Preoperative Tunnel Measurement in Hidradenitis Suppurativa: Comparison of Palpation and Ultrasound
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Tunnel Length Measurement Methods
2.4. Data Collection for Secondary Objectives
2.5. Ethical Approval
2.6. Sample Size
2.7. Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HS | Hidradenitis Suppurativa |
| USG | Ultrasound |
| BMI | Body Mass Index |
| IHS4 | International Hidradenitis Suppurativa Severity Score System |
| SOS-HS | Severity of Skin in Hidradenitis Suppurativa |
| ICC | Intraclass Correlation Coefficient |
| LoA | Limits of agreement |
| HiSCR | Hidradenitis Suppurativa Clinical Response |
References
- Sabat, R.; Jemec, G.B.E.; Matusiak, Ł.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis Suppurativa. Nat. Rev. Dis. Primers 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Navrazhina, K.; Frew, J.W.; Gilleaudeau, P.; Sullivan-Whalen, M.; Garcet, S.; Krueger, J.G. Epithelialized Tunnels Are a Source of Inflammation in Hidradenitis Suppurativa. J. Allergy Clin. Immunol. 2021, 147, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Karagaiah, P.; Patil, A.; Farnbach, K.; Ortega-Loayza, A.G.; Tzellos, T.; Szepietowski, J.C.; Giulini, M.; Schepler, H.; Grabbe, S.; et al. Surgical Treatment in Hidradenitis Suppurativa. J. Clin. Med. 2022, 11, 2311. [Google Scholar] [CrossRef]
- Martorell, A.; Giovanardi, G.; Gomez-Palencia, P.; Sanz-Motilva, V. Defining Fistular Patterns in Hidradenitis Suppurativa: Impact on the Management. Dermatol. Surg. 2019, 45, 1237–1244. [Google Scholar] [CrossRef]
- Magalhães, R.F.; Rivitti-Machado, M.C.; Duarte, G.V.; Souto, R.; Nunes, D.H.; Chaves, M.; Hirata, S.H.; Ramos, A.M.C. Consensus on the Treatment of Hidradenitis Suppurativa—Brazilian Society of Dermatology. An. Bras. Dermatol. 2019, 94, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Ezanno, A.-C.; Texier, G.; Marchi, J.; Fougerousse, A.-C. Factors Affecting Wound Healing after the Wide Surgical Excision of Hidradenitis Suppurativa Lesions. J. Clin. Med. 2024, 13, 5598. [Google Scholar] [CrossRef]
- Cuenca-Barrales, C.; Salvador-Rodríguez, L.; Arias-Santiago, S.; Molina-Leyva, A. Pre-Operative Ultrasound Planning in the Surgical Management of Patients with Hidradenitis Suppurativa. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2362–2367. [Google Scholar] [CrossRef]
- Vergilio, M.M.; Monteiro e Silva, S.A.; Jales, R.M.; Leonardi, G.R. High-Frequency Ultrasound as a Scientific Tool for Skin Imaging Analysis. Exp. Dermatol. 2021, 30, 897–910. [Google Scholar] [CrossRef]
- Di Cesare, A.; Rosi, E.; Amerio, P.; Prignano, F. Clinical and Ultrasonographic Characterization of Hidradenitis Suppurativa in Female Patients: Impact of Early Recognition of the Disease. Life 2023, 13, 1630. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Desai, N.; Emtestam, L.; Hunger, R.E.; Ioannides, D.; Juhász, I.; Lapins, J.; Matusiak, L.; Prens, E.P.; Revuz, J.; et al. European S1 Guideline for the Treatment of Hidradenitis Suppurativa/Acne Inversa. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 619–644. [Google Scholar] [CrossRef]
- Hurley, H.; Roenigk, R.; Roenigk, H. Dermatologic Surgery, Principles and Practice; Marcel: New York, NY, USA, 1989. [Google Scholar]
- Zouboulis, C.C.; Tzellos, T.; Kyrgidis, A.; Jemec, G.B.E.; Bechara, F.G.; Giamarellos-Bourboulis, E.J.; Ingram, J.R.; Kanni, T.; Karagiannidis, I.; Martorell, A.; et al. Development and Validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a Novel Dynamic Scoring System to Assess HS Severity. Br. J. Dermatol. 2017, 177, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X.; Moreno, C.; Soto, R.; Arellano, J.; Pezo, C.; Wortsman, J. Ultrasound In-Depth Characterization and Staging of Hidradenitis Suppurativa. Dermatol. Surg. 2013, 39, 1835. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G. Practical Statistics for Medical Research; Chapman and Hall/CRC: New York, NY, USA, 1990; ISBN 978-0-429-25858-9. [Google Scholar]
- Nguyen, T.V.; Damiani, G.; Orenstein, L.A.V.; Hamzavi, I.; Jemec, G.B. Hidradenitis Suppurativa: An Update on Epidemiology, Phenotypes, Diagnosis, Pathogenesis, Comorbidities and Quality of Life. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Saunte, D.M.L.; Jemec, G.B.E. Hidradenitis Suppurativa: Advances in Diagnosis and Treatment. JAMA 2017, 318, 2019–2032. [Google Scholar] [CrossRef]
- Tatliparmak, A.C.; Yilmaz, S. Agreement of Oscillometric and Auscultatory Blood Pressure Measurement Methods: An Ambulance Noise Simulation Study. Am. J. Emerg. Med. 2023, 67, 120–125. [Google Scholar] [CrossRef]
- Doğan, N.Ö. Bland-Altman Analysis: A Paradigm to Understand Correlation and Agreement. Turk. J. Emerg. Med. 2018, 18, 139–141. [Google Scholar] [CrossRef]
- Bilić-Zulle, L. Comparison of Methods: Passing and Bablok Regression. Biochem. Med. 2011, 21, 49–52. [Google Scholar] [CrossRef]
- Martorell, A.; Alfageme Roldán, F.; Vilarrasa Rull, E.; Ruiz-Villaverde, R.; Romaní De Gabriel, J.; García Martínez, F.; Vidal Sarro, D.; Velasco Pastor, M.; Ciudad Blanco, C.; Segura Palacios, J.M.; et al. Ultrasound as a Diagnostic and Management Tool in Hidradenitis Suppurativa Patients: A Multicentre Study. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X.; Calderon, P.; Castro, A. Seventy-MHz Ultrasound Detection of Early Signs Linked to the Severity, Patterns of Keratin Fragmentation, and Mechanisms of Generation of Collections and Tunnels in Hidradenitis Suppurativa. J. Ultrasound Med. 2020, 39, 845–857. [Google Scholar] [CrossRef]
- Gogate, S.; Aggarwal, R.; Sardana, K.; Yadav, S.; Boyidi, B.B.; Siddharth, S.; Sharma, P. A Pilot Real-World Study of Ultrasonography Findings of Hidradenitis Suppurativa in Indian Patients and Its Diagnostic and Therapeutic Implications. Indian J. Radiol. Imaging 2024, 34, 603–611. [Google Scholar] [CrossRef]
- Kumar, P.; Mardon, M.; Bradley, M.; Gray, S.; Swinkels, A. Assessment of Glenohumeral Subluxation in Poststroke Hemiplegia: Comparison between Ultrasound and Fingerbreadth Palpation Methods. Phys. Ther. 2014, 94, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Cessford, T.; Meneilly, G.S.; Arishenkoff, S.; Eddy, C.; Chen, L.Y.C.; Kim, D.J.; Ma, I.W.Y. Comparing Physical Examination With Sonographic Versions of the Same Examination Techniques for Splenomegaly. J. Ultrasound Med. 2018, 37, 1621–1629. [Google Scholar] [CrossRef]
- Ambulkar, R.; Patil, V.; Doctor, J.R.; Desai, M.; Shetty, N.; Agarwal, V. Accuracy of Ultrasound Imaging versus Manual Palpation for Locating the Intervertebral Level. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 348–352. [Google Scholar] [CrossRef]
- Mota, P.; Pascoal, A.G.; Sancho, F.; Carita, A.I.; Bø, K. Reliability of the Inter-Rectus Distance Measured by Palpation. Comparison of Palpation and Ultrasound Measurements. Man. Ther. 2013, 18, 294–298. [Google Scholar] [CrossRef]
- Michelucci, A.; Fidanzi, C.; Manzo Margiotta, F.; Granieri, G.; Salvia, G.; Janowska, A.; Romanelli, M.; Dini, V. Presurgical Mapping With Ultra-High Frequency Ultrasound of Hidradenitis Suppurativa Lesions Treated with Wide Local Excision and Secondary Intention Healing. Dermatol. Surg. 2025, 51, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Sharma, A.; Kumaran, M.S.; Narang, T.; Sinha, A.; Dogra, S. Disease Severity Assessment in Hidradenitis Suppurativa: A Single-Centre Cross-Sectional Study Utilising Clinical Evaluation, High-Resolution Ultrasound and Colour Doppler. Indian J. Dermatol. Venereol. Leprol. 2025, 91, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Tsentemeidou, A.; Vakirlis, E.; Bakirtzi, K.; Chatzi-Sotiriou, T.; Lallas, A.; Kiritsi, D.; Sotiriou, E. Diagnostic Delay in Hidradenitis Suppurativa: A Systematic Review and Novel Data from a Greek Cohort. Australas. J. Dermatol. 2024, 65, 378–380. [Google Scholar] [CrossRef]
- Prens, L.M.; Bouwman, K.; Aarts, P.; Arends, S.; van Straalen, K.R.; Dudink, K.; Horváth, B.; Prens, E.P. Adalimumab and Infliximab Survival in Patients with Hidradenitis Suppurativa: A Daily Practice Cohort Study. Br. J. Dermatol. 2021, 185, 177–184. [Google Scholar] [CrossRef]
- Van Straalen, K.R.; Piguet, V.; Gudjonsson, J.E. Hidradenitis Suppurativa: Key Insights into Treatment Success and Failure. J. Clin. Investig. 2024, 134, e186744. [Google Scholar] [CrossRef]
- Alsadhan, H.; Alfawzan, A.I.; Yaqoub, A.; Almoneef, A.; Almohideb, M. Hidradenitis Suppurativa: Estimated Prevalence, Clinical Features, and Risk Factors in Riyadh, Saudi Arabia. Cureus 2022, 14, e23029. [Google Scholar] [CrossRef]
- Hladiuk, M.; Huchcroft, S.; Temple, W.; Schnurr, B.E. Arm Function after Axillary Dissection for Breast Cancer: A Pilot Study to Provide Parameter Estimates. J. Surg. Oncol. 1992, 50, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Jemec, G.B.E.; Yang, M.; Kageleiry, A.; Signorovitch, J.E.; Okun, M.M.; Gu, Y.; Wang, K.; Mulani, P.; Sundaram, M. Assessing the Validity, Responsiveness and Meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the Clinical Endpoint for Hidradenitis Suppurativa Treatment. Br. J. Dermatol. 2014, 171, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Daoud, M.; Suppa, M.; Benhadou, F.; Daxhelet, M.; Njimi, H.; White, J.; Jemec, G.; del Marmol, V. Overview and Comparison of the Clinical Scores in Hidradenitis Suppurativa: A Real-Life Clinical Data. Front. Med. 2023, 10, 1145152. [Google Scholar] [CrossRef] [PubMed]
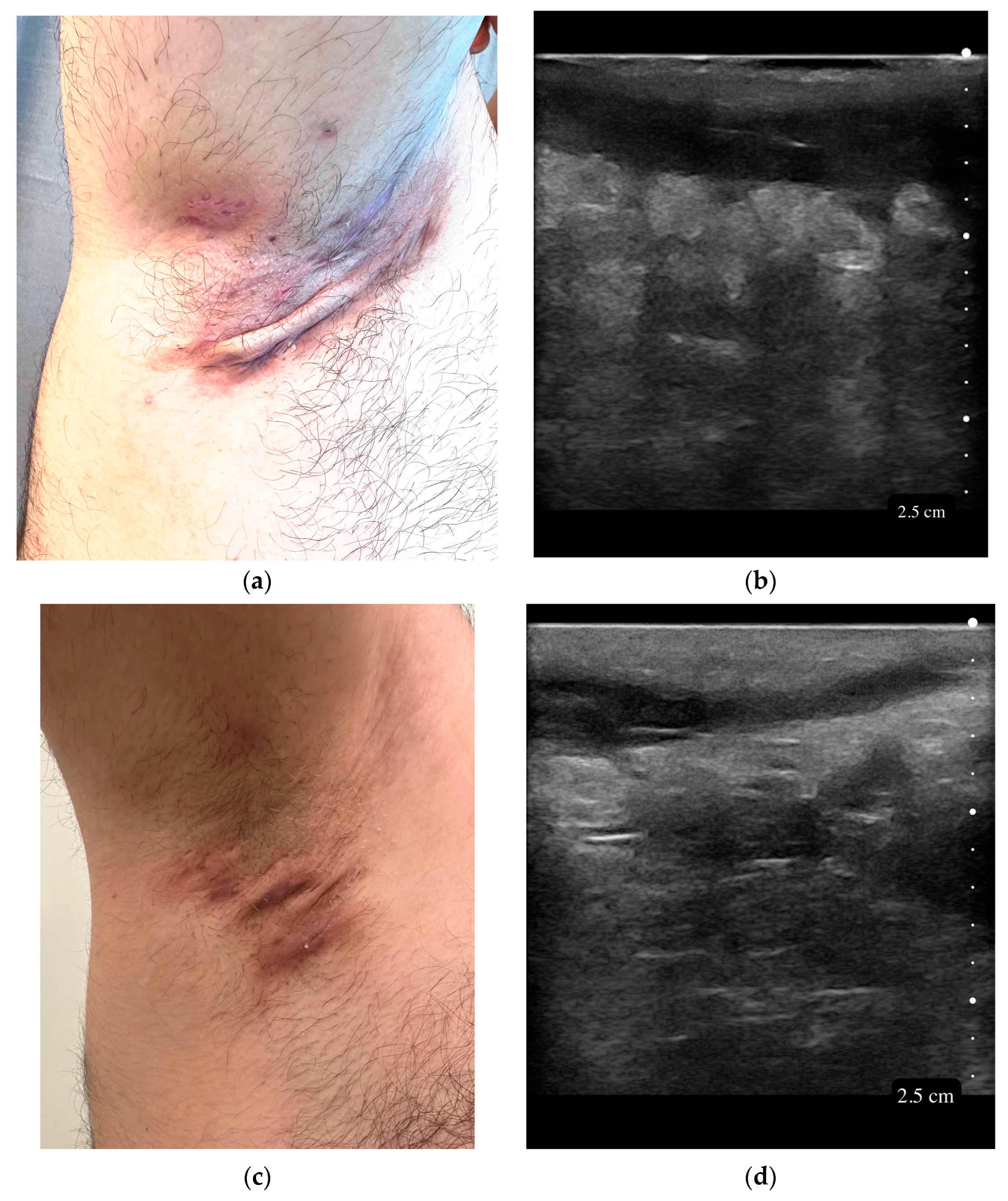

| Variable | All Patients (n = 51) |
|---|---|
| Age | 32 ± 9.4 |
| Sex (female) | 29 (56.9%) |
| Family history | 20 (39.2%) |
| Time since diagnosis (months) | 48 [24–96] |
| Smoking | 31 (60.8%) |
| BMI | 22.5 [21–25.4] |
| Use of antibiotics | 37 (72.5%) |
| Use of isotretinoin | 24 (47.1%) |
| Use of adalimumab | 15 (29.4%) |
| Duration of adalimumab treatment (months) | 26 ± 16 |
| Treated with ≥2 therapies | 24 (47.1%) |
| Treated with ≥3 therapies | 1 (2%) |
| Variable | Category | Lesions (n = 121) |
|---|---|---|
| Lesion Site | Axilla | 65 (53.7%) |
| Inguinal | 39 (32.2%) | |
| Sacral | 8 (6.6%) | |
| Gluteal | 5 (4.1%) | |
| Areolar | 1 (0.8%) | |
| Perineal | 1 (0.8%) | |
| Pubic | 1 (0.8%) | |
| Sternal | 1 (0.8%) | |
| Hurley Stage | Stage 1 | 4 (3.3%) |
| Stage 2 | 72 (59.5%) | |
| Stage 3 | 45 (37.2%) | |
| IHS4 | Score | 8 [7–11] |
| SOS-HS Stage | Stage 1 | 1 (0.8%) |
| Stage 2 | 46 (38.0%) | |
| Stage 3 | 74 (61.2%) | |
| Tunnel Length (mm) | By ultrasound | 36 [24–51.5] |
| By palpation | 30 [18–40] |
| Lesion Site | Hurley Stage 1 | Hurley Stage 2 | Hurley Stage 3 |
|---|---|---|---|
| Axilla | 1.6% (1) | 61.9% (39) | 36.5% (23) |
| Gluteal | 0.0% (0) | 25.0% (1) | 75.0% (3) |
| Inguinal | 5.0% (2) | 55.0% (22) | 40.0% (16) |
| Other | 50.0% (1) | 50.0% (1) | 0.0% (0) |
| Perineal | 0.0% (0) | 0.0% (0) | 100.0% (1) |
| Pubic | 0.0% (0) | 0.0% (0) | 100.0% (1) |
| Sacral | 0.0% (0) | 88.9% (8) | 11.1% (1) |
| Sternal | 0.0% (0) | 100.0% (1) | 0.0% (0) |
| Total | 3.3% (4) | 59.5% (72) | 37.2% (45) |
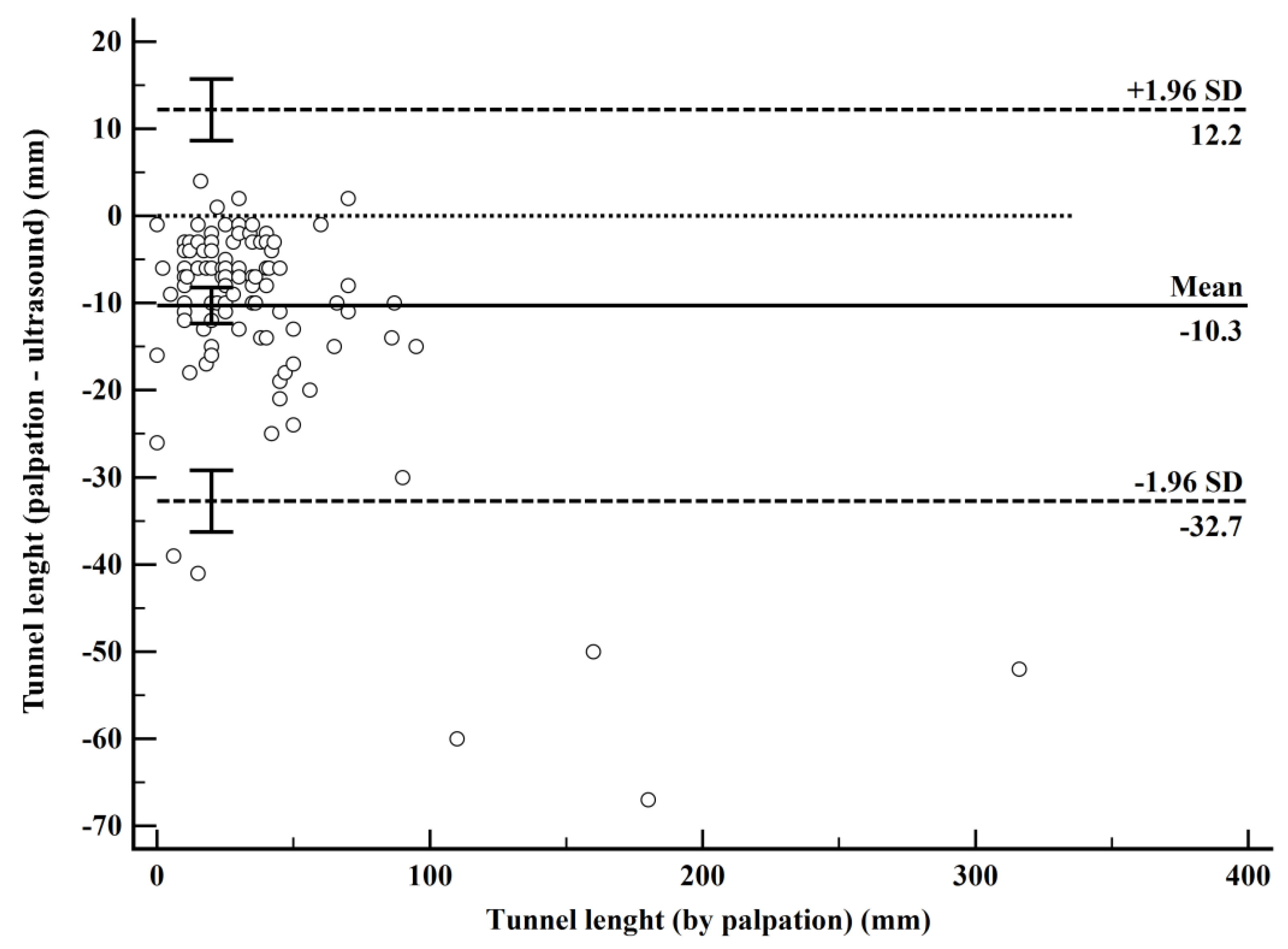
| Comparison | Mean Bias (95% CI) | p | Lower LoA (95% CI) | Upper LoA (95% CI) |
|---|---|---|---|---|
| Palpation-USG | −10.3 | <0.001 | −32.7 (−36.3 to −29.2) | 12.2 (8.6 to 15.7) |
| Comparison | Intercept A (95% CI) | Slope B (95% CI) | RSD (±1.96 SD) |
|---|---|---|---|
| Palpation-USG | −3.2 (−5 to −1.67) | 0.88 (0.83 to 0.94) | 6.3 (−12.4 to 12.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatlıparmak, A.; Doğan, M.; Türkoğlu, Z. Preoperative Tunnel Measurement in Hidradenitis Suppurativa: Comparison of Palpation and Ultrasound. Diagnostics 2025, 15, 1442. https://doi.org/10.3390/diagnostics15111442
Tatlıparmak A, Doğan M, Türkoğlu Z. Preoperative Tunnel Measurement in Hidradenitis Suppurativa: Comparison of Palpation and Ultrasound. Diagnostics. 2025; 15(11):1442. https://doi.org/10.3390/diagnostics15111442
Chicago/Turabian StyleTatlıparmak, Aslı, Murat Doğan, and Zafer Türkoğlu. 2025. "Preoperative Tunnel Measurement in Hidradenitis Suppurativa: Comparison of Palpation and Ultrasound" Diagnostics 15, no. 11: 1442. https://doi.org/10.3390/diagnostics15111442
APA StyleTatlıparmak, A., Doğan, M., & Türkoğlu, Z. (2025). Preoperative Tunnel Measurement in Hidradenitis Suppurativa: Comparison of Palpation and Ultrasound. Diagnostics, 15(11), 1442. https://doi.org/10.3390/diagnostics15111442






