Standardisation and Optimisation of Chest and Pelvis X-Ray Imaging Protocols Across Multiple Radiography Systems in a Radiology Department
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Settings and Image Acquisition
2.2. Quantitative Image Quality Assessment
2.3. Visual Image Quality Assessment
2.4. Statistical Analysis Method
3. Results
3.1. Quantitative Image Quality and Radiation Dose Results
3.2. Visual Image Assessment Results
3.3. Statistical Analysis Results
3.3.1. Statistical Comparison of the Radiation Doses
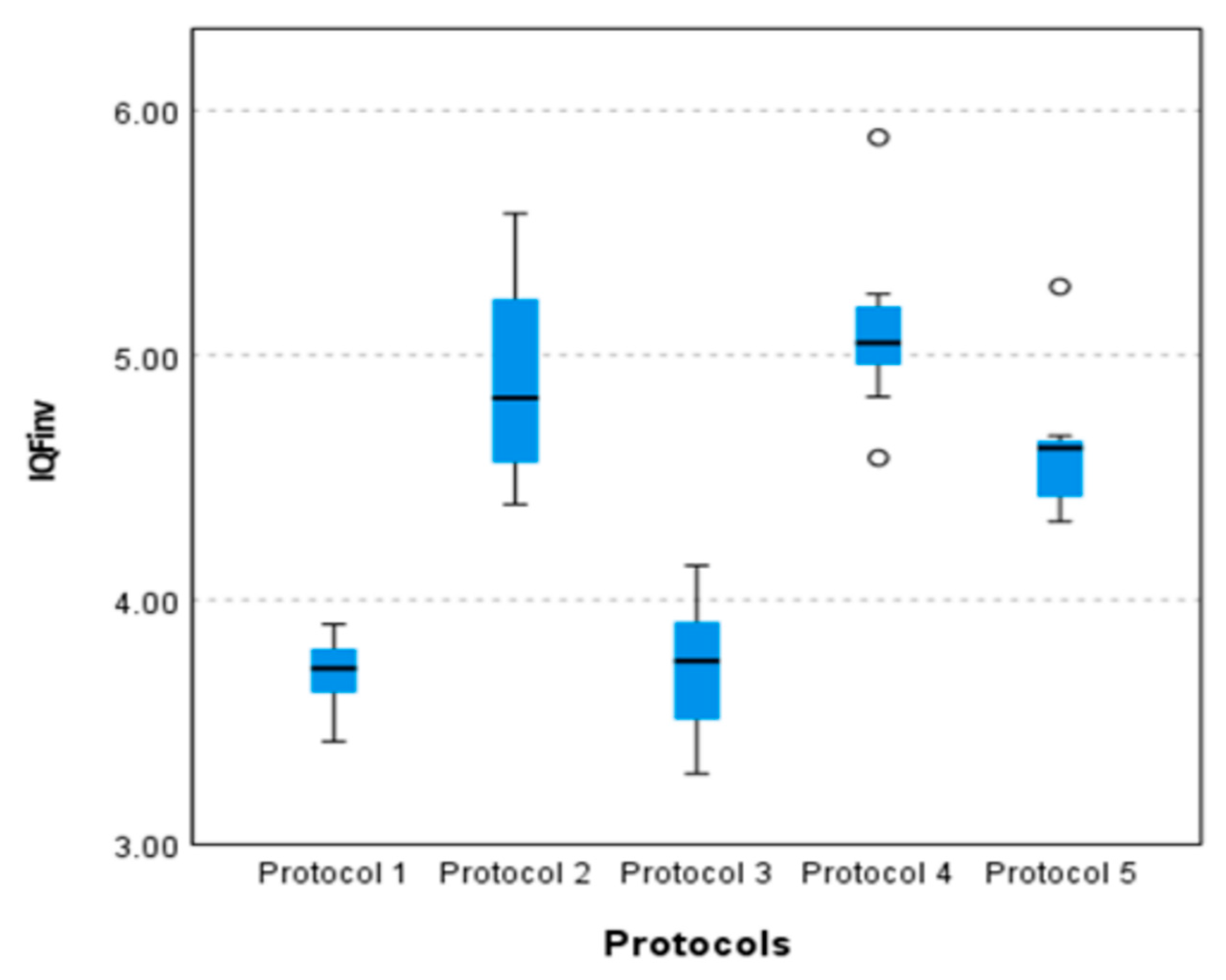
3.3.2. Statistical Comparison of the Quantitative Image Quality Metric, IQFinv
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of ionizing radiation on biological molecules—Mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Feinendegen, L.E.; Pollycove, M.; Sondhaus, C.A. Responses to Low Doses of Ionizing Radiation in Biological Systems. Nonlinearity Biol. Toxicol. Med. 2004, 2, 154014204905074. [Google Scholar] [CrossRef] [PubMed]
- Hoheisel, M. Review of medical imaging with emphasis on X-ray detectors. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip. 2006, 563, 215–224. [Google Scholar] [CrossRef]
- Hussain, S.; Mubeen, I.; Ullah, N.; Shah, S.S.U.D.; Khan, B.A.; Zahoor, M.; Ullah, R.; Khan, F.A.; Sultan, M.A. Modern Diagnostic Imaging Technique Applications and Risk Factors in the Medical Field: A Review. BioMed Res. Int. 2022, 2022, 5164970. [Google Scholar] [CrossRef]
- Oakley, P.A.; Harrison, D.E. Are Continued Efforts to Reduce Radiation Exposures from X-Rays Warranted? Dose-Response 2021, 19, 1559325821995653. [Google Scholar] [CrossRef]
- Wall, B.F.; Kendall, G.M.; Edwards, A.A.; Bouffler, S.; Muirhead, C.R.; Meara, J.R. What are the risks from medical X-rays and other low dose radiation? Br. J. Radiol. 2006, 79, 285–294. [Google Scholar] [CrossRef]
- Mallya, S.M. Effective and Safe Use of X-Rays: Understanding the Risks for Practical Decision-Making. J. Calif. Dent. Assoc. 2021, 49, 301–309. [Google Scholar] [CrossRef]
- Joseph, B.B.; George, S. The road to radiation safety and ALARA: A review. IP Int. J. Maxillofac. Imaging 2021, 6, 89–92. [Google Scholar] [CrossRef]
- Fernandez, R.; International Radiation Protection Association (IRPA); Sociedad Argentina de Radioproteccion (SAR); International Atomic Energy Agency (IAEA); Pan American Health Organization (PAHO); World Health Organization (WHO). The Principle of Optimisation: Reasons for Success and Legal Criticism; SAR: Buenos Aires, Argentina, 2010. [Google Scholar]
- Martin, C.J. Optimisation in general radiography. Biomed. Imaging Interv. J. 2007, 3, e18. [Google Scholar] [CrossRef]
- Vassileva, J. A phantom for dose-image quality optimization in chest radiography. Br. J. Radiol. 2002, 75, 837–842. [Google Scholar] [CrossRef]
- Moore, C.S.; Wood, T.J.; Beavis, A.W.; Saunderson, J.R. Correlation of the clinical and physical image quality in chest radiography for average adults with a computed radiography imaging system. Br. J. Radiol. 2013, 86, 20130077. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Pérez, S.; Marshall, N.W.; Struelens, L.; Bosmans, H. Characterization and validation of the thorax phantom Lungman for dose assessment in chest radiography optimization studies. J. Med. Imaging 2018, 5, 013504. [Google Scholar] [CrossRef] [PubMed]
- Ofori, E.K.; Antwi, W.K.; Scutt, D.N.; Ward, M. Optimization of patient radiation protection in pelvic X-ray examination in Ghana. J. Appl. Clin. Med. Phys. 2012, 13, 160–171. [Google Scholar] [CrossRef]
- Toussie, D.; Voutsinas, N.; Finkelstein, M.; Cedillo, M.A.; Manna, S.; Maron, S.Z.; Jacobi, A.; Chung, M.; Bernheim, A.; Eber, C.; et al. Clinical and chest radiography features determine patient outcomes in young and middle-aged adults with COVID-19. Radiology 2020, 297, E197–E206. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yao, D.; Shi, Y.; Song, Z. Computer-aided detection in chest radiography based on artificial intelligence: A survey. Biomed. Eng. Online 2018, 17, 113. [Google Scholar] [CrossRef]
- Abdi, A.J.; Jensen, K.R.; Madsen, C.G. PS03.08 optimisation and harminisation of image quality and radiation dose in chest X-ray protocols using quantitative image quality evaluation phantom. Phys. Medica 2024, 125, 104107. [Google Scholar] [CrossRef]
- Abdi, A.J.; Mussmann, B.; Mackenzie, A.; Klaerke, B.; Andersen, P.E. Comparison of Radiation Exposure to the Patient and Contrast Detail Resolutions Across Low Dose 2D/3D Slot Scanner and Two Conventional Digital Radiography X-ray Imaging Systems. Radiat. Prot. Dosim. 2019, 185, 252–265. [Google Scholar] [CrossRef]
- Petoussi-Henss, N.; Bolch, W.E.; Eckerman, K.F.; Endo, A.; Hertel, N.; Hunt, J.; Menzel, H.G.; Pelliccioni, M.; Schlattl, H.; Zankl, M. ICRP Publication 116—The first ICRP/ICRU application of the male and female adult reference computational phantoms. Phys. Med. Biol. 2014, 59, 5209. [Google Scholar] [CrossRef]
- Vano, E.; Gonzalez, L.; Fernandez, J.M.; Prieto, C.; Guibelalde, E. Influence of patient thickness and operation modes on occupational and patient radiation doses in interventional cardiology. Radiat. Prot. Dosim. 2006, 118, 325–330. [Google Scholar] [CrossRef]
- Kuramoto, T.; Takarabe, S.; Kanzaki, Y.; Shibayama, Y.; Yamasaki, Y. Correlation Between Contrast-Detail Analysis and Clinical Image Quality Assessment of Intrapulmonary Lesions in Dual-Energy Subtraction Chest Radiography Using the Two- Shot Method: A Phantom Study clinical image quality assessment introduction. Acad. Radiol. 2025, 31, 2118–2127. [Google Scholar] [CrossRef]
- Rama, K.; Esser, M.; Spogis, J.; Wanninger, F.; Hoberg, B.; Radiology, I.; Healthcare, A.; Radiography, T.; Infant, N.; Phantoms, I.; et al. Dose-Optimized Image Acquisition Parameters for Neonatal Chest Radiography: A Phantom Study Comparing Computed Radiography and Wireless Digital Radiography Needle Detectors. RoFo 2025, 186, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Sun, Y.; Li, M. Use of an anthropomorphic chest model to evaluate multiple scanning protocols for high- definition and standard-definition computed tomography to detect small pulmonary nodules. Med. Sci. Monit. 2019, 25, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.C.; McDonnell, S.; O’Leary, D. Increasing film-focus distance (ffd) reduces radiation dose for X-ray examinations. Radiat. Prot. Dosim. 2004, 108, 263–268. [Google Scholar] [CrossRef]
- Svalkvist, A.; Svensson, S.; Håkansson, M.; Båth, M.; Månsson, L.G. VIEWDEX: A STATUS REPORT. Radiat. Prot. Dosim. 2016, 169, 38–45. [Google Scholar] [CrossRef]
- Håkansson, M.; Svensson, S.; Zachrisson, S.; Svalkvist, A.; Båth, M.; Månsson, L.G. VIEWDEX: An Efficient and Easy-To-Use Software for observer performance studies. Radiat. Prot. Dosim. 2010, 139, 42–51. [Google Scholar] [CrossRef]
- Båth, M.; Månsson, L.G. Visual grading characteristics (VGC) analysis: A non-parametric rank-invariant statistical method for image quality evaluation. Br. J. Radiol. 2007, 80, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.J.; Mussmann, B.; Mackenzie, A.; Gerke, O.; Jørgensen, G.M.; Bechsgaard, T.E.; Jensen, J.; Olsen, L.B.; Andersen, P.E. Visual Evaluation of Image Quality of a Low Dose 2D/3D Slot Scanner Imaging System Compared to Two Conventional Digital Radiography X-ray Imaging Systems. Diagnostics 2021, 11, 1932. [Google Scholar] [CrossRef]
- Precht, H.; Hansson, J.; Outzen, C.; Hogg, P.; Tingberg, A. Radiographers’ perspectives’ on Visual Grading Analysis as a scientific method to evaluate image quality. Radiography 2019, 25, S14–S18. [Google Scholar] [CrossRef]
- Yvert, M.; Diallo, A.; Bessou, P.; Rehel, J.L.; Lhomme, E.; Chateil, J.F. Radiography of scoliosis: Comparative dose levels and image quality between a dynamic flat-panel detector and a slot-scanning device (EOS system). Diagn. Interv. Imaging 2015, 96, 1177–1188. [Google Scholar] [CrossRef]
- Doktor, K.; Vilholm, M.L.; Hardardóttir, A.; Christensen, H.W.; Lauritsen, J. European guidelines on quality criteria for diagnostic radiographic images of the lumbar spine—An intra- and inter-observer reproducibility study. Chiropr. Man. Ther. 2019, 27, 2–7. [Google Scholar] [CrossRef]
- European Commission. European Guidelines on Quality Criteria for Diagnostic Radiographic Images; EU Publications: Luxembourg, 1996; ISBN 9282772845. [Google Scholar]
- Abdi, A.J.; Mussmann, B.R.; Mackenzie, A.; Gerke, O.; Klaerke, B.; Andersen, P.E. Quantitative Image Quality Metrics of the Low-Dose 2D/3D Slot Scanner Compared to Two Conventional Digital Radiography X-ray Imaging Systems. Diagnostics 2021, 11, 1699. [Google Scholar] [CrossRef] [PubMed]
- Almen, A.; Tingberg, A.; Mattsson, S.; Besjakov, J.; Kheddache, S.; Lanhede, B.; Mansson, L.G.; Zankl, M. The influence of different technique factors on image quality of lumbar spine radiographs as evaluated by established CEC image criteria. Br. J. Radiol. 2000, 73, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Sandborg, M.; Dance, D.R.; Carlsson, G.A.; Persliden, J. Monte Carlo study of grid performance in diagnostic radiology: Factors which affect the selection of tube potential and grid ratio. Br. J. Radiol. 1993, 66, 1164–1176. [Google Scholar] [CrossRef]
- Precht, H.; Tingberg, A.; Waaler, D.; Outzen, C.B. New developed DR detector performs radiographs of hand, pelvic and premature chest anatomies at a lower radiation dose and/or a higher image quality. J. Digit. Imaging 2014, 27, 68–76. [Google Scholar] [CrossRef]
- Barba, J.; Culp, M. Copper filtration and kVp: Effect on entrance skin exposure. Radiol. Technol. 2015, 86, 603–609. [Google Scholar]
- Brosi, P.; Stuessi, A.; Verdun, F.R.; Vock, P.; Wolf, R. Copper filtration in pediatric digital X-ray imaging: Its impact on image quality and dose. Radiol. Phys. Technol. 2011, 4, 148–155. [Google Scholar] [CrossRef]
- Seeram, E. Digital Radiography: An Introduction for Technologists; Delmar Learning: Clifton Park, NY, USA, 2010. [Google Scholar]
- Sundhedsstyrelsen Vejledning om patientdoser og referencedoser for røntgenundersøgelser Konventionelle røntgenundersøgelser. Sundhedsstyrelsen 2012, 2. Available online: https://www.sst.dk/-/media/Udgivelser/2012/Vejledning-om-patientdoser-og-referencedoser-for-røntgenundersøgelser.ashx (accessed on 1 June 2025).
- Precht, H.; Hansen, D.L.; Ring-Pedersen, B.M.; Møller Hansen, L.F.; Waaler, D.; Tingberg, A.; Midtgaard, M.; Jensen Ohlsen, M.G.; Juhl Hankelbjerg, S.T.; Ravn, P.; et al. Comparison of image quality in chest, hip and pelvis examinations between mobile equipment in nursing homes and static indirect radiography equipment in the hospital. Radiography 2020, 26, e31–e37. [Google Scholar] [CrossRef]
- Peacock, N.E.; Steward, A.L.; Riley, P.J. An evaluation of the effect of tube potential on clinical image quality using direct digital detectors for pelvis and lumbar spine radiographs. J. Med. Radiat. Sci. 2020, 67, 260–268. [Google Scholar] [CrossRef]

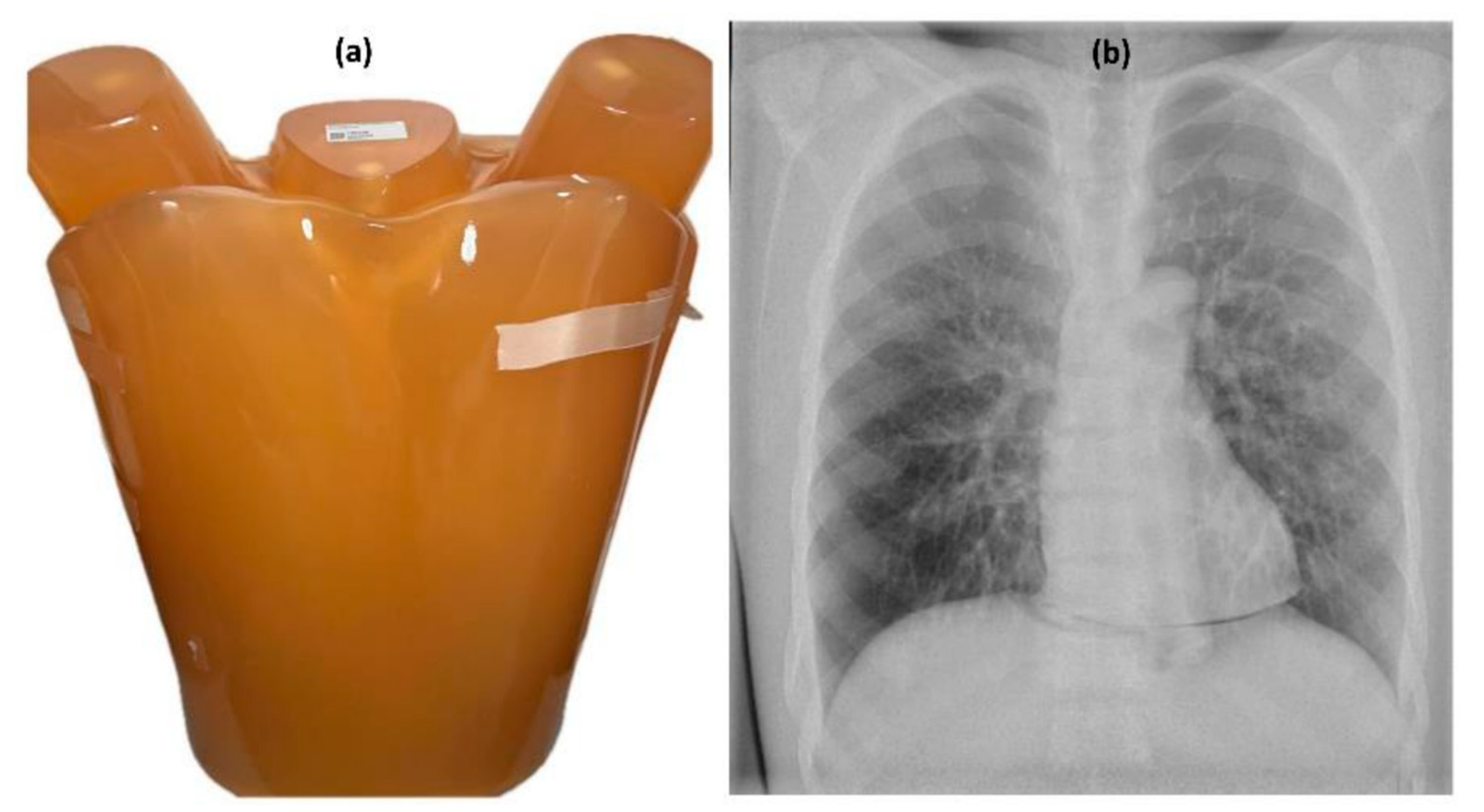
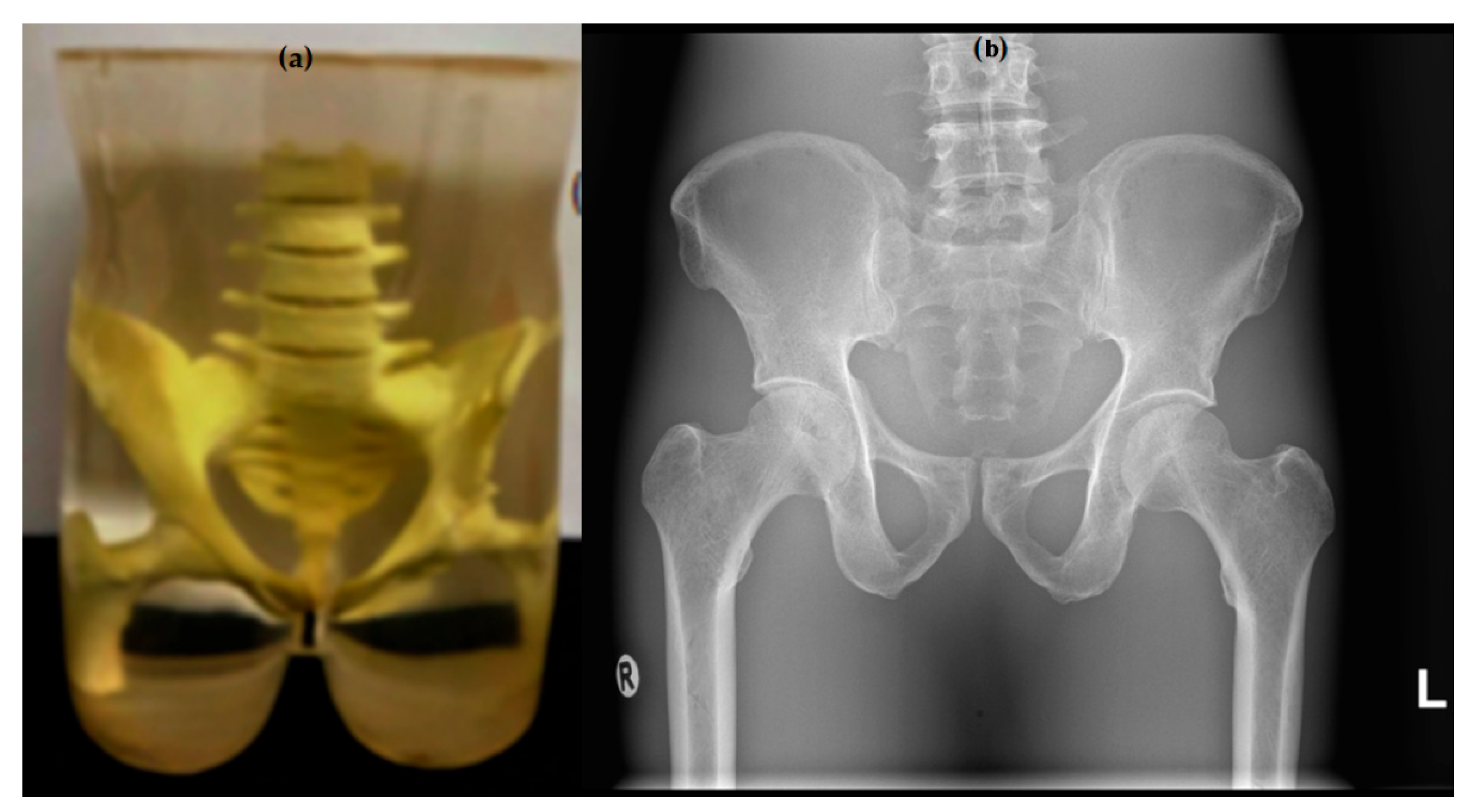
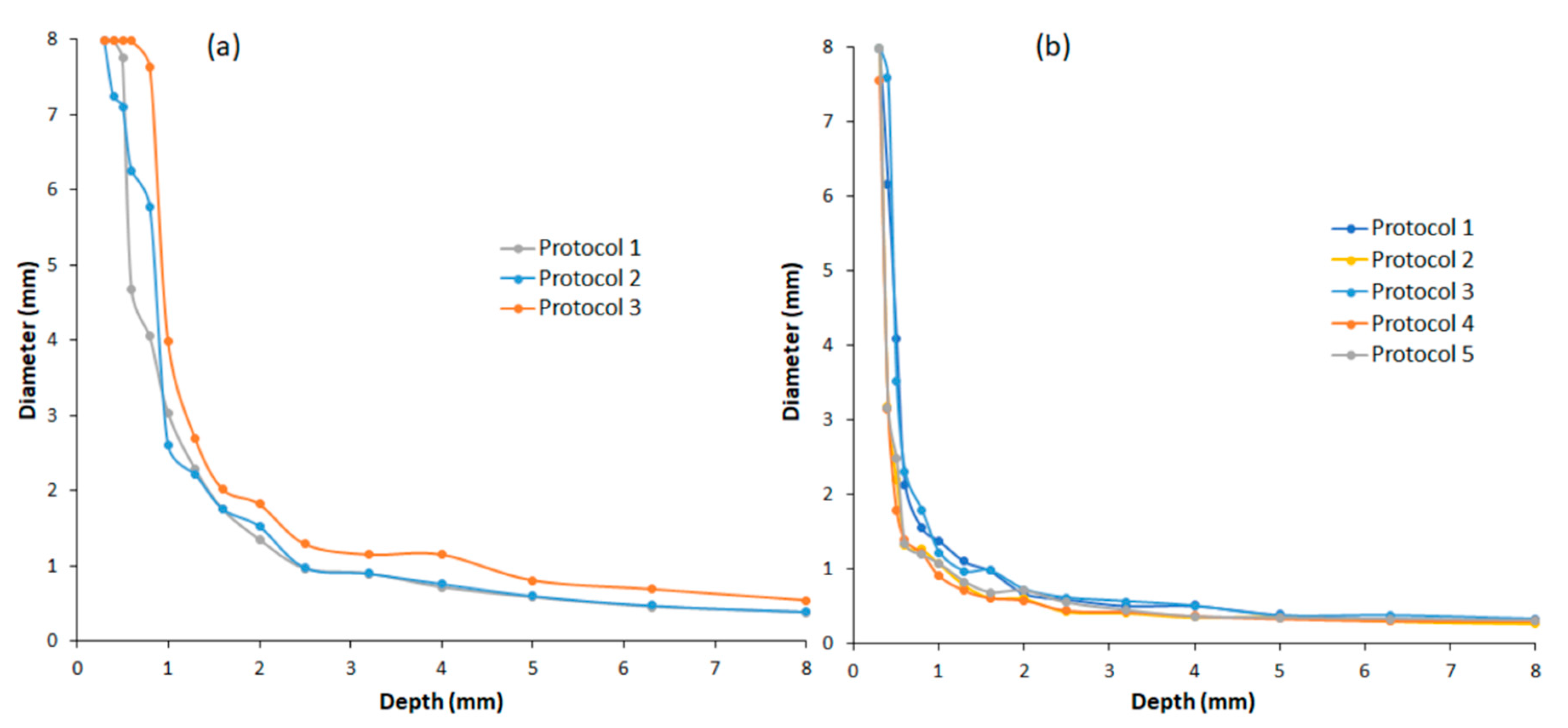

| Parameters | Specifications |
|---|---|
| Tube voltage (kV) | 40–150 |
| Tube current max (mA) | 1000 |
| Tube load (mAs) | 0.5–800 |
| Exposure time (s) | 0.001–5 |
| Generator power (kW) | 80 |
| Focal spot (mm) | 0.6/1.0 |
| Target angle (degree) | 12 |
| Detector type | CsI, a-Si TFT |
| Detector size (inch) | 14 × 17 |
| Detector pitch (μm) | 148 |
| Active matrix (pixel) | 2354 × 2872 |
| DQE at 1 lp/mm | 50% |
| MTF at 1 lp/mm | 61% |
| # Protocol | Tube Voltage (kVp) | Tube Load (mAs) | Tube Current (mA) | Filtration (Cu) | Dose/Speed | SID (cm) | Protocols |
|---|---|---|---|---|---|---|---|
| Protocol 1 | 125 | 2 | 621 | 0.0 | 2.50 | 180 | Chest |
| Protocol 2 | 125 | 2 | 686 | 0.2 | 2.50 | 180 | |
| Protocol 3 | 150 | 2 | 594 | 0.3 | 3.57 | 300 | |
| Protocol 1 | 81 | 5 | 831 | 0.0 | 2.50 | 115 | Pelvis |
| Protocol 2 | 81 | 12 | 897 | 0.1 | 5.00 | 115 | |
| Protocol 3 | 81 | 8 | 926 | 0.2 | 2.50 | 115 | |
| Protocol 4 | 81 | 15 | 844 | 0.2 | 5.00 | 115 | |
| Protocol 5 | 75 | 20 | 805 | 0.3 | 3.57 | 115 |
| # Protocol | Tube Voltage (kVp) | Filtration (Cu) | Dose/Speed | SID (cm) | Protocol |
|---|---|---|---|---|---|
| Protocol 1 | 125 | 0.00 | 2.50 | 180 | chest |
| Protocol 2 | 125 | 0.20 | 2.50 | 180 | |
| Protocol 3 | 150 | 0.30 | 3.57 | 300 | |
| Protocol 1 | 81 | 0.00 | 2.50 | 115 | pelvis |
| Protocol 3 | 81 | 0.20 | 2.50 | 115 | |
| Protocol 5 | 75 | 0.30 | 3.57 | 115 |
| Scale | Description | Interpretation |
|---|---|---|
| 1 | Poor image quality | The image is not usable and causes loss of information |
| 2 | Limited image quality | Limited relevance for clinical use and significant loss of information |
| 3 | Adequate image quality | Moderate restrictions for clinical use without significant loss of info |
| 4 | Good image quality | Minimal limitations for clinical use |
| 5 | Excellent image quality | No restrictions on clinical use |
| # Criteria | Criteria (Visualisation of) |
|---|---|
| 1 | Pulmonary vasculature and peripheral vessels (PA—Posterior–Anterior, an X-ray taken from the back to the front of the body). |
| 2 | Trachea and proximal bronchi. |
| 3 | Borders of the heart and the aorta. |
| 4 | Diaphragm and lateral costophrenic angles (the areas where the rib cage meets the diaphragm). |
| 5 | The spine is visible through the shadow of the heart. |
| # Criteria | Criteria |
|---|---|
| 1 | Symmetrical reproduction of the pelvis. |
| 2 | Visualisation of the sacrum and its intervertebral spaces. |
| 3 | Visualisation of sacroiliac joints. |
| 4 | Reproduction of the femoral neck, which should not be distorted by shortening or rotation. |
| 5 | Reproduction of spongiosa and corticalis as well as visualisation of the trochanters. |
| # Protocol | Tube Voltage (kV) | Tube Current (mA) | Filtration (Cu) | Dose/Speed | SID (cm) | IQFinv | DAP (dGycm2) | Protocols |
|---|---|---|---|---|---|---|---|---|
| Protocol 1 | 125 | 621 | 0.00 | 2.50 | 180 | 2.26 | 0.75 | Chest |
| Protocol 2 | 125 | 686 | 0.20 | 2.50 | 180 | 2.17 | 0.46 | |
| Protocol 3 | 150 | 594 | 0.30 | 3.75 | 300 | 1.69 | 0.43 | |
| Protocol 1 | 81 | 831 | 0.00 | 2.50 | 115 | 3.72 | 2.98 | Pelvis |
| Protocol 2 | 81 | 897 | 0.10 | 5.00 | 115 | 4.87 | 4.28 | |
| Protocol 3 | 81 | 926 | 0.20 | 2.50 | 115 | 3.63 | 1.78 | |
| Protocol 4 | 81 | 844 | 0.20 | 5.00 | 115 | 4.97 | 3.63 | |
| Protocol 5 | 75 | 805 | 0.30 | 3.75 | 115 | 4.63 | 2.89 |
| # Protocol | Criteria/VGA | Mean VGA | Raters | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Protocol 1 | 4.70 | 4.90 | 5.00 | 5.00 | 4.50 | 4.82 | R1 |
| Protocol 2 | 4.90 | 4.90 | 5.00 | 5.00 | 4.80 | 4.92 | |
| Protocol 3 | 4.90 | 4.90 | 4.80 | 5.00 | 4.60 | 4.84 | |
| Protocol 1 | 5.00 | 4.90 | 5.00 | 5.00 | 4.90 | 4.96 | R2 |
| Protocol 2 | 4.90 | 4.80 | 4.70 | 5.00 | 4.60 | 4.80 | |
| Protocol 3 | 4.80 | 4.50 | 4.90 | 5.00 | 4.40 | 4.72 | |
| # Protocols | Criteria/VGA | Mean VGA | Raters | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Protocol 1 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | R1 |
| Protocol 3 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | |
| Protocol 5 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | |
| Protocol 1 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | R2 |
| Protocol 3 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | |
| Protocol 5 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | |
| Protocol Settings | Test Statistic | Std. Error | Std. Test Statistic | p-Values | Protocols |
|---|---|---|---|---|---|
| Protocol 3 vs. Protocol 2 | 8.00 | 3.878 | 2.06 | 0.039 | Chest |
| Protocol 3 vs. Protocol 1 | 19.00 | 3.878 | 4.90 | <0.001 | |
| Protocol 2 vs. Protocol 1 | 11.00 | 3.878 | 2.84 | 0.005 | |
| Protocol 3 vs. Protocol 5 | −10.00 | 6.39 | −1.57 | 0.117 | Pelvis |
| Protocol 3 vs. Protocol 1 | 20.00 | 6.39 | 3.13 | 0.002 | |
| Protocol 3 vs. Protocol 4 | −29.50 | 6.56 | −4.50 | <0.001 | |
| Protocol 3 vs. Protocol 2 | 39.00 | 6.39 | 6.11 | <0.001 | |
| Protocol 5 vs. Protocol 1 | 10.00 | 6.39 | 1.57 | 0.117 | |
| Protocol 5 vs. Protocol 4 | 19.50 | 6.56 | 2.97 | 0.003 | |
| Protocol 5 vs. Protocol 2 | 29.00 | 6.39 | 4.54 | <0.001 | |
| Protocol 1 vs. Protocol 4 | −9.50 | 6.56 | −1.45 | 0.148 | |
| Protocol 1 vs. Protocol 2 | −19.00 | 6.39 | −2.97 | 0.003 | |
| Protocol 4 vs. Protocol 2 | 9.50 | 6.56 | 1.45 | 0.148 |
| Protocols | Test Statistic | Std. Error | Std. Test Statistic | p-Values |
|---|---|---|---|---|
| Protocol 3 vs. Protocol 2 | 12.45 | 3.93 | 3.16 | 0.002 |
| Protocol 3 vs. Protocol 1 | 17.25 | 3.93 | 4.38 | <0.001 |
| Protocol 2 vs. Protocol 1 | 4.80 | 3.93 | 1.22 | 0.220 |
| Protocol Settings | Test Statistic | Std. Error | Std. Test Statistic | p-Values |
|---|---|---|---|---|
| Protocol 1 vs. Protocol 3 | −0.40 | 6.39 | −0.06 | 0.950 |
| Protocol 1 vs. Protocol 5 | −19.20 | 6.39 | −3.00 | 0.003 |
| Protocol 1 vs. Protocol 2 | −25.90 | 6.39 | −4.05 | <0.001 |
| Protocol 1 vs. Protocol 4 | −29.48 | 6.56 | −4.49 | <0.001 |
| Protocol 3 vs. Protocol 5 | −18.80 | 6.39 | −2.94 | 0.003 |
| Protocol 3 vs. Protocol 2 | 25.50 | 6.39 | 3.99 | <0.001 |
| Protocol 3 vs. Protocol 4 | −29.08 | 6.56 | −4.43 | <0.001 |
| Protocol 5 vs. Protocol 2 | 6.70 | 6.39 | 1.05 | 0.294 |
| Protocol 5 vs. Protocol 4 | 10.28 | 6.56 | 1.57 | 0.117 |
| Protocol 2 vs. Protocol 4 | −3.58 | 6.56 | −0.54 | 0.586 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdi, A.J.; Jensen, K.R.; Pietersen, P.I.; Jensen, J.; Hovgaard, R.L.; Holmboe, A.K.A.; Gregersen, S. Standardisation and Optimisation of Chest and Pelvis X-Ray Imaging Protocols Across Multiple Radiography Systems in a Radiology Department. Diagnostics 2025, 15, 1450. https://doi.org/10.3390/diagnostics15121450
Abdi AJ, Jensen KR, Pietersen PI, Jensen J, Hovgaard RL, Holmboe AKA, Gregersen S. Standardisation and Optimisation of Chest and Pelvis X-Ray Imaging Protocols Across Multiple Radiography Systems in a Radiology Department. Diagnostics. 2025; 15(12):1450. https://doi.org/10.3390/diagnostics15121450
Chicago/Turabian StyleAbdi, Ahmed Jibril, Kasper Rørdam Jensen, Pia Iben Pietersen, Janni Jensen, Rune Lau Hovgaard, Ask Kristian Aas Holmboe, and Sofie Gregersen. 2025. "Standardisation and Optimisation of Chest and Pelvis X-Ray Imaging Protocols Across Multiple Radiography Systems in a Radiology Department" Diagnostics 15, no. 12: 1450. https://doi.org/10.3390/diagnostics15121450
APA StyleAbdi, A. J., Jensen, K. R., Pietersen, P. I., Jensen, J., Hovgaard, R. L., Holmboe, A. K. A., & Gregersen, S. (2025). Standardisation and Optimisation of Chest and Pelvis X-Ray Imaging Protocols Across Multiple Radiography Systems in a Radiology Department. Diagnostics, 15(12), 1450. https://doi.org/10.3390/diagnostics15121450







