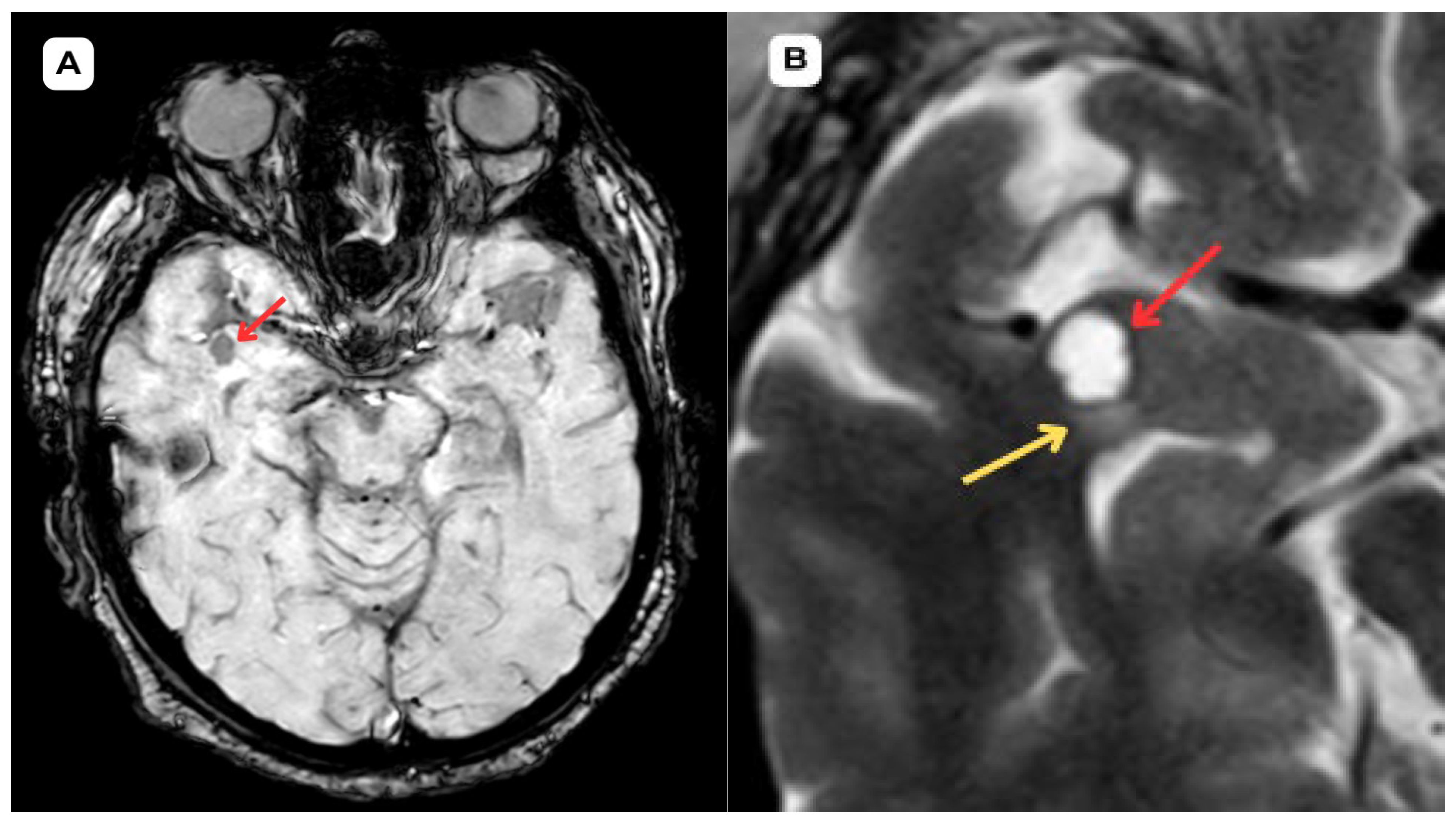
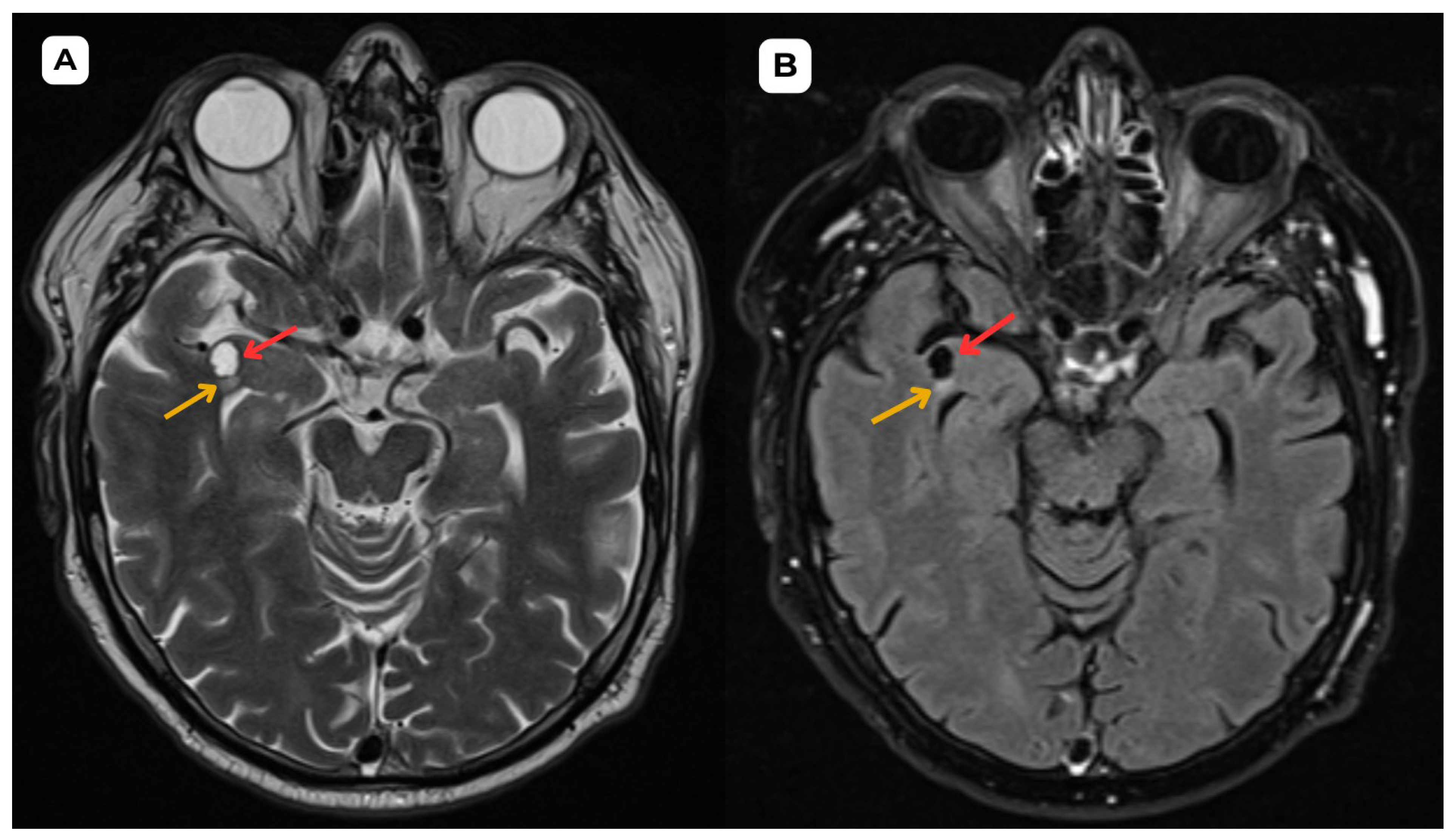
Opercular Perivascular Space Mimicking a Space-Occupying Brain Lesion: A Short Case Series
Abstract
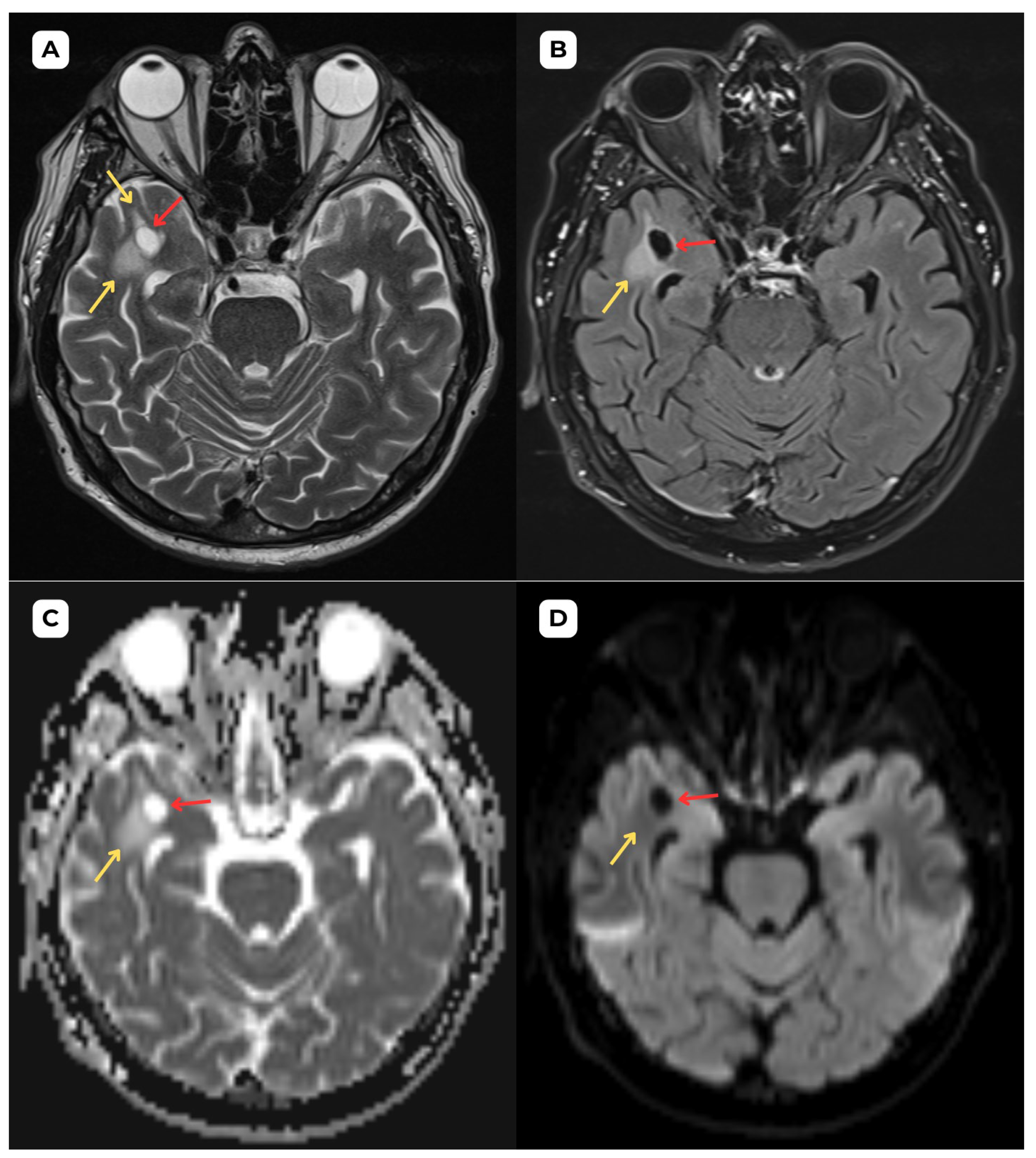
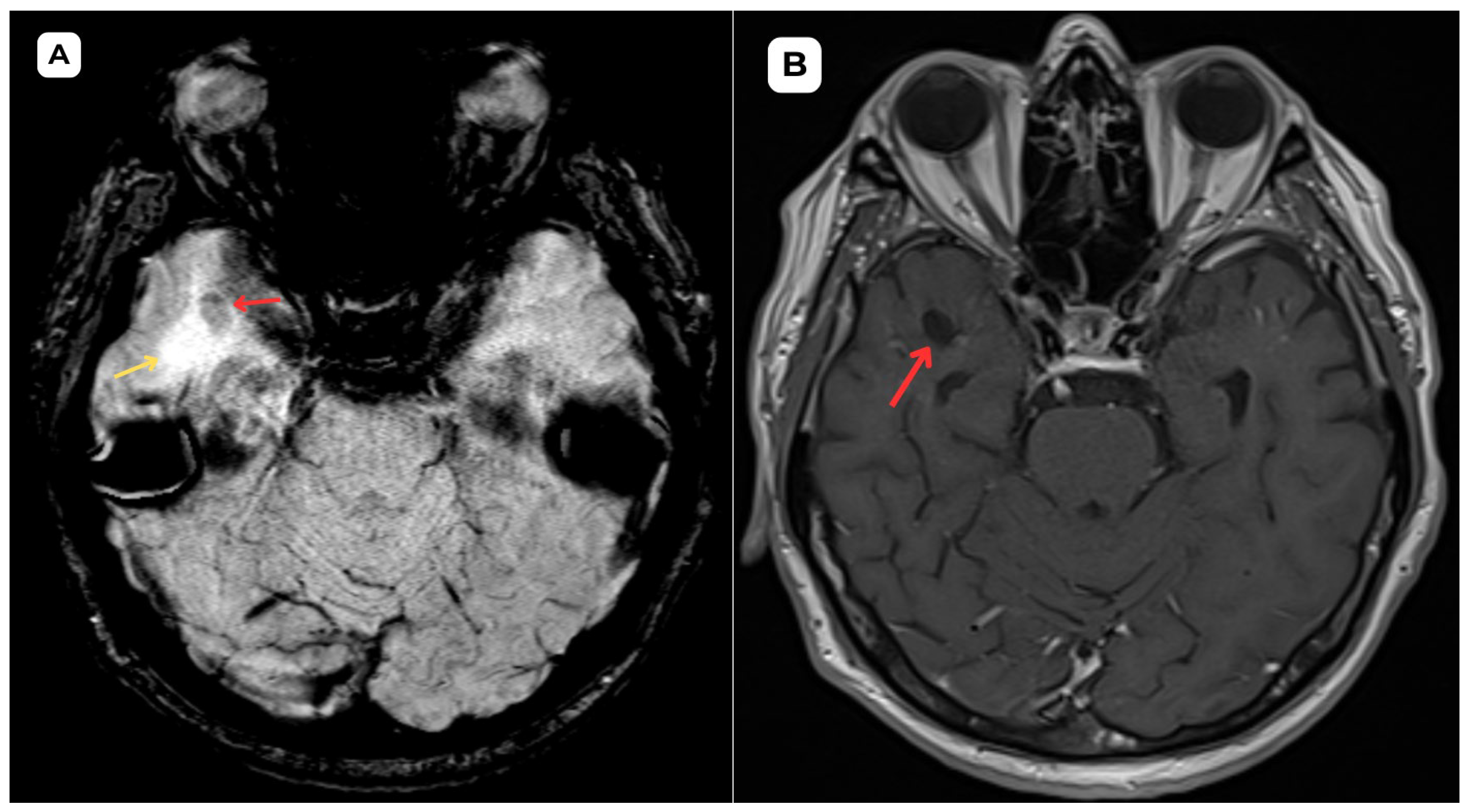
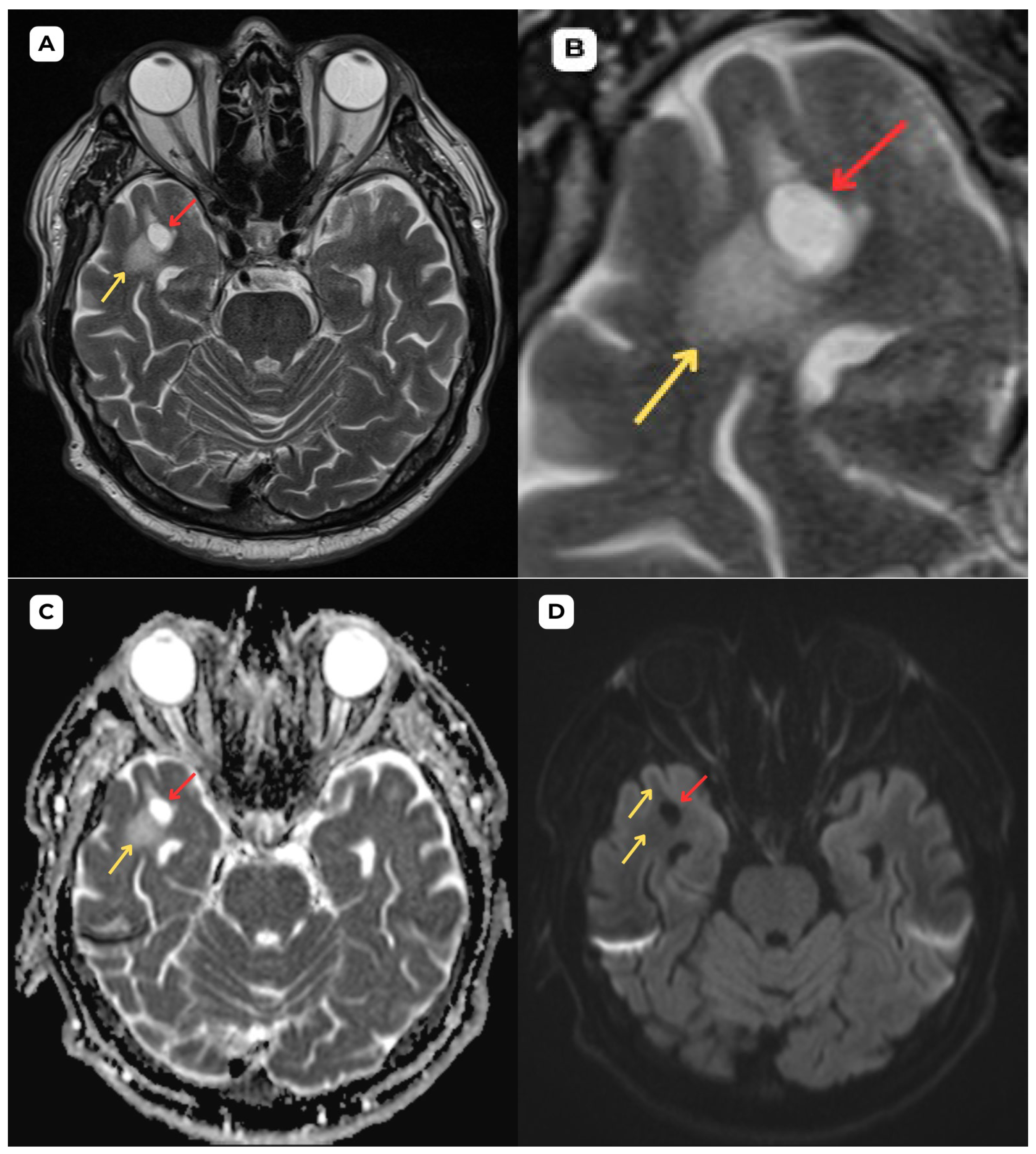
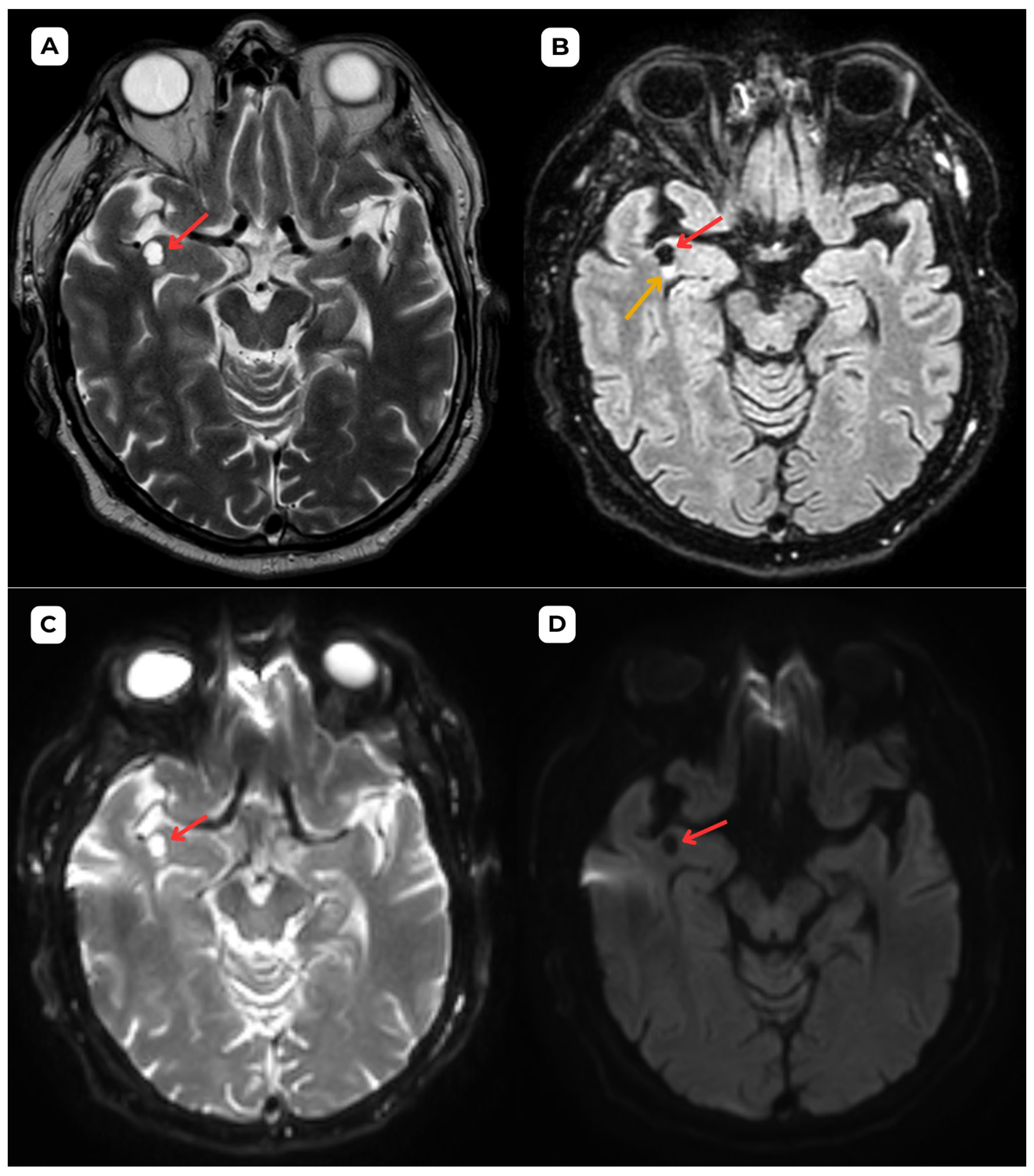
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McArdle, D.J.T.; Lovell, T.J.H.; Lekgabe, E.; Gaillard, F. Opercular perivascular cysts: A proposed new subtype of dilated perivascular spaces. Eur. J. Radiol. 2020, 124, 108838. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Negro, A.; Cirillo, S.; Iovine, S.; Puoti, G.; Cirillo, M.; Conforti, R. Large anterior temporal Virchow–Robin spaces: Evaluating MRI features over the years—Our experience and literature review. Clin. Transl. Neurosci. 2020, 4, 2514183X2090525. [Google Scholar] [CrossRef]
- Doubal, F.N.; MacLullich, A.M.J.; Ferguson, K.J.; Dennis, M.S.; Wardlaw, J.M. Enlarged Perivascular Spaces on MRI are a Feature of Cerebral Small Vessel Disease. Stroke 2010, 41, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.T.; Chandra, R.V.; Trost, N.M.; McKelvie, P.A.; Stuckey, S.L. Large anterior temporal Virchow-Robin spaces: Unique MR imaging features. Neuroradiology 2015, 57, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Page, I.; McArdle, D.J.T.; Gaillard, F. Opercular Perivascular Cyst: Old Entity, New Location. Neurographics 2021, 11, 186–188. [Google Scholar] [CrossRef]
- Yu, L.; Hu, X.; Li, H.; Zhao, Y. Perivascular Spaces, Glymphatic System and MR. Front. Neurol. 2022, 13, 844938. [Google Scholar] [CrossRef] [PubMed]
- Conforti, R.; Capasso, R.; Franco, D.; Russo, C.; Rinaldi, F.O.; Pezzullo, G.; Coluccino, S.; Brunese, M.C.; Caiazzo, C.; Caranci, F.; et al. Giant Tumefactive Perivascular Space: Advanced Fusion MR Imaging and Tractography Study—A Case Report and a Systematic Review. Diagnostics 2023, 13, 1602. [Google Scholar] [CrossRef] [PubMed]
- Guillevin, R.; Herpe, G.; Verdier, M.; Guillevin, C. Low-grade gliomas: The challenges of imaging. Diagn. Interv. Imaging 2014, 95, 957–963. [Google Scholar] [CrossRef] [PubMed]
- de la Gala, D.H.; Casagranda, S.; Mathon, B.; Mandonnet, E.; Nichelli, L. High perilesional T2-FLAIR signal around anterior temporal perivascular spaces: How can fluid suppressed Amide Proton Transfer weighted imaging further comfort the diagnosis. Magn. Reson. Imaging 2023, 103, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Cheraya, G.; Bajaj, S.; Sharma, S.; Gandhi, D.; Shah, J.; Parikh, N.; Gupta, N. Giant perivascular spaces in brain: Case report with a comprehensive literature review. Precis. Cancer Med. 2022, 5, 29. [Google Scholar] [CrossRef]
- Ahmad, M.; Narayanasamy, S.; Siddiqui, M.; Ahmad, I. Giant perivascular spaces: Utility of MR in differentiation from other cystic lesions of the brain. J. Belg. Soc. Radiol. 2014, 97, 364–365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mandonnet, E.; Delattre, J.-Y.; Tanguy, M.-L.; Swanson, K.R.; Carpentier, A.F.; Duffau, H.; Cornu, P.; Van Effenterre, R.; Alvord, E.C., Jr.; Capelle, L. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann. Neurol. 2003, 53, 524–528. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumelkans, R.; Eraslan, C.; Balodis, A. Opercular Perivascular Space Mimicking a Space-Occupying Brain Lesion: A Short Case Series. Diagnostics 2025, 15, 1486. https://doi.org/10.3390/diagnostics15121486
Tumelkans R, Eraslan C, Balodis A. Opercular Perivascular Space Mimicking a Space-Occupying Brain Lesion: A Short Case Series. Diagnostics. 2025; 15(12):1486. https://doi.org/10.3390/diagnostics15121486
Chicago/Turabian StyleTumelkans, Roberts, Cenk Eraslan, and Arturs Balodis. 2025. "Opercular Perivascular Space Mimicking a Space-Occupying Brain Lesion: A Short Case Series" Diagnostics 15, no. 12: 1486. https://doi.org/10.3390/diagnostics15121486
APA StyleTumelkans, R., Eraslan, C., & Balodis, A. (2025). Opercular Perivascular Space Mimicking a Space-Occupying Brain Lesion: A Short Case Series. Diagnostics, 15(12), 1486. https://doi.org/10.3390/diagnostics15121486




