Evolving Paradigm in Radioactive Iodine Therapy for Differentiated Thyroid Cancer: Historical Perspectives, Current Practices and Future Directions
Abstract
1. Introduction
2. Risk Stratification
3. Historical Overview
3.1. Beginnings of Radioactive Iodine-131Administration in DTC
3.2. Beginnings of Radioiodine Ablation of DTC
3.3. Radioiodine Activity Selection
3.4. Former Guidelines and Recommendations for DTC Management
4. Current Approaches for DTC Management
4.1. Current Guidelines and Recommendations
4.2. DTC Patient’s Risk Assessment
4.2.1. Patients with a Low Risk of Relapse/Resistant Disease
4.2.2. Patients with Intermediate Risk of Relapse/Resistant Disease
4.2.3. Patients with a High Risk of Relapse/Resistant Disease
4.3. Radioiodine Treatment Strategies
4.3.1. Risk Adapted Treatment Approach
4.3.2. Theranostic Approach
Dosimetry-Guided Approach
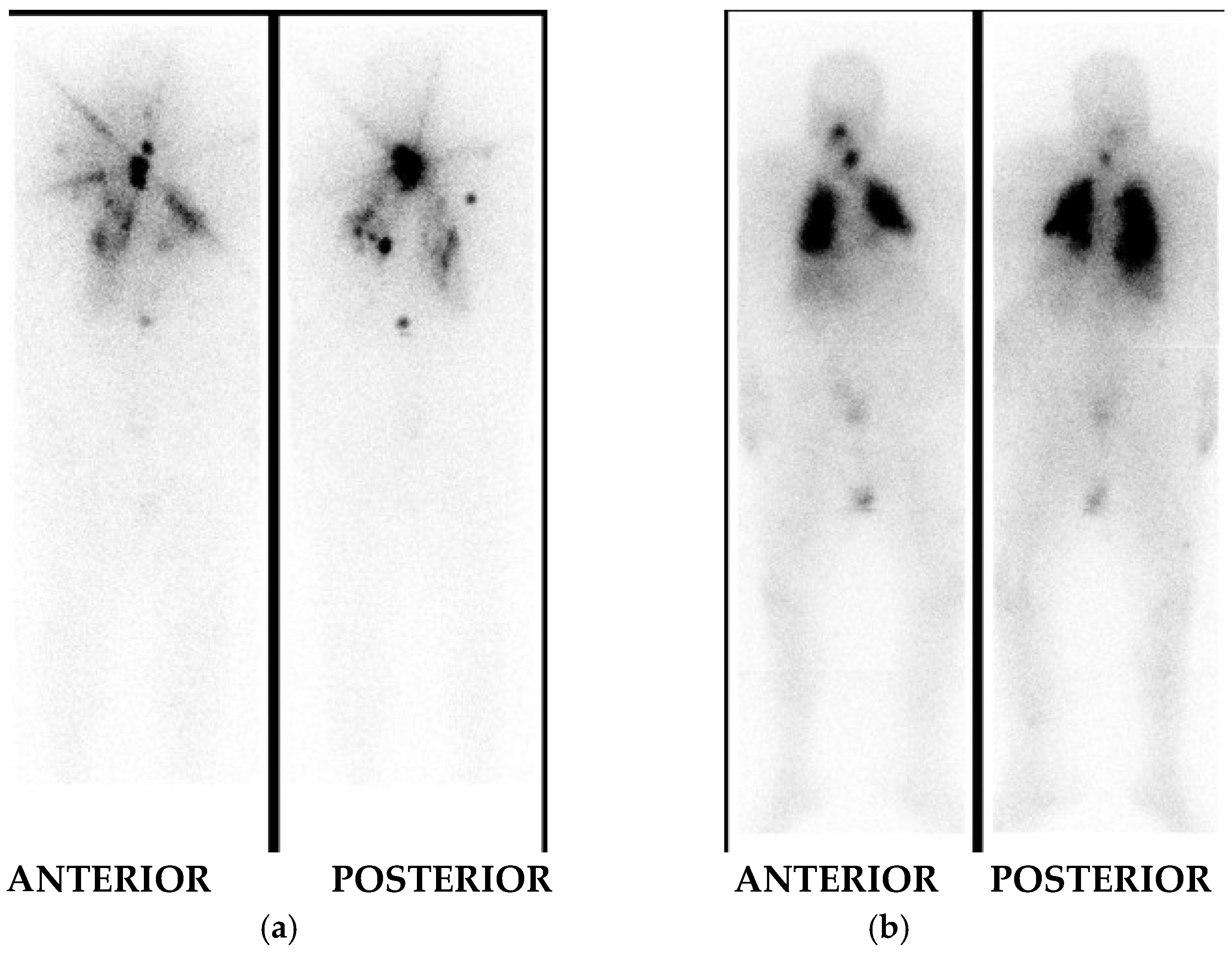
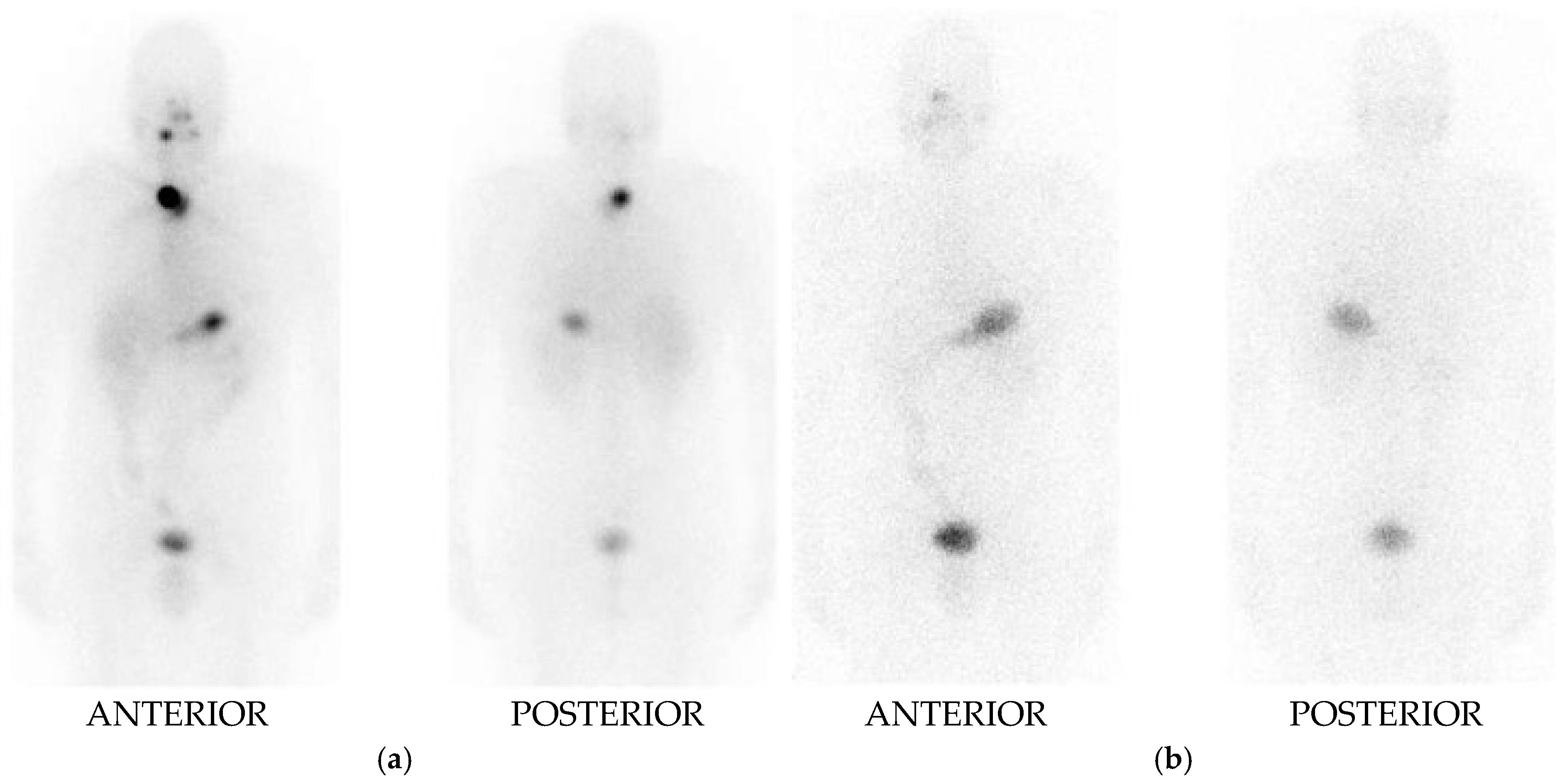
I-131 Whole Body Imaging
4.4. Preparation Methods for Radioiodine Therapy
4.4.1. Low-Iodine Diet
4.4.2. TSH Stimulation
4.5. Dynamic Risk Stratification
4.6. Molecular Profiling of Thyroid Cancer
5. Future Directions
6. Conclusions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Megwalu, U.C.; Moon, P.K. Thyroid Cancer Incidence and Mortality Trends in the United States: 2000–2018. Thyroid 2022, 32, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Schneider, A.B. Epidemiology of Thyroid Cancer. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1284–1297. [Google Scholar] [CrossRef]
- Luis, P.O.; Lucía, M.A.; Hugo, R.C.; Ramiro, R.M.; Stalin, C.Q. Differentiated Thyroid Carcinoma Long-Term Prognostic Factors. Int. J. Surg. Oncol. 2024, 2024, 1067447. [Google Scholar] [CrossRef]
- Liang, N.; Zhang, H.; Sui, C.; Du, R.; Li, C.; Li, J.; Dionigi, G.; Zhang, D.; Sun, H. Surgical resection of recurrent differentiated thyroid cancer: Patterns, detection, staging, and treatment of 683 patients. Front. Endocrinol. 2023, 14, 1301620. [Google Scholar] [CrossRef] [PubMed]
- Coca-Pelaz, A.; Rodrigo, J.P.; Shah, J.P.; Nixon, I.J.; Hartl, D.M.; Robbins, K.T.; Kowalski, L.P.; Mäkitie, A.A.; Hamoir, M.; López, F.; et al. Recurrent Differentiated Thyroid Cancer: The Current Treatment Options. Cancers 2023, 15, 2692. [Google Scholar] [CrossRef]
- Cavalheiro, B.G.; Shah, J.P.; Randolph, G.W.; Medina, J.E.; Tufano, R.P.; Zafereo, M.; Hartl, D.M.; Nixon, I.J.; Guntinas-Lichius, O.; Vander Poorten, V.; et al. Management of Recurrent Well-Differentiated Thyroid Carcinoma in the Neck: A Comprehensive Review. Cancers 2023, 15, 923. [Google Scholar] [CrossRef]
- Cavalheiro, B.G.; de Matos, L.L.; Leite, A.K.N.; Kulcsar, M.A.V.; Cernea, C.R.; Kowalski, L.P. Survival in differentiated thyroid carcinoma: Comparison between the 7th and 8th editions of the AJCC/UICC TNM staging system and the ATA initial risk stratification system. Head Neck 2021, 43, 2913–2922. [Google Scholar] [CrossRef]
- Klubo-Gwiezdzinska, J. Staging and Prognosis of thyroid cancer. In The Thyroid and Its Disease; Luster, M., Duntas, L.H., Wartofsky, L., Eds.; Springer International Publishing AG: Cham, Switzerland, 2019; pp. 595–610. [Google Scholar]
- Byar, D.P.; Green, S.B.; Dor, P.; Williams, E.D.; Colon, J.; van Gilse, H.A.; Mayer, M.; Sylvester, R.J.; van Glabbeke, M. A prognostic index for thyroid carcinoma: A study of the EORTC Thyroid Cancer Cooperative Group. Eur. J. Cancer 1979, 15, 1033–1041. [Google Scholar] [CrossRef]
- Cady, B.; Rossi, R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 1988, 104, 947–953. [Google Scholar] [PubMed]
- Hay, I.D.; Grant, C.S.; Taylor, W.F.; McConahey, W.M. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: A retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery 1987, 102, 1088–1095. [Google Scholar]
- Hay, I.D.; Bergstralh, E.J.; Goellner, J.R.; Ebersold, J.R.; Grant, C.S. Predicting outcome in papillary thyroid carcinoma: Development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 1993, 114, 1050–1058. [Google Scholar]
- Shaha, A.R.; Loree, T.R.; Shah, J.P. Intermediate-risk group for differentiated carcinoma of the thyroid. Surgery 1994, 116, 1036–1040. [Google Scholar] [PubMed]
- Sobin, L.H.; Wittekind, C. TNM Classification of Malignant Tumours, 6th ed.; Wiley-Liss: New York, NY, USA, 2002. [Google Scholar]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 7th ed.; Wiley–Blackwell Publishing: West Sussex, UK, 2010; pp. 58–62. [Google Scholar]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; Wiley Blackwell: Chichester, UK, 2017. [Google Scholar]
- Schlumberger, M.; Pacini, F. Thyroid Tumors, 3rd ed.; Editions Nucleon: Paris, France, 2006. [Google Scholar]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009, 19, 1167–1214, Erratum in Thyroid 2010, 20, 942; Erratum in Thyroid 2010, 20, 674–675. [Google Scholar]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, J.; Chmielik, E.; Jarząb, B. Dynamic risk stratification in the follow-up of thyroid cancer: What is still to be discovered in 2017? Endocr.-Relat. Cancer 2017, 24, R387–R402. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Tala, H.; Shah, J.; Leboeuf, R.; Ghossein, R.; Gonen, M.; Brokhin, M.; Omry, G.; Fagin, J.A.; Shaha, A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: Using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010, 20, 1341–1349. [Google Scholar]
- Ehrhardt, J.D., Jr.; Güleç, S. A Review of the History of Radioactive Iodine Theranostics: The Origin of Nuclear Ontology. Mol. Imaging Radionucl. Ther. 2020, 29, 88–97. [Google Scholar] [CrossRef]
- Hertz, S.; Roberts, A.; Evans, R.D.; Jackson, H. Radioactive iodine as an indicator in the study of thyroid physiology. Proc. Soc. Exp. Biol. Med. 1938, 38, 510–513. [Google Scholar] [CrossRef]
- Hertz, S.; Roberts, A. Radioactive iodine in the study of thyroid physiology VII. The use of radioactive iodine therapy in hyperthyroidism. JAMA 1946, 131, 181. [Google Scholar]
- Keston, A.S.; Ball, R.P.; Frantz, V.K.; Palmer, W.W. Storage of radioactive iodine in a metastasis from thyroid carcinoma. Science 1942, 95, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Seidlin, S.M.; Marinelli, L.D.; Oshry, E. Radioactive iodine therapy: Effect on functioning metastases of adenocarcinoma of the thyroid. JAMA 1946, 14, 838–847. [Google Scholar] [CrossRef]
- Siegel, E. The beginnings of radioiodine therapy of metastatic thyroid carcinoma: A memoir of Samuel M. Seidlin, M.D. (1895-1955) and his celebrated patient. Cancer Biother. Radiopharm. 1999, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Blahd, W.H.; Nordyke, R.A.; Bauer, F.K. Radioactive iodine (I 131) in the postoperative treatment of thyroid cancer. Cancer 1960, 13, 745–756. [Google Scholar] [CrossRef]
- Varma, V.M.; Beierwaltes, W.H.; Nofal, M.H.; Nishiyama, R.H.; Copp, J.E. Treatment of thyroid cancer: Death rates after surgery and after surgery followed by 131-I. JAMA 1970, 214, 1437–1442. [Google Scholar] [CrossRef]
- Krishnamurthy, G.T.; Blahd, W.H. Radioiodine 1-131 therapy in the management of thyroid cancer. A prospective study. Cancer 1977, 40, 195–202. [Google Scholar] [CrossRef]
- Beierwaltes, W.H.; Johnson, P.C.; Solari, A.J. Clinical Use of Radioisotopes; W.B. Saunders Co.: Philadelphia, PA, USA, 1957; p. 150. [Google Scholar]
- Beierwaltes, W.H.; Rabbani, R.; Dmuchowski, C.; Lloyd, R.V.; Eyre, P.; Mallette, S. An analysis of ‘ablation of thyroid remnants’ with I-131 in 511 patients from 1947-1984: Experience at University of Michigan. J. Nucl. Med. 1984, 25, 1287–1293. [Google Scholar]
- Mazzaferri, E.L.; Young, R.L.; Oertel, J.E.; Kemmerer, W.T.; Page, C.P. Papillary thyroid carcinoma: The impact of therapy in 576 patients. Medicine 1977, 56, 171–196. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Jhiang, S.M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 1994, 97, 418–428. [Google Scholar] [CrossRef]
- Mazzaferri, E.L. Thyroid remanant 131-I ablation for papillary and follicular thyroid carcinoma. Thyroid 1997, 7, 265–271. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Kloos, R.T. Current approaches to primary therapy for papillary and follicular thyroid cancer. J. Clin. Endocrinol. Metab. 2001, 86, 1447–1463. [Google Scholar] [CrossRef]
- Sisson, J.C. Applying the radioactive eraser: I-131 to ablate normal thyroid tissue in patients from whom thyroid cancer has been resected. J. Nucl. Med. 1983, 24, 743–745. [Google Scholar]
- Snyder, J.; Gorman, C.; Scanlon, P. Thyroid remnant ablation: Questionable pursuit of an ill-defined goal. J. Nucl. Med. 1983, 24, 659–665. [Google Scholar]
- Hay, I.D.; McConahey, W.M.; Goellner, J.R. Managing patients with papillary thyroid carcinoma: Insights gained from the Mayo Clinic’s experience of treating 2, 512 consecutive patients during 1940 through 2000. Trans. Am. Clin. Climatol. Assoc. 2002, 113, 241–260. [Google Scholar]
- Hay, I.D.; Thompson, G.B.; Grant, C.S.; Bergstralh, E.J.; Dvorak, C.E.; Gorman, C.A.; Maurer, M.S.; McIver, B.; Mullan, B.P.; Oberg, A.L.; et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): Temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J. Surg. 2002, 26, 879–885. [Google Scholar] [CrossRef]
- Wartofsky, L.; Sherman, S.I.; Gopal, J.; Schlumberger, M.; Hay, I.D. The use of radioactive iodine in patients with papillary and follicular thyroid cancer. J. Clin. Endocrinol. Metab. 1998, 83, 4195–4203. [Google Scholar] [CrossRef]
- Hay, I.D. Selective use of radioactive iodine in the postoperative management of patients with papillary and follicular thyroid carcinoma. J. Surg. Oncol. 2006, 94, 692–700. [Google Scholar] [CrossRef]
- Hay, I.D. Management of patients with low-risk papillary thyroid carcinoma. Endocr. Pract. 2007, 13, 521–533. [Google Scholar] [CrossRef]
- Hay, I.D.; Kaggal, S.; Iniguez-Ariza, N.M.; Reinalda, M.S.; Wiseman, G.A.; Thompson, G.B. Inability of Radioiodine Remnant Ablation to Improve Postoperative Outcome in Adult Patients with Low-Risk Papillary Thyroid Carcinoma. Mayo Clin. Proc. 2021, 96, 1727–1745. [Google Scholar] [CrossRef]
- Doi, S.A.; Woodhouse, N.J. Ablation of the thyroid remnant and 131I dose in differentiated thyroid cancer. Clin. Endocrinol. 2000, 52, 765–773. [Google Scholar] [CrossRef]
- Kukulska, A.; Krajewska, J.; Gawkowska-Suwinska, M.; Puch, Z.; Paliczka-Cieslik, E.; Roskosz, J.; Handkiewicz-Junak, D.; Jarzab, M.; Gubała, E.; Jarzab, B. Radioiodine thyroid remnant ablation in patients with differentiated thyroid carcinoma (DTC): Prospective comparison of long-term outcomes of treatment with 30, 60 and 100 mCi. Thyroid. Res. 2010, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Prpic, M.; Dabelic, N.; Stanicic, J.; Jukic, T.; Milosevic, M.; Kusic, Z. Adjuvant thyroid remnant ablation in patients with differentiated thyroid carcinoma confined to the thyroid: A comparison of ablation success with different activities of radioiodine (I-131). Ann. Nucl. Med. 2012, 26, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Molinaro, E.; Castagna, M.G.; Lippi, F.; Ceccarelli, C.; Agate, L.; Elisei, R.; Pinchera, A. Ablation of thyroid residues with 30 mCi (131)I: A comparison in thyroid cancer patients prepared with recombinant human TSH or thyroid hormone withdrawal. J. Clin. Endocrinol. Metab. 2002, 87, 4063–4068. [Google Scholar] [CrossRef]
- McCowen, K.D.; Adler, R.A.; Ghaed, N.; Verdon, T.; Hofeldt, F.D. Low dose radioiodine thyroid ablation in postsurgical patients with thyroid cancer. Am. J. Med. 1976, 61, 52–58. [Google Scholar] [CrossRef]
- Bal, C.S.; Kumar, A.; Pant, G.S. Radioiodine dose for remnant ablation in differentiated thyroid carcinoma: A randomized clinical trial in 509 patients. J. Clin. Endocrinol. Metab. 2004, 89, 1666–1673. [Google Scholar] [CrossRef]
- Bal, C.; Chandra, P.; Kumar, A.; Dwivedi, S.A. randomized equivalence trial to determine the optimum dose of iodine-131 for remnant ablation in differentiated thyroid cancer. Nucl. Med. Commun. 2012, 33, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.; Woodhouse, N.J.; Odugbesan, O. Comparison of 1073 and 3700MBq iodine-131 in postoperative ablation of residual thyroid tissue in patients with differentiated thyroid cancer. J. Nucl. Med. 1991, 32, 252–254. [Google Scholar]
- Taube, A.; Lundell, G. Prospective randomized clinical trial to evaluate the optimal dose of 131I for remnant ablation in patients with differentiated thyroid carcinoma. Cancer 1997, 79, 190–191. [Google Scholar] [CrossRef]
- Maenpaa, H.O.; Heikkonen, J.; Vaalavirta, L.; Tenhunen, M.; Joensuu, H. Low vs. high radioiodine activity to ablate the thyroid after thyroidectomy for cancer: A randomized study. PLoS ONE 2008, 3, e1885. [Google Scholar] [CrossRef]
- Fallahi, B.; Beiki, D.; Takavar, A.; Fard-Esfahani, A.; Gilani, K.A.; Saghari, M.; Eftekhari, M. Low versus high radioiodine dose in postoperative ablation of residual thyroid tissue in patients with differentiated thyroid carcinoma: A large randomized clinical trial. Nucl. Med. Commun. 2012, 33, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Caglar, M.; Bozkurt, F.M.; Akca, C.K.; Vargol, S.E.; Bayraktar, M.; Ugur, O.; Karaağaoğlu, E. Comparison of 800 and 3700 MBq iodine-131 for the postoperative ablation of thyroid remnant in patients with low-risk differentiated thyroid cancer. Nucl. Med. Commun. 2012, 33, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Mallick, U.; Harmer, C.; Yap, B.; Wadsley, J.; Clarke, S.; Moss, L.; Nicol, A.; Clark, P.M.; Farnell, K.; McCready, R.; et al. Ablation with low-dose radioiodine and thyrotropinalfa in thyroid cancer. N. Engl. J. Med. 2012, 366, 1674–1685. [Google Scholar] [CrossRef]
- Schlumberger, M.; Catargi, B.; Borget, I.; Deandreis, D.; Zerdoud, S.; Bridji, B.; Bardet, S.; Leenhardt, L.; Bastie, D.; Schvartz, C.; et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N. Engl. J. Med. 2012, 366, 1663–1673. [Google Scholar] [CrossRef]
- Buscombe, J. Controversies in the Radioiodine Treatment of Patients With Differentiated Thyroid Cancer. Semin. Nucl. Med. 2023, 53, 475–480. [Google Scholar] [CrossRef]
- Van De Velde, C.J.H.; Hamming, J.F.; Goslings, B.M.; Schelfhout, L.J.D.M.; Clark, O.H.; Smeds, S.; Bruining, H.A.; Krenning, E.P.; Cady, B. Report of the consensus development conference on the management of differentiated thyroid cancer in the Netherlands. Eur. J. Cancer Clin. Oncol. 1988, 24, 287–292. [Google Scholar] [CrossRef]
- Singer, P.A.; Cooper, D.S.; Daniels, G.H.; Ladenson, P.W.; Greenspan, F.S.; Levy, E.G.; Braverman, L.E.; Clark, O.H.; McDougall, I.R.; Ain, K.V.; et al. Treatment guidelines for patients with thyroid nodules and well differentiated thyroid cancer. Arch. Intern. Med. 1996, 156, 2165. [Google Scholar] [CrossRef]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Sherman, S.I.; Tuttle, R.M. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2006, 16, 109–142. [Google Scholar] [CrossRef]
- Silberstein, E.B.; Alavi, A.; Balon, H.R.; Clarke, S.E.M.; Divgi, C.; Gelfand, M.J.; Goldmisth, S.J.; Jadvar, H.; Marcus, C.S.; Martin, W.H.; et al. The SNMMI Practice Guideline for therapy of thyroid disease with I-131 3.0. J. Nucl. Med. 2012, 53, 1633–1651. [Google Scholar] [CrossRef]
- Luster, M.; Clarke, S.E.; Dietlin, M.; Lassmann, M.; Lind, P.; Oyen, W.J.; Tennvall, J.; Bombardieri, E. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef]
- Harris, P.E. The management of thyroid cancer in adults: A review of new guidelines. Clin. Med. 2002, 2, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Schlumberger, M.; Dralle, H.; Elisei, R.; Smit, J.W.A.; Wiersinga, W. European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 2006, 154, 787–803. [Google Scholar] [CrossRef] [PubMed]
- British Thyroid Association. Royal College of Physicians: Guidelines for the Management of Thyroid Cancer, 2nd ed.; Perros, P., Ed.; Report of the Thyroid Cancer Guidelines Update Group; Royal College of Physicians: London, UK, 2007. [Google Scholar]
- Perros, P.; Boelaert, K.; Colley, S.; Evans, C.; Evans, R.M.; Gerrard, B.G.; Gilbert, J.; Harrison, B.; Johnson, S.J.; Giles, T.E.; et al. British Thyroid Association. Guidelines for the management of thyroid cancer. Clin. Endocrinol. 2014, 81 (Suppl. 1), 1–122. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.L.; Gandhi, A.; Scott-Coombes, D.; Perros, P. Management of thyroid cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S150–S160. [Google Scholar] [CrossRef]
- Verburg, F.A.; Aktolun, C.; Chiti, A.; Frangos, S.; Giovanella, L.; Hoffmann, M.; Iakovou, I.; Mihailovic, J.; Krause, B.J.; Langsteger, W.; et al. EANM and the EANM Thyroid Committee. Why the European Association of Nuclear Medicine has declined to endorse the 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1001–1005. [Google Scholar] [CrossRef]
- Pacini, F.; Fuhrer, D.; Elisei, R.; Handkiewicz-Junak, D.; Leboulleux, S.; Luster, M.; Schlumberger, M.; Smit, J.W. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur. Thyroid. J. 2022, 11, e210046. [Google Scholar] [CrossRef]
- Schmidt, M.; Bartenstein, P.; Bucerius, J.; Dietlein, M.; Drzezga, A.; Herrmann, K.; Lapa, C.; Lorenz, K.; Musholt, T.J.; Nagarajah, J.; et al. Individualized treatment of differentiated thyroid cancer: The value of surgery in combination with radioiodine imaging and therapy-A German position paper from surgery and nuclear medicine. Nuklearmedizin 2022, 61, 87–96. [Google Scholar]
- Avram, A.M.; Giovanella, L.; Greespan, B.; Lawson, S.A.; Luster, M.; van Nostrand, D.; Peacock, J.G.; Ovčariček, P.P.; Silberstein, E.; Tulchinsky, M.; et al. SNMMI procedure standard/EANM practice guideline for nuclear medicine evaluation and therapy of differentiated thyroid cancer:abbreviated version. J. Nucl. Med. 2022, 63, 15N–35N. [Google Scholar]
- Thyroid Cancer: Assessment and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2022. [PubMed]
- Tuttle, R.M.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Daniels, G.H.; Dillehay, G.; Draganescu, C.; Flux, G.; Führer, D.; et al. Controversies, Consensus, and Collaboration in the Use of 131I Therapy in Differentiated Thyroid Cancer: A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid 2019, 29, 461–470. [Google Scholar]
- Carhill, A.A.; Litofsky, D.R.; Ross, D.S.; Jonklaas, J.; Cooper, D.S.; Brierley, J.D.; Ladenson, P.W.; Ain, K.B.; Fein, H.G.; Haugen, B.R.; et al. Long-Term Outcomes Following Therapy in Differentiated Thyroid Carcinoma: NTCTCS Registry Analysis 1987–2012. J. Clin. Endocrinol. Metab. 2015, 100, 3270–3279. [Google Scholar] [CrossRef]
- Schvartz, C.; Bonnetain, F.; Dabakuyo, S.; Gauthier, M.; Cueff, A.; Fieffe, S.; Pochart, J.M.; Cochet, I.; Crevisy, E.; Dalac, A.; et al. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J. Clin. Endocrinol. Metab. 2012, 97, 1526–1535. [Google Scholar] [CrossRef]
- Jonklaas, J.; Sarlis, N.J.; Litofsky, D.; Ain, K.B.; Bigos, S.T.; Brierley, J.D.; Cooper, D.S.; Haugen, B.R.; Ladenson, P.W.; Magner, J.; et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006, 16, 1229–1242. [Google Scholar] [CrossRef]
- Jonklaas, J.; Cooper, D.S.; Ain, K.B.; Bigos, T.; Brierley, J.D.; Haugen, B.R.; Ladenson, P.W.; Magner, J.; Ross, D.S.; Skarulis, M.C.; et al. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid 2010, 20, 1423–1424. [Google Scholar] [CrossRef]
- Lamartina, L.; Durante, C.; Filetti, S.; Cooper, D.S. Low-risk differentiated thyroid cancer and radioiodine remnant ablation: A systematic review of the literature. J. Clin. Endocrinol. Metab. 2015, 100, 1748–1761. [Google Scholar] [CrossRef]
- Sacks, W.; Fung, C.H.; Chang, J.T.; Waxman, A.; Braunstein, G.D. The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: A systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid 2010, 20, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Sawka, A.M.; Brierley, J.D.; Tsang, R.W.; Thabane, L.; Rotstein, L.; Gafni, A.; Straus, S.; Goldstein, D.P. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol. Metab. Clin. N. Am. 2008, 37, 457–480. [Google Scholar] [CrossRef]
- Verburg, F.A.; Flux, G.; Giovanella, L.; van Nostrand, D.; Muylle, K.; Luster, M. Differentiated thyroid cancer patients potentially benefitting from postoperative I-131 therapy: A review of the literature of the past decade. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 78–83. [Google Scholar] [CrossRef]
- James, D.L.; Ryan, É.J.; Davey, M.G.; Quinn, A.J.; Heath, D.P.; Garry, S.J.; Boland, M.R.; Young, O.; Lowery, A.J.; Kerin, M.J. Radioiodine Remnant Ablation for Differentiated Thyroid Cancer: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Head. Neck Surg. 2021, 147, 544–552. [Google Scholar] [CrossRef]
- Mallick, U.; Harmer, C.; Hackshaw, A.; Moss, L. IoN Trial Management Group. Iodine or Not (IoN) for low-risk differentiated thyroid cancer: The next UK National Cancer Research Network randomised trial following HiLo. Clin. Oncol. (R. Coll. Radiol.) 2012, 24, 159–161. [Google Scholar] [CrossRef]
- Leboulleux, S.; Bournaud, C.; Chougnet, C.N.; Zerdoud, S.; Al Ghuzlan, A.; Catargi, B.; Do Cao, C.; Kelly, A.; Barge, M.L.; Lacroix, L.; et al. Thyroidectomy without Radioiodine in Patients with Low-Risk Thyroid Cancer. N. Engl. J. Med. 2022, 386, 923–932. [Google Scholar] [CrossRef]
- Leboulleux, S.; Bournaud, C.; Chougnet, C.N.; Lamartina, L.; Zerdoud, S.; Do Cao, C.; Catargi, B.; Dygai, I.; Kelly, A.; Barge, M.L.; et al. Thyroidectomy without radioiodine in patients with low-risk thyroid cancer: 5 years of follow-up of the prospective randomised ESTIMABL2 trial. Lancet Diabetes Endocrinol. 2025, 13, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Tulchinsky, M. Thyroidectomy without Radioiodine in Low-Risk Thyroid Cancer. N. Engl. J. Med. 2022, 386, 2153–2154. [Google Scholar] [PubMed]
- Greenspan, B.S. Thyroidectomy without Radioiodine in Low-Risk Thyroid Cancer. N. Engl. J. Med. 2022, 386, 2154. [Google Scholar] [PubMed]
- Gulec, S.A.; McGoron, A.J. Thyroidectomy without Radioiodine in Low-Risk Thyroid Cancer. N. Engl. J. Med. 2022, 386, 2154–2155. [Google Scholar] [PubMed]
- Ruel, E.; Thomas, S.; Dinan, M.; Perkins, J.M.; Roman, S.A.; Sosa, J.A. Adjuvant radioactive iodine therapy is associated with improved survival for patients with intermediate-risk papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2015, 100, 1529–1536. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, Y.; Zheng, L.; Zhang, Z.; Jiang, N. Postoperative radioactive iodine-131 ablation is not necessary among patients with intermediate-risk differentiated thyroid carcinoma: A population-based study. Hell. J. Nucl. Med. 2017, 20, 3–10. [Google Scholar]
- Kim, S.K.; Woo, J.W.; Lee, J.H.; Park, I.; Choe, J.H.; Kim, J.H.; Kim, J.S. Radioactive iodine ablation may not decrease the risk of recurrence in intermediate-risk papillary thyroid carcinoma. Endocr. Relat. Cancer 2016, 23, 367–376. [Google Scholar] [CrossRef]
- Podnos, Y.D.; Smith, D.D.; Wagman, L.D.; Ellenhorn, J.D. Survival in patients with papillary thyroid cancer is not affected by the use of radioactive isotope. J. Surg. Oncol. 2007, 96, 3–7. [Google Scholar] [CrossRef]
- Podnos, Y.D.; Smith, D.; Wagman, L.D.; Ellenhorn, J.D. Radioactive iodine offers survival improvement in patients with follicular carcinoma of the thyroid. Surgery 2005, 138, 1072–1076. [Google Scholar] [CrossRef]
- Yang, Z.; Flores, J.; Katz, S.; Nathan, C.A.; Mehta, V. Comparison of Survival Outcomes Following Postsurgical Radioactive Iodine Versus External Beam Radiation in Stage IV Differentiated Thyroid Carcinoma. Thyroid 2017, 27, 944–952. [Google Scholar] [CrossRef]
- Benua, R.S.; Cicale, N.R.; Sonenberg, M.; Rawson, R.W. The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1962, 87, 171–182. [Google Scholar] [PubMed]
- Lassmann, M.; Hänscheid, H.; Chiesa, C.; Hindorf, C.; Flux, G.; Luster, M. EANM Dosimetry Committee series on standard operational procedures for pre-therapeutic dosimetry I: Blood and bone marrow dosimetry in differentiated thyroid cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Maxon, H.R.; Thomas, S.R.; Hertzberg, V.S.; Kereiakes, J.G.; Chen, I.W.; Sperling, M.I.; Saenger, E.L. Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. N. Engl. J. Med. 1983, 309, 937–941. [Google Scholar] [CrossRef]
- Flux, G.D.; Haq, M.; Chittenden, S.J.; Buckley, S.; Hindorf, C.; Newbold, K.; Harmer, C.L. A dose-effect correlation for radioiodine ablation in differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 270–275. [Google Scholar] [CrossRef]
- Wierts, R.; Brans, B.; Havekes, B.; Kemerink, G.J.; Halders, S.G.; Schaper, N.N.; Backes, W.H.; Mottaghy, F.M.; Jentzen, W. Dose-Response Relationship in Differentiated Thyroid Cancer Patients Undergoing Radioiodine Treatment Assessed by Means of 124-I PET/CT. J. Nucl. Med. 2016; 57, 1027–1032. [Google Scholar]
- Maxon, H.R., II; Englaro, W.W.; Thomas, S.R.; Hertzberg, V.S.; Hinnefeld, J.D.; Chen, L.S.; Smith, H.; Cummings, D.; Aden, M.D. Radioiodine-131 therapy for well-differentiated thyroid cancer-a quantitative radiation dosimetric approach: Outcome and validation in 85 patients. J. Nucl. Med. 1992, 33, 1132–1136. [Google Scholar]
- Sgouros, G.; Song, H.; Ladenson, P.W.; Wahl, R.L. Lung toxicity in radioiodine therapy of thyroid carcinoma: Development of a dose-rate method and dosimetric implications of the 80-mCi rule. J. Nucl. Med. 2006, 47, 1977–1984. [Google Scholar]
- Jentzen, W.; Nahum, A.E.; Bockisch, A.; Binse, I.; Tulchinsky, M. Fixed 3.7-GBq (131)I Activity for Metastatic Thyroid Cancer Therapy Ignores Science and History. J. Nucl. Med. 2017, 58, 1530. [Google Scholar] [CrossRef]
- Verburg, F.A.; Luster, M.; Giovanella, L.; Lassmann, M.; Chiesa, C.; Chouin, N.; Flux, G. The “reset button” revisited: Why high activity (131)I therapy of advanced differentiated thyroid cancer after dosimetry is advantageous for patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Klubo-Gwiezdzinska, J.; Van Nostrand, D.; Atkins, F.; Burman, K.; Jonklaas, J.; Mete, M.; Wartofsky, L. Efficacy of dosimetric versus empiric prescribed activity of 131I for therapy of differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2011, 96, 3217–3225. [Google Scholar] [CrossRef]
- Mihailović, J. Pre-Treatment and Post-Treatment I-131 Imaging in Differentiated Thyroid Carcinoma. J. Clin. Med. 2024, 13, 1984. [Google Scholar] [CrossRef]
- de Koster, E.J.; Sulaiman, T.; Hamming, J.F.; Schepers, A.; Snel, M.; van Velden, F.H.P.; de Geus-Oei, L.F.; Vriens, D. Radioiodine in Differentiated Thyroid Carcinoma: Do We Need Diagnostic Pre-Ablation Iodine-123 Scintigraphy to Optimize Treatment? Diagnostics 2021, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.; Kuker, R. Radioactive Iodine Remnant Ablation: The Beta-knife Completion Thyroidectomy. Mol. Imaging. Radionucl. Ther. 2017, 26 (Suppl. S1), 16–23. [Google Scholar] [CrossRef] [PubMed]
- Rawson, R.W.; Rall, J.E.; Peacock, W. Limitations and indications in the treatment of cancer of the thyroid with radioactive iodine. J. Clin. Endocrinol. Metab. 1951, 11, 1128–1131. [Google Scholar] [CrossRef]
- Park, H.M. Stunned thyroid after high-dose I-131 imaging. Clin. Nucl. Med. 1992, 17, 501–502. [Google Scholar] [CrossRef]
- Park, H.M.; Perkins, O.W.; Edmondson, J.W.; Schnute, R.B.; Manatunga, A. Influence of diagnostic radioiodines on the uptake of ablative dose of iodine131. Thyroid 1994, 4, 49–54. [Google Scholar] [CrossRef]
- Verburg, F.A.; Verkoiijen, R.B.; Stokkel, M.P.; van Isselt, J.W. The success of 131-I ablation in thyroid cancer patients is significantly reduced after a diagnostic activity of 40 MBq 131-I. Nuklearmedizin 2009, 48, 138–142. [Google Scholar] [PubMed]
- Chen, M.K.; Yasrebi, M.; Samii, J.; Staib, L.H.; Doddamane, I.; Cheng, D.W. The Utility of I-123 Pretherapy Scan in I-131 Radioiodine Therapy for Thyroid Cancer. Thyroid 2012, 22, 304–309. [Google Scholar] [CrossRef]
- Song, H.; Mosci, C.; Akatsu, H.; Basina, M.; Dosiou, C.; Iagaru, A. Diagnostic 123-I whole body scan prior to ablation of thyroid remnant in patients with papillary thyroid cancer: Implications for clinical management. Clin. Nucl. Med. 2018, 43, 705–709. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Thyroid Carcinoma Version 1.2025-27 March 2025; 2025 National Comprehensive Cancer Network, Inc. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (accessed on 8 April 2025).
- Herbert, G.; England, C.; Perry, R.; Whitmarsh, A.; Moore, T.; Searle, A.; Chotaliya, S.; Ness, A.; Beasley, M.; Atkinson, C. Impact of low iodine diets on ablation success in differentiated thyroid cancer: A mixed-methods systematic review and meta-analysis. Clin. Endocrinol. 2022, 97, 702–729. [Google Scholar] [CrossRef]
- Herbert, G.; Searle, A.; England, C.Y.; Ness, A.; Beasley, M.; Haupt-Schott, I.; Moss, L.; Wescott, J.; Atkinson, C. Experiences of low iodine diets in the treatment of differentiated thyroid cancer with radioactive iodine ablation therapy. Clin. Nutr. ESPEN 2020, 39, 190–197. [Google Scholar] [CrossRef]
- Luo, H.; Tobey, A.; Auh, S.; Cochran, C.; Behairy, N.; Merino, M.; Zemskova, M.; Klubo-Gwiezdzinska, J. The utility of low-iodine diet in preparation for thyroid cancer therapy with radioactive iodine-A cohort study. Front. Pharmacol. 2022, 30, 791710. [Google Scholar]
- Pluijmen, M.J.; Eustatia-Rutten, C.; Goslings, B.M.; Stokkel, M.P.; Arias, A.M.; Diamant, M.; Romijn, J.A.; Smit, J.W. Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin. Endocrinol. 2003, 58, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Dekker, B.L.; Links, M.H.; Muller Kobold, A.C.; Swart-Busscher, L.G.; Kars, M.; Bons, J.A.P.; Brouwers, A.H.; Links, T.P.; van der Horst-Schrivers, A.N.A. Low-Iodine Diet of 4 Days Is Sufficient Preparation for 131I Therapy in Differentiated Thyroid Cancer Patients. J. Clin. Endocrinol. Metab. 2022, 107, e604–e611. [Google Scholar] [CrossRef]
- Edmonds, C.J.; Hayes, S.; Kermode, J.C.; Thompson, B.D. Measurement of serum TSH and thyroid hormones in the ATA Thyroid Nodule/DTC Guidelines 117 management of treatment of thyroid carcinoma with radioiodine. Br. J. Radiol. 1977, 50, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J.; Driedger, A.; Magner, J. Recombinant human thyrotropin-assisted radioiodine therapy for patients with metastatic thyroid cancer who could not elevate endogenous thyrotropin or be withdrawn from thyroxine. Thyroid 2006, 16, 1121–1130. [Google Scholar] [CrossRef]
- Coerts, H.I.; de Keizer, B.; Marlowe, R.J.; Verburg, F.A. Recombinant or endogenous thyroid-stimulating hormone for radioactive iodine therapy in thyroid cancer: State of knowledge and current controversies. Eur. J. Endocrinol. 2023, 188, lvad006. [Google Scholar] [CrossRef] [PubMed]
- Pitoia, F.; Marlowe, R.J.; Abelleira, E.; Faure, E.N.; Bueno, F.; Schwarzstein, D.; Lutfi, R.J.; Niepomniszcze, H. Radioiodine thyroid remnant ablation after recombinant human thyrotropin or thyroid hormone withdrawal in patients with high-risk differentiated thyroid cancer. J. Thyroid. Res. 2012, 2012, 481568. [Google Scholar] [CrossRef]
- Tsai, J.R.; Wu, S.T.; Chi, S.Y.; Yang, Y.T.; Chan, Y.C.; Lim, L.S.; Chiew, Y.E.W.; Chen, W.C.; Chen, Y.N.; Chou, C.K. Recombinant human thyrotropin versus thyroid hormone withdrawal preparation for radioiodine ablation in differentiated thyroid cancer: Experience in a South Taiwanese medical center. Kaohsiung J. Med. Sci. 2023, 39, 175–181. [Google Scholar] [CrossRef]
- Gomes-Lima, C.J.; Chittimoju, S.; Wehbeh, L.; Dia, S.; Pagadala, P.; Al-Jundi, M.; Jhawar, S.; Tefera, E.; Mete, M.; Klubo-Gwiezdzinska, J.; et al. Metastatic Differentiated Thyroid Cancer Survival Is Unaffected by Mode of Preparation for 131I Administration. J. Endocr. Soc. 2022, 6, bvac032. [Google Scholar] [CrossRef]
- Tsai, H.C.; Ho, K.C.; Chen, S.H.; Tseng, J.R.; Yang, L.Y.; Lin, K.J.; Cheng, J.C.; Liou, M.J. Feasibility of recombinant human TSH as a preparation for radioiodine therapy in patients with distant metastases from papillary thyroid cancer: Comparison of long-term survival outcomes with thyroid hormone withdrawal. Diagnostics 2022, 12, 221. [Google Scholar] [CrossRef]
- Lin, Y.S.; Yang, H.; Li, X.Y.; Wu, L.Q.; Xu, J.G.; Yang, A.M.; Gao, Z.R.; Ding, Y.; Zhang, Y.Q.; Chen, K.; et al. Novel recombinant human thyroid-stimulating hormone in aiding postoperative assessment of patients with differentiated thyroid cancer-phase I/II study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Gu, Y.; Xiu, Y.; Han, X.; Wen, Q.; Lv, Z.; Fan, W.; Li, S.; Tan, J.; Wang, F.; et al. Recombinant Human Thyrotropin Plus Radioactive Iodine Among Patients With Thyroid Cancer: A Noninferiority Randomized Clinical Trial. JAMA Netw. Open. 2024, 7, e2443407. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Cosma, C.; Plebani, M. Letter to the Editor: What Is the Role of Serum Thyroglobulin Measurement in Patients With Differentiated Thyroid Cancer Treated Without Radioactive Iodine? J. Clin. Endocrinol. Metab. 2017, 102, 2113–2114. [Google Scholar] [CrossRef] [PubMed]
- Bellini, P.; Dondi, F.; Gatta, E.; Zilioli, V.; Albano, D.; Cappelli, C.; Bertagna, F. Prognostic role and characteristics of the indeterminate response in differentiated thyroid cancer: A systematic review. Endocrine 2024, 84, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, F.; Momesso, D.; Bulzico, D.A.; Pessoa, C.H.; Dias, F.; Corbo, R.; Vaisman, M.; Tuttle, R.M. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin. Endocrinol. 2012, 77, 132–138. [Google Scholar] [CrossRef]
- Gulec, S.A.; Meneses, E. Theranostic Risk Stratification for Thyroid Cancer in the Genomic Paradigm. Cancers 2024, 16, 1585. [Google Scholar] [CrossRef]
- Liu, X.; Qu, S.; Liu, R.; Sheng, C.; Shi, X.; Zhu, G.; Murugan, A.K.; Guan, H.; Yu, H.; Wang, Y.; et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 99, E1130e6. [Google Scholar] [CrossRef]
- Landa, I. InTERTwined: How TERT promoter mutations impact BRAFV600E-driven thyroid cancers. Curr. Opin. Endocr. Metab. Res. 2023, 30, 100460. [Google Scholar] [CrossRef]
- Argyropoulou, M.; Veskoukis, A.S.; Karanatsiou, P.M.; Manolakelli, A.; Kostoglou-Athanassiou, I.; Vilaras, G.; Karameris, A.; Liadaki, K. Low Prevalence of TERT Promoter, BRAF and RAS Mutations in Papillary Thyroid Cancer in the Greek Population. Pathol. Oncol. Res. 2020, 26, 347–354. [Google Scholar] [CrossRef]
- Bikas, A.; Ahmadi, S.; Pappa, T.; Marqusee, E.; Wong, K.; Nehs, M.A.; Cho, N.L.; Haase, J.; Doherty, G.M.; Sehgal, K.; et al. Additional Oncogenic Alterations in RAS-Driven Differentiated Thyroid Cancers Associate with Worse Clinicopathologic Outcomes. Clin. Cancer Res. 2023, 29, 2678–2685. [Google Scholar] [CrossRef]
- Riccio, I.; Laforteza, A.; Landau, M.B.; Hussein, M.H.; Linhuber, J.; Staav, J.; Issa, P.P.; Toraih, E.A.; Kandil, E. Decoding RAS mutations in thyroid cancer: A meta-analysis unveils specific links to distant metastasis and increased mortality. Am. J. Otolaryngol. 2025, 46, 104570. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, J.K.; Kim, G.J.; Kang, S.W.; Lee, J.; Jeong, J.J.; Chung, W.Y.; Kim, D.; Nam, K.H. TERT Promoter and BRAF V600E Mutations in Papillary Thyroid Cancer: A Single-Institution Experience in Korea. Cancers 2022, 14, 4928. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Choi, S.W.; Yi, J.W. Prospective Analysis of TERT Promoter Mutations in Papillary Thyroid Carcinoma at a Single Institution. J. Clin. Med. 2021, 10, 2179. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Boucai, L.; Seshan, V.; Williams, M.; Knauf, J.A.; Saqcena, M.; Ghossein, R.A.; Fagin, J.A. Characterization of subtypes of BRAF-mutant papillary thyroid cancer defined by their thyroid differentiation score. J. Clin. Endocrinol. Metab. 2022, 107, 1030–1039. [Google Scholar] [CrossRef]
- McGoron, A.J.; Garcia, J.M.; Uluvar, B.; Gulec, S.A. Thyroid differentiation profile for differentiated thyroid cancer. Endocr. Oncol. 2025, 5, e240072. [Google Scholar] [CrossRef]
| TNM | 6th Edition | 7th Edition | 8th Edition * |
|---|---|---|---|
| T | |||
| Tx | T cannot be assessed | T cannot be assessed | T cannot be assessed |
| T0 | No evidence of T | No evidence of T | No evidence of T |
| T1 | Tumor ≤ 2 cm limited to the thyroid | Tumors of ≤2 cm are limited to the thyroid | Tumors of ≤2 cm are limited to the thyroid |
| T1a | - | Tumor size ≤ 1 cm, limited to the thyroid | Tumor ≤ 1 cm, limited to the thyroid |
| T1b | - | Tumor size > 1 cm ≤ 2 cm, limited to the thyroid | Tumor > 1 cm ≤ 2 cm, limited to the thyroid |
| T2 | Tumor > 2 < 4 cm limited to the thyroid | Tumor size ≤ 4 cm limited to the thyroid | Tumor size > 2 cm but ≤4 cm, limited to the thyroid. |
| T3 | Tumor > 4 cm limited to the thyroid or any T with minimal ETE (extension to sternohyoid muscle or perithyroid soft tissues) | Tumor size > 4 cm limited to the thyroid or any T with minimal ETE (e.g., extension to sternohyoid muscle or perithyroid soft tissue) | Tumor size > 4 cm, limited to the thyroid or any T with gross ETE invading only strap muscles |
| T3a | - | - | Tumor > 4 cm, limited to the thyroid |
| T3b | - | - | Any T with gross ETE invading only strap muscles (e.g., extension to sternothyroid, sternohyoid, thyrohyoid, or omohyoid muscles) |
| T4a | Any T extending beyond the thyroid capsule to invade subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve | Any T extending beyond the thyroid capsule to invade subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve—moderately advanced disease | Any T with gross ETE invading subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve |
| T4b | Tumor invades prevertebral fascia or encases carotid artery or mediastinal vessels | Tumor invades prevertebral fascia or encases the carotid artery or mediastinal vessels-very advanced disease | Any T with gross ETE invading prevertebral fascia or encasing the carotid artery or mediastinal vessels |
| N | |||
| Nx | RLN cannot be assessed | RLN cannot be assessed | RLN cannot be assessed |
| N0 | No RLN metastasis | No RLN metastasis | No RLN metastasisOne/more cytological or histologically confirmed benign LN |
| N0a | - | - | No radiologic or clinical evidence of locoregional metastasis |
| N1 | RLN metastasis | RLN metastasis | RLN metastasis |
| N1a | Metastasis to level VI (pretracheal, paratracheal, and prelaryngeal/Delphian LN) | Metastasis to level VI (pretracheal, paratracheal, and prelaryngeal/Delphian LN) | Metastases to level VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian, or upper mediastinal) lymph nodes. This can be unilateral or bilateral disease |
| N1b | Metastasis to unilateral, bilateral, or contralateral cervical or superior mediastinal LN | Metastasis to unilateral, bilateral, or contralateral cervical or superior mediastinal LN (level VII) | Metastasis to unilateral, bilateral, or contralateral lateral neck lymph nodes (levels I, II, III, IV or V) or retropharyngeal lymph nodes |
| M | |||
| Mx | Distant metastasis cannot be assessed | - | - |
| M0 | No distant metastasis | No distant metastasis is found | No distant metastasis is found |
| M1 | Distant metastasis is present | Distant metastasis is present | Distant metastasis is present |
| STAGING GROUPS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6th Edition | 7th Edition | 8th Edition | |||||||||
| <45 years | <45 years | <55 years | |||||||||
| S | T | N | M | S | T | N | M | S | T | N | M |
| I | Any T | Any N | M0 | I | Any T | Any N | M0 | I | Any T | Any N | M0 |
| II | Any T | Any N | M1 | II | Any T | Any N | M1 | II | Any T | Any N | M1 |
| III | - | - | - | III | - | - | - | III | - | - | - |
| IVa | - | - | - | IVa | - | IVa | - | - | - | ||
| IVb | - | - | - | IVb | - | - | - | IVb | - | - | - |
| IVc | - | - | - | IVc | - | - | - | IVc | - | - | - |
| ≥45 years | ≥45 years | ≥55 years | |||||||||
| S | T | N | M | S | T | N | M | S | T | N | M |
| I | T1 | N0 | M0 | I | T1a, T1b | N0 | M0 | I | T1a, T1b, T2 | N0 | M0 |
| II | T2 | N0 | M0 | II | T2 | N0 | M0 | II | T1, T2 T3 | N1 N0, N1 | M0 M0 |
| III | T3 T1, T2, T3 | N0 N1a | M0 M0 | III | T3 T1, T2, T3 | N0 N1a | M0 M0 | III | T4a | Any N | M0 |
| IVa | T1, T2, T3 T4a | N1b N0, N1a, N1b | M0 M0 | IVa | T1, T2, T3 T4a | N1b N0, N1 | M0 M0 | IVa | T4b | Any T | M0 |
| IVb | T4b | Any N | M0 | IVb | T4b | Any N | M0 | IVb | Any T | Any N | M1 |
| IVc | Any T | Any N | M1 | IVc | Any T | Any N | M1 | IVc | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihailović, J. Evolving Paradigm in Radioactive Iodine Therapy for Differentiated Thyroid Cancer: Historical Perspectives, Current Practices and Future Directions. Diagnostics 2025, 15, 1438. https://doi.org/10.3390/diagnostics15111438
Mihailović J. Evolving Paradigm in Radioactive Iodine Therapy for Differentiated Thyroid Cancer: Historical Perspectives, Current Practices and Future Directions. Diagnostics. 2025; 15(11):1438. https://doi.org/10.3390/diagnostics15111438
Chicago/Turabian StyleMihailović, Jasna. 2025. "Evolving Paradigm in Radioactive Iodine Therapy for Differentiated Thyroid Cancer: Historical Perspectives, Current Practices and Future Directions" Diagnostics 15, no. 11: 1438. https://doi.org/10.3390/diagnostics15111438
APA StyleMihailović, J. (2025). Evolving Paradigm in Radioactive Iodine Therapy for Differentiated Thyroid Cancer: Historical Perspectives, Current Practices and Future Directions. Diagnostics, 15(11), 1438. https://doi.org/10.3390/diagnostics15111438






