.9 | 56.0 |
| hippocampal-fissure | −3.0 | 18.5 | −3.6 | 19.9 |
| Whole_hippocampal_body | −10.6 | 74.5 | −13.8 | 70.6 |
| Whole_hippocampal_head | 13.9 | 107.0 | 13.5 | 99.5 |
| Whole_hippocampus | 4.0 | 194.9 | 0.6 | 181.9 |
| Region | T2 TSE a vs. T2 TSE DRB b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right Hippocampus | Left Hippocampus | |||||||||
| Mean Volume T2 TSE (mm3) | Mean Volume T2 TSE DRB (mm3) | Mean Volume Difference (mm3) | SD c (mm3) | p-Value | Mean Volume T2 TSE (mm3) | Mean Volume T2 TSE DRB (mm3) | Mean Volume Difference (mm3) | SD c (mm3) | p-Value | |
| Parasubiculum | 63.9 | 64 | 0.2 | 1.6 | 1 | 66.9 | 65.9 | −1 | 2.1 | 0.726 |
| Presubiculum-Head | 124.4 | 123.5 | −0.9 | 4 | 1 | 128.6 | 129.5 | 0.9 | 5.9 | 1 |
| Subiculum-Head | 186.4 | 185.8 | −0.6 | 4.2 | 1 | 188.4 | 188.3 | −0.04 | 4.2 | 0.958 |
| CA1-Head | 566 | 569.9 | 3.9 | 9.6 | 0.55 | 556.8 | 559.2 | 2.4 | 9.6 | 1 |
| CA2/3-Head | 137.3 | 136.9 | −0.4 | 3.7 | 1 | 130 | 128.7 | −1.3 | 3.5 | 0.871 |
| CA4-Head | 141.2 | 139.7 | −1.5 | 2.9 | 0.158 | 134.9 | 134.6 | −0.2 | 3.9 | 1 |
| GC-ML-DG-head | 174.2 | 172.5 | −1.7 | 3.5 | 0.234 | 166.8 | 166.7 | −0.1 | 4.7 | 1 |
| molecular_layer_HP-head | 323.5 | 324.6 | 1.1 | 10.3 | 1 | 341 | 337.6 | −3.4 | 11.3 | 1 |
| HATA | 66.1 | 65.4 | −0.6 | 1.9 | 1 | 67.4 | 64.6 | −2.8 | 12.8 | 1 |
| Presubiculum-body | 139.3 | 140.2 | 0.9 | 3.9 | 1 | 157.2 | 158.8 | 1.5 | 4.7 | 1 |
| Subiculum-body | 232.9 | 232.7 | −0.3 | 4.3 | 1 | 250.1 | 249.3 | −0.8 | 6 | 1 |
| CA1-Body | 133.2 | 135.2 | 2 | 2.6 | 0.003 | 130.1 | 128.9 | −1.1 | 4.3 | 1 |
| CA2/3-body | 92.4 | 91.5 | −0.9 | 4 | 1 | 89.2 | 87 | −2.2 | 4.1 | 0.126 |
| CA4-body | 116.1 | 113.1 | −3 | 3.3 | 0.0002 | 116.3 | 114.8 | −1.5 | 3.5 | 0.55 |
| GC-ML-DG-body | 129.6 | 130.5 | 0.9 | 20.5 | 1 | 130.8 | 130.2 | −0.6 | 4.5 | 1 |
| molecular_layer_HP-body | 250 | 248.2 | −1.8 | 23.3 | 1 | 239.9 | 238.2 | −1.8 | 6.9 | 1 |
| fimbria | 73 | 74.3 | 1.4 | 3.4 | 0.681 | 72.6 | 74.9 | 2.3 | 4.1 | 0.079 |
| Hippocampal_tail | 564.5 | 564.7 | 0.2 | 6.4 | 1 | 561.7 | 563.1 | 1.3 | 6.4 | 1 |
| hippocampal-fissure | 138.8 | 138.5 | −0.4 | 4.3 | 1 | 135.6 | 137.5 | 1.9 | 17.1 | 1 |
| Whole_hippocampal_body | 1166.5 | 1158.7 | −7.8 | 13.8 | 0.012 | 1186.2 | 1182 | −4.2 | 15.7 | 0.491 |
| Whole_hippocampal_head | 1782.9 | 1782.5 | −0.4 | 19.1 | 0.897 | 1778.6 | 1777.1 | −1.5 | 19.9 | 1 |
| Whole_hippocampus | 3513.9 | 3505.9 | −8 | 27.8 | 0.487 | 3526.5 | 3522.1 | −4.3 | 33.6 | 1 |
| Region | Right Hippocampus vs. Left Hippocampus | |
|---|---|---|
| T2 TSE a | T2 TSE a DRB c | |
| p-Value | ||
| Parasubiculum | 0.927 | 0.774 |
| Presubiculum-Head | 1 | 1 |
| Subiculum-Head | 1 | 1 |
| CA1-Head | 1 | 1 |
| CA2/3-Head | 1 | 0.929 |
| CA4-Head | 0.76 | 1 |
| GC-ML-DG-head | 1 | 1 |
| molecular_layer_HP-head | 1 | 1 |
| HATA | 1 | 1 |
| Presubiculum-body | 0.018 | 0.045 |
| Subiculum-body | 0.004 | 0.117 |
| CA1-Body | 1 | 1 |
| CA2/3-body | 1 | 1 |
| CA4-body | 1 | 1 |
| GC-ML-DG-body | 1 | 1 |
| molecular_layer_HP-body | 1 | 1 |
| fimbria | 1 | 1 |
| Hippocampal_tail | 1 | 1 |
| hippocampal-fissure | 1 | 1 |
| Whole_hippocampal_body | 1 | 1 |
| Whole_hippocampal_head | 1 | 1 |
| Whole_hippocampus | 0.988 | 1 |
| Region | Patient 01—Hippocampal Sclerosis Right | |||
|---|---|---|---|---|
| Right Hippocampus (mm3) | Left Hippocampus (mm3) | RH *-LH † (mm3) | z-Value | |
| Parasubiculum | 33.0 | 53.7 | −20.7 | −0.9 |
| Presubiculum-Head | 70.6 | 108.1 | −37.6 | −1.9 |
| Subiculum-Head | 101.7 | 148.1 | −46.4 | −2.3 |
| CA1-Head | 251.1 | 437.4 | −186.3 | −5.4 |
| CA2/3-Head | 62.8 | 99.8 | −37.0 | −2.6 |
| CA4-Head | 53.4 | 99.9 | −46.5 | −3.7 |
| GC-ML-DG-head | 67.3 | 123.1 | −55.8 | −3.3 |
| molecular_layer_HP-head | 151.9 | 251.8 | −99.9 | −3.9 |
| HATA | 44.7 | 53.3 | −8.6 | −1.1 |
| Presubiculum-body | 73.2 | 132.1 | −58.9 | −1.8 |
| Subiculum-body | 121.9 | 216.7 | −94.8 | −4.5 |
| CA1-Body | 67.2 | 130.3 | −63.1 | −3.1 |
| CA2/3-body | 39.2 | 82.3 | −43.1 | −3.1 |
| CA4-body | 40.6 | 103.4 | −62.7 | −5.2 |
| GC-ML-DG-body | 46.7 | 117.4 | −70.7 | −4.7 |
| molecular_layer_HP-body | 145.8 | 239.4 | −93.7 | −4.0 |
| fimbria | 24.4 | 32.9 | −8.5 | −0.5 |
| Hippocampal_tail | 288.5 | 486.2 | −197.6 | −3.5 |
| hippocampal-fissure | 101.1 | 126.3 | −25.2 | −1.2 |
| Whole_hippocampal_body | 559.2 | 1054.5 | −495.3 | −6.5 |
| Whole_hippocampal_head | 836.5 | 1375.2 | −538.8 | −5.2 |
| Whole_hippocampus | 1684.2 | 2915.9 | −1231.8 | −6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clodi, N.; Bender, B.; Hecke, G.; Hauptvogel, K.; Gohla, G.; Hauser, T.-K.; Ghibes, P.; Hergan, K.; Ernemann, U.; Estler, A. Enhancing Hippocampal Subfield Visualization Through Deep Learning Reconstructed MRI Scans. Diagnostics 2025, 15, 1523. https://doi.org/10.3390/diagnostics15121523
Clodi N, Bender B, Hecke G, Hauptvogel K, Gohla G, Hauser T-K, Ghibes P, Hergan K, Ernemann U, Estler A. Enhancing Hippocampal Subfield Visualization Through Deep Learning Reconstructed MRI Scans. Diagnostics. 2025; 15(12):1523. https://doi.org/10.3390/diagnostics15121523
Chicago/Turabian StyleClodi, Nikolaus, Benjamin Bender, Gretha Hecke, Karolin Hauptvogel, Georg Gohla, Till-Karsten Hauser, Patrick Ghibes, Klaus Hergan, Ulrike Ernemann, and Arne Estler. 2025. "Enhancing Hippocampal Subfield Visualization Through Deep Learning Reconstructed MRI Scans" Diagnostics 15, no. 12: 1523. https://doi.org/10.3390/diagnostics15121523
APA StyleClodi, N., Bender, B., Hecke, G., Hauptvogel, K., Gohla, G., Hauser, T.-K., Ghibes, P., Hergan, K., Ernemann, U., & Estler, A. (2025). Enhancing Hippocampal Subfield Visualization Through Deep Learning Reconstructed MRI Scans. Diagnostics, 15(12), 1523. https://doi.org/10.3390/diagnostics15121523






