Whole-Brain Confocal Imaging Provides an Accurate Global View of the Nigral Dopamine System
Abstract
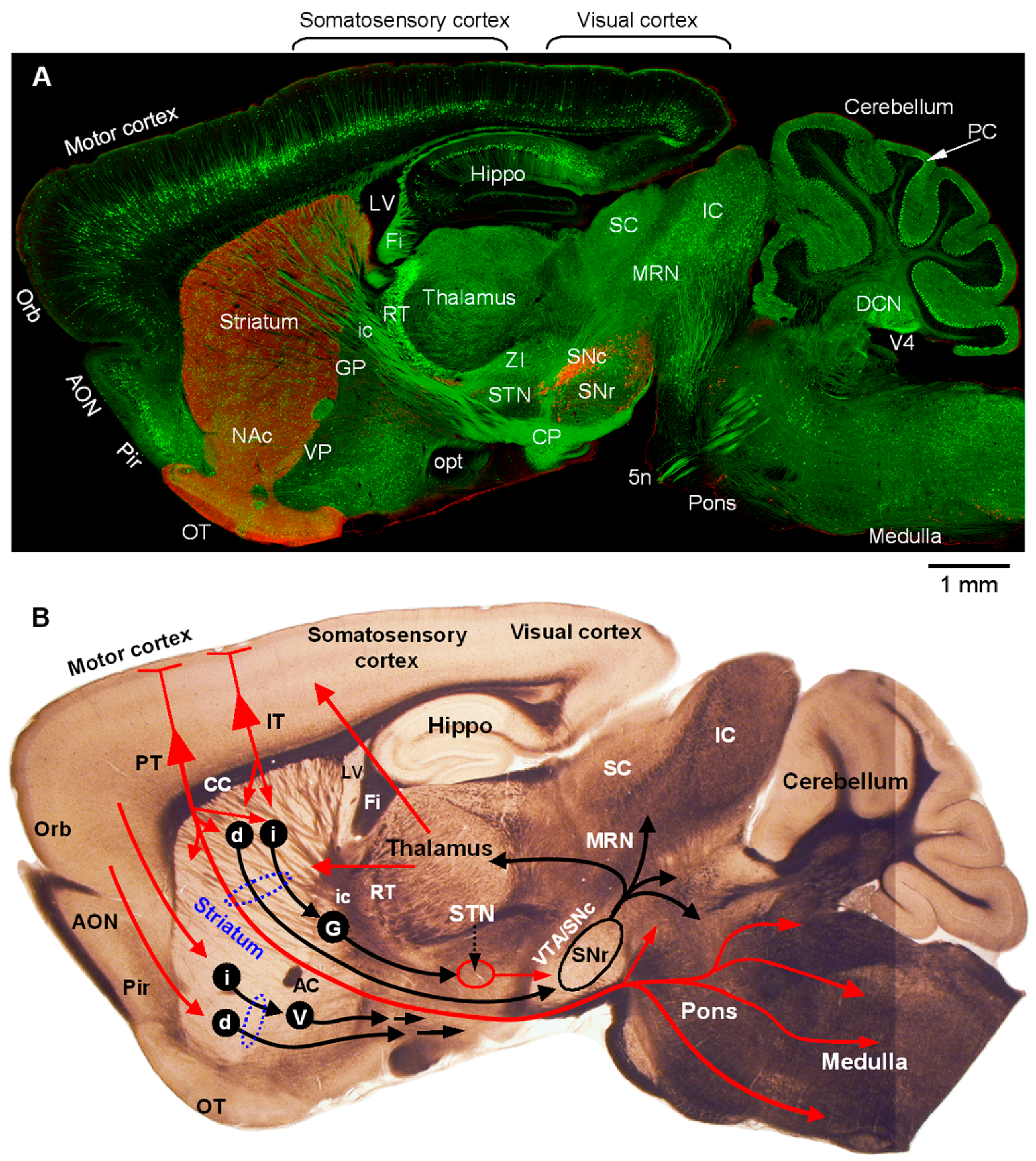

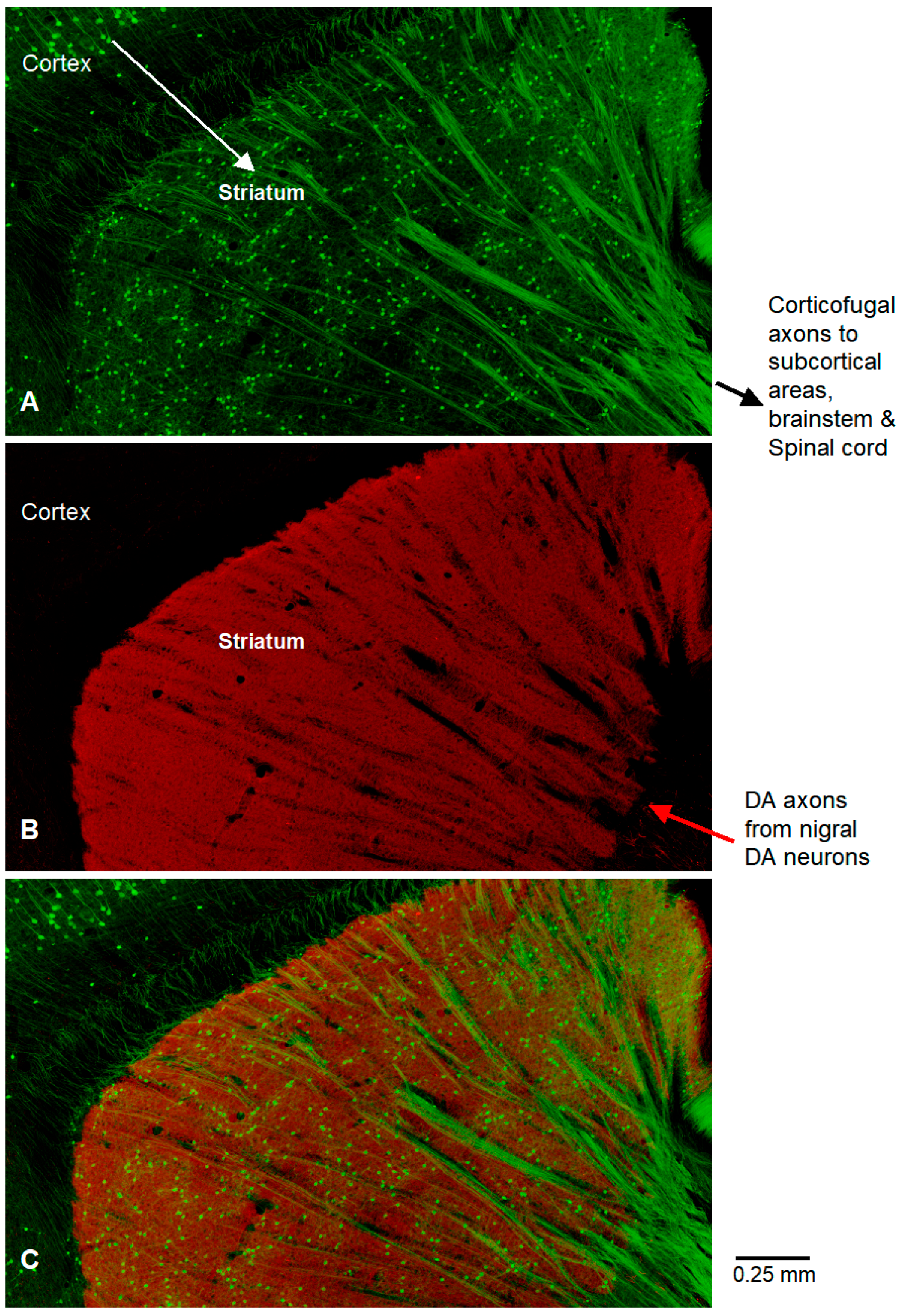
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Haber, S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016, 18, 7–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McColgan, P.; Joubert, J.; Tabrizi, S.J.; Rees, G. The human motor cortex microcircuit: Insights for neurodegenerative disease. Nat. Rev. Neurosci. 2020, 21, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Sesack, S.R.; Grace, A.A. Cortico-Basal Ganglia reward network: Microcircuitry. Neuropsychopharmacology 2010, 35, 27–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shepherd, G.M.G.; Yamawaki, N. Untangling the cortico-thalamo-cortical loop: Cellular pieces of a knotty circuit puzzle. Nat. Rev. Neurosci. 2021, 22, 389–406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hornykiewicz, O. Chemical neuroanatomy of the basal ganglia—Normal and in Parkinson’s disease. J. Chem. Neuroanat. 2001, 22, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kita, H.; Kita, T. Cortical stimulation evokes abnormal responses in the dopamine-depleted rat basal ganglia. J. Neurosci. 2011, 31, 10311–10322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCutcheon, R.A.; Abi-Dargham, A.; Howes, O.D. Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 2019, 42, 205–220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Q.; Wang, Y.; Liao, F.F.; Zhou, F.M. Dopaminergic inhibition of the inwardly rectifying potassium current in direct pathway medium spiny neurons in normal and parkinsonian striatum. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, W.; Ding, S.; Zhou, F.M. Dopaminergic treatment weakens medium spiny neuron collateral inhibition in the parkinsonian striatum. J. Neurophysiol. 2017, 117, 987–999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, W.; Li, L.; Yu, G.; Ding, S.; Li, C.; Zhou, F.M. Supersensitive presynaptic dopamine D2 receptor inhibition of the striatopallidal projection in nigrostriatal dopamine-deficient mice. J. Neurophysiol. 2013, 110, 2203–2216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, S.; Li, L.; Zhou, F.M. Nigral dopamine loss induces a global upregulation of presynaptic dopamine D1 receptor facilitation of the striatonigral GABAergic output. J. Neurophysiol. 2015, 113, 1697–1711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, Y.; Lanciego, J.; Kerkerian-Le-Goff, L.; Coulon, P.; Salin, P.; Kachidian, P.; Lei, W.; Del Mar, N.; Reiner, A. Differential organization of cortical inputs to striatal projection neurons of the matrix compartment in rats. Front. Syst. Neurosci. 2015, 9, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, T.; Wilson, C.J. Corticostriatal combinatorics: The implications of corticostriatal axonal arborizations. J. Neurophysiol. 2002, 87, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Economo, M.N.; Viswanathan, S.; Tasic, B.; Bas, E.; Winnubst, J.; Menon, V.; Graybuck, L.T.; Nguyen, T.N.; Smith, K.A.; Yao, Z.; et al. Distinct descending motor cortex pathways and their roles in movement. Nature 2018, 563, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Althobaiti, Y.S.; Almalki, A.H.; Das, S.C.; Alshehri, F.S.; Sari, Y. Effects of repeated high-dose methamphetamine and ceftriaxone post-treatments on tissue content of dopamine and serotonin as well as glutamate and glutamine. Neurosci. Lett. 2016, 634, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Muneoka, K.; Kuwagata, M.; Ogawa, T.; Shioda, S. Sex-specific effects of early neonatal progesterone treatment on dopamine and serotonin metabolism in rat striatum and frontal cortex. Life Sci. 2010, 87, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.; Sedvall, G.; Magnusson, O.; Kopp, J.; Halldin, C.; Farde, L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology 1994, 11, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Schmidt, P.; Dettmeyer, R.; Priemer, F.; Wittig, H.; Madea, B. A systematic regional study of dopamine and dopamine-derived salsolinol and norsalsolinol levels in human brain areas. Forensic Sci. Int. 1999, 105, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Seamans, J.K.; Yang, C.R. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004, 74, 1–58, Erratum in Prog. Neurobiol. 2004, 74, 321. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.-M. Whole-Brain Confocal Imaging Provides an Accurate Global View of the Nigral Dopamine System. Diagnostics 2025, 15, 1436. https://doi.org/10.3390/diagnostics15111436
Zhou F-M. Whole-Brain Confocal Imaging Provides an Accurate Global View of the Nigral Dopamine System. Diagnostics. 2025; 15(11):1436. https://doi.org/10.3390/diagnostics15111436
Chicago/Turabian StyleZhou, Fu-Ming. 2025. "Whole-Brain Confocal Imaging Provides an Accurate Global View of the Nigral Dopamine System" Diagnostics 15, no. 11: 1436. https://doi.org/10.3390/diagnostics15111436
APA StyleZhou, F.-M. (2025). Whole-Brain Confocal Imaging Provides an Accurate Global View of the Nigral Dopamine System. Diagnostics, 15(11), 1436. https://doi.org/10.3390/diagnostics15111436





