The Lung Microbiome and Its Impact on Obstructive Sleep Apnea: A Diagnostic Frontier
Abstract
1. Introduction
2. The Lung Microbiome in Health and Disease
2.1. Composition of the Lung Microbiome in Healthy Individuals
2.2. Potential Origin of Lung Microbiome
2.3. Pathological Dysbiosis and Its Implications for Respiratory Inflammation and Immune Response
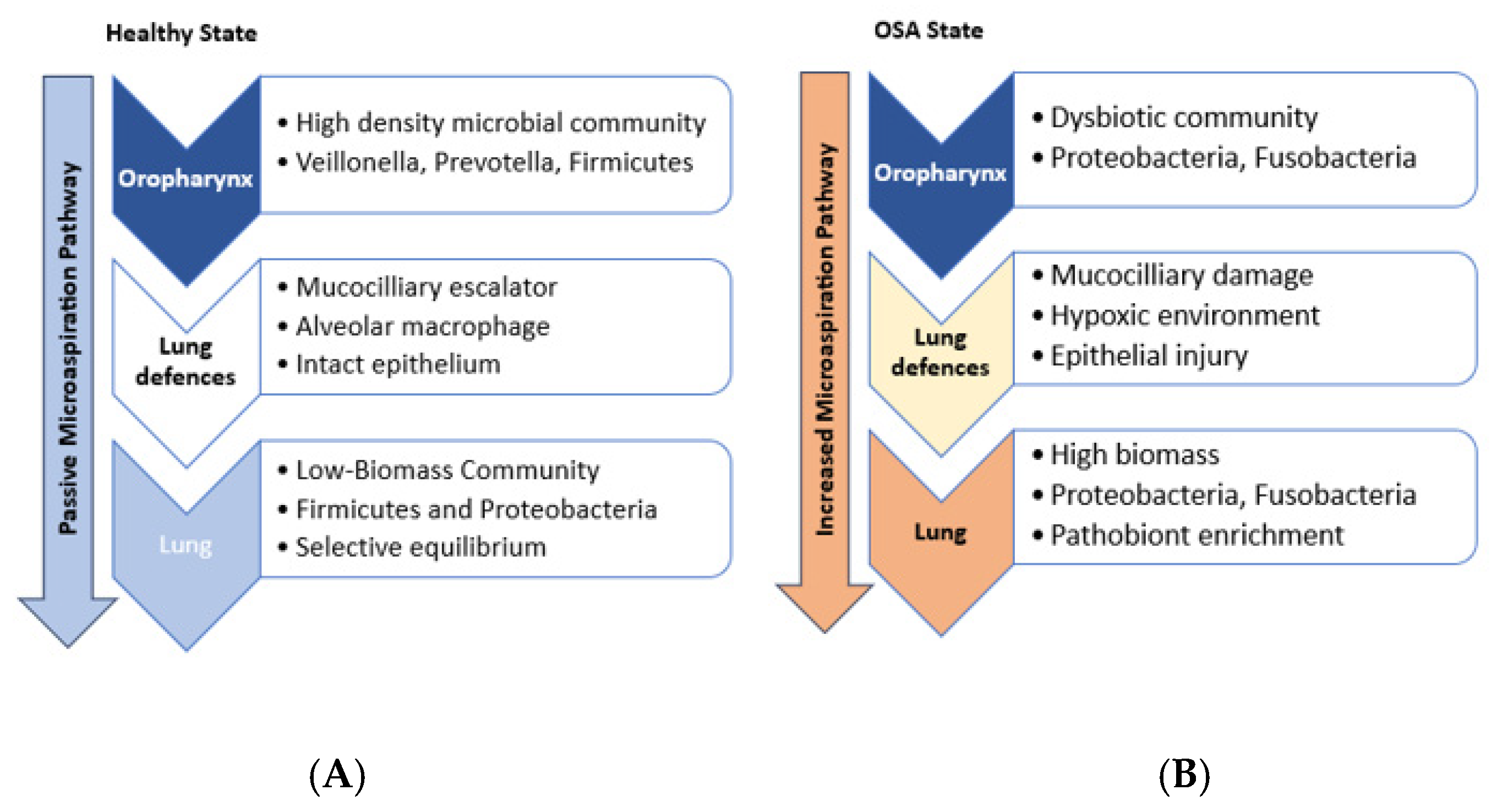
3. Microbiome Dysbiosis and OSA
3.1. Emerging Research on Microbial Profiles in OSA Patients
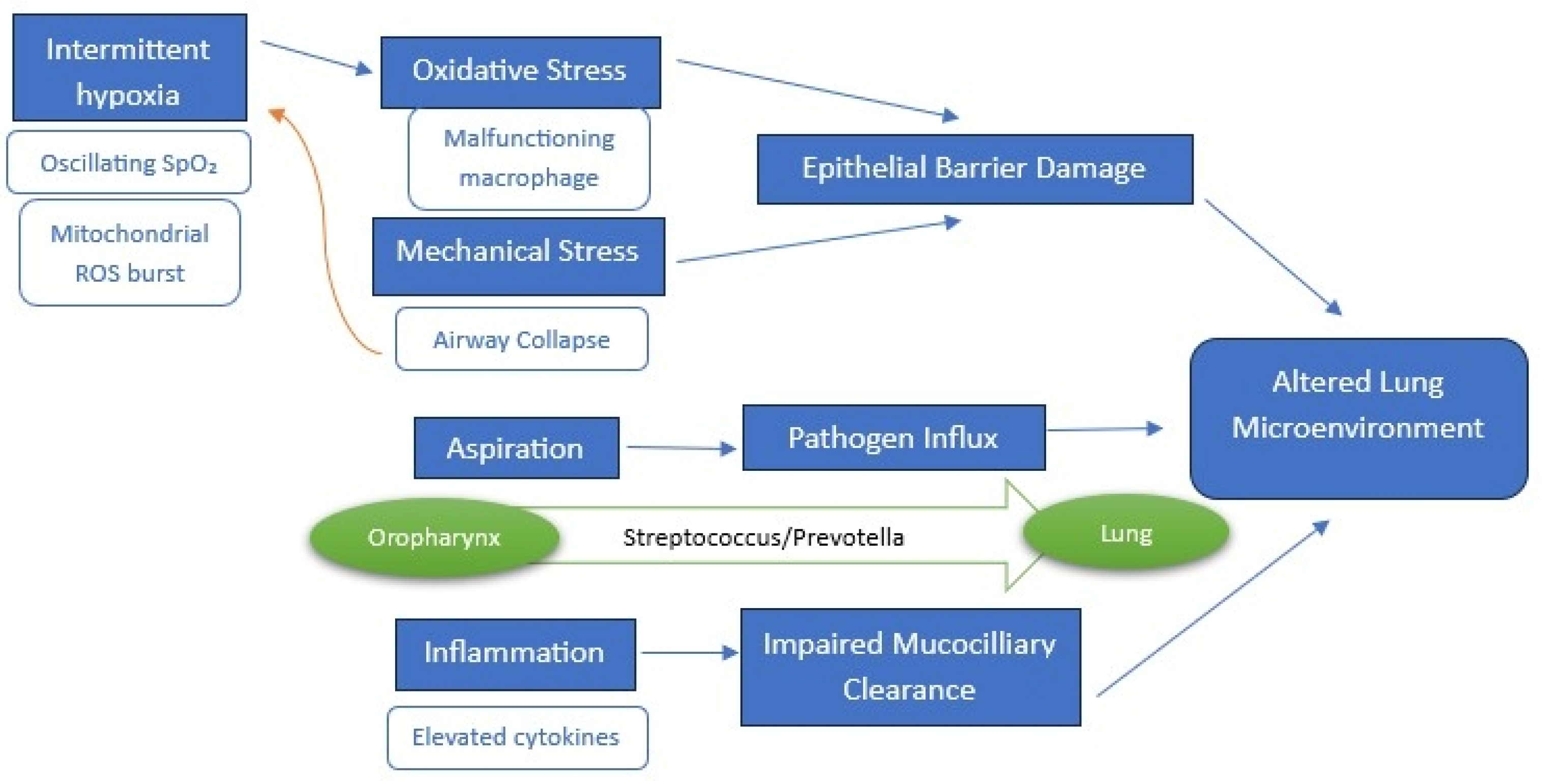
3.2. Mechanistic Links Between the Lung Microbiome and OSA
3.2.1. Intermittent Hypoxia and Systemic Inflammation
3.2.2. Mechanical Stress and Aspiration
3.2.3. Could Baseline Lung Microbiome Composition Increase OSA Risk or Severity?
4. Limitations
5. Conclusions and Future Research Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OSA | Obstructive Sleep Apnea |
| URT | Upper Respiratory Tract |
| IH | Intermittent Hypoxia |
| CPAP | Continuous Positive Airway Pressure |
| AHI | Apnea Hypopnea Index |
| LRT | Lower Respiratory Tract |
| SCFAs | Short Chain Fatty Acids |
| IL | Interleukin |
| SDB | Sleep Disordered Breathing |
| TNF-α | Tumor Necrosis Factor α |
| CRP | C Reactive Protein |
| EDS | Excessive Daytime Sleepiness |
| PHAL | Pharyngeal Lavage |
| COPD | Chronic Obstructive Pulmonary Disease |
References
- Whiteside, S.A.; McGinniss, J.E.; Collman, R.G. The lung microbiome: Progress and promise. J. Clin. Investig. 2021, 131, e150473. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, J.; Zhou, X. Lung microbiome: New insights into the pathogenesis of respiratory diseases. Signal Transduct. Target. Ther. 2024, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Bassis, C.M.; Erb-Downward, J.R.; Dickson, R.P.; Freeman, C.M.; Schmidt, T.M.; Young, V.B.; Beck, J.M.; Curtis, J.L.; Huffnagle, G.B. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 2015, 6, e00037. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Dickson, R.P.; Lukacs, N.W. The respiratory tract microbiome and lung inflammation: A two-way street. Mucosal Immunol. 2017, 10, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.; Garay-Malpartida, M.; Faintuch, J. Lung microbiome and origins of the respiratory diseases. Curr. Res. Immunol. 2023, 4, 100065. [Google Scholar] [CrossRef]
- Durack, J.; Huang, Y.; Nariya, S.; Christian, L.; Ansel, K.M.; Beigelman, A.; Castro, M.; Dyer, A.M.; Israel, E.; Kraft, M.; et al. Bacterial biogeography of adult airways in atopic asthma. Microbiome 2018, 6, 104. [Google Scholar] [CrossRef]
- Erb-Downward, J.R.; Thompson, D.L.; Han, M.K.; Freeman, C.M.; McCloskey, L.; Schmidt, L.A.; Young, V.B.; Toews, G.B.; Curtis, J.L.; Sundaram, B.; et al. Analysis of the lung microbiome in the ‘healthy’ smoker and in COPD. PLoS ONE 2011, 6, e16384. [Google Scholar] [CrossRef]
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population-A review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar] [CrossRef]
- Alakörkkö, I.; Törmälehto, S.; Leppänen, T.; McNicholas, W.T.; Arnardottir, E.S.; Sund, R. The economic cost of obstructive sleep apnea: A systematic review. Sleep Med. Rev. 2023, 72, 101854. [Google Scholar] [CrossRef]
- Pataka, A. ‘One Size Doesn’t Fit for All’: There Is a Need for Targeted Personalized Therapy in Obstructive Sleep Apnea Syndrome. J. Clin. Med. 2022, 11, 3595. [Google Scholar] [CrossRef]
- Wu, B.G.; Segal, L.N. Lung Microbiota and Its Impact on the Mucosal Immune Phenotype. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shao, L.; Heizhati, M.; Wu, T.; Yao, X.; Wang, W.; Wang, L.; Li, N. Oropharyngeal microbiome in obstructive sleep apnea: Decreased diversity and abundance. J. Clin. Sleep Med. 2019, 15, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.N.; Clemente, J.C.; Tsay, J.-C.J.; Koralov, S.B.; Keller, B.C.; Wu, B.G.; Li, Y.; Shen, N.; Ghedin, E.; Morris, A.; et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat. Microbiol. 2016, 1, 16031. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Erb-Downward, J.R.; Huffnagle, G.B. Homeostasis and its disruption in the lung microbiome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, 1047–1055. [Google Scholar] [CrossRef]
- Man, W.H.; De Steenhuijsen Piters, W.A.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef]
- Bach, L.L.; Ram, A.; Ijaz, U.Z.; Evans, T.J.; Lindström, J. A Longitudinal Study of the Human Oropharynx Microbiota Over Time Reveals a Common Core and Significant Variations with Self-Reported Disease. Front. Microbiol. 2021, 11, 573969. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Freeman, C.M.; McCloskey, L.; Falkowski, N.R.; Huffnagle, G.B.; Curtis, J.L. Bacterial topography of the healthy human lower respiratory tract. mBio 2017, 8, e02287-16. [Google Scholar] [CrossRef]
- Carmody, L.A.; Zhao, J.; Schloss, P.D.; Petrosino, J.F.; Murray, S.; Young, J.B.; Li, J.Z.; LiPuma, J.J. Changes in cystic fibrosis airway microbiota at pulmonary exacerbation. Ann. Am. Thorac. Soc. 2013, 10, 179–187. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Du, W.; Liu, W.; Dai, R.; Tang, W.; Wang, P.; Zhang, C.; Shi, G. Fungal and bacterial microbiome dysbiosis and imbalance of trans-kingdom network in asthma. Clin. Transl. Allergy 2020, 10, 42. [Google Scholar] [CrossRef]
- Sharma, A.; Laxman, B.; Naureckas, E.T.; Hogarth, D.K.; Sperling, A.I.; Solway, J.; Ober, C.; Gilbert, J.A.; White, S.R. Associations between fungal and bacterial microbiota of airways and asthma endotypes. J. Allergy Clin. Immunol. 2019, 144, 1214–1227.e7. [Google Scholar] [CrossRef]
- Charlson, E.S.; Bittinger, K.; Chen, J.; Diamond, J.M.; Li, H.; Collman, R.G.; Bushman, F.D. Assessing Bacterial Populations in the Lung by Replicate Analysis of Samples from the Upper and Lower Respiratory Tracts. PLoS ONE 2012, 7, e42786. [Google Scholar] [CrossRef] [PubMed]
- Marsland, B.J.; Gollwitzer, E.S. Host-microorganism interactions in lung diseases. Nat. Rev. Immunol. 2014, 14, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.W.; Evangelista III, J.S.; Schmieder, R.; Bailey, B.; Haynes, M.; Furlan, M.; Maughan, H.; Edwards, R.; Rohwer, F.; Conrad, D. Clinical insights from metagenomic analysis of sputum samples from patients with cystic fibrosis. J. Clin. Microbiol. 2014, 52, 425–437. [Google Scholar] [CrossRef]
- Lysholm, F.; Wetterbom, A.; Lindau, C.; Darban, H.; Bjerkner, A.; Fahlander, K.; Lindberg, A.M.; Perrson, B.; Allander, T.; Andersson, B. Characterization of the viral microbiome in patients with severe lower respiratory tract infections, using metagenomic sequencing. PLoS ONE 2012, 7, e30875. [Google Scholar] [CrossRef]
- Abbas, A.A.; Taylor, L.J.; Dothard, M.I.; Leiby, J.S.; Fitzgerald, A.S.; Khatib, L.A.; Collman, R.G.; Bushman, F.D. Redondoviridae, a Family of Small, Circular DNA Viruses of the Human Oro-Respiratory Tract Associated with Periodontitis and Critical Illness. Cell Host Microbe 2019, 25, 719–729.e4. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, N.; Li, Y.; Lu, R.; Wang, H.; Liu, G.; Zou, X.; Xie, Z.; Tan, W. Metagenomic analysis of viral genetic diversity in respiratory samples from children with severe acute respiratory infection in China. Clin. Microbiol. Infect. 2016, 22, 458.e1–458.e9. [Google Scholar] [CrossRef]
- MacDuff, D.A.; Reese, T.A.; Kimmey, J.M.; Weiss, L.A.; Song, C.; Zhang, X.; Kambal, A.; Duan, E.; Carrero, J.A.; Boisson, B.; et al. Phenotypic complementation of genetic immunodeficiency by chronic herpesvirus infection. Elife 2015, 4, e04494. [Google Scholar] [CrossRef]
- Sun, L.; Miyoshi, H.; Origanti, S.; Nice, T.J.; Barger, A.C.; Manieri, N.A.; Fogel, L.A.; French, A.R.; Piwnica-Worms, D.; Piwnica-Worms, H.; et al. Type I interferons link viral infection to enhanced epithelial turnover and repair. Cell Host Microbe 2015, 17, 85–97. [Google Scholar] [CrossRef]
- Willner, D.; Furlan, M.; Haynes, M.; Schmieder, R.; Angly, F.E.; Silva, J.; Tammadoni, S.; Nosrat, B.; Conrad, D.; Rowher, F. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS ONE 2009, 4, e7370. [Google Scholar] [CrossRef]
- Lim, Y.W.; Schmieder, R.; Haynes, M.; Willner, D.; Furlan, M.; Youle, M.; Abbott, K.; Edwards, R.; Evangelista, J.; Conrad, D.; et al. Metagenomics and metatranscriptomics: Windows on CF-associated viral and microbial communities. J. Cyst. Fibros. 2013, 12, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Garaci, E.; Pariano, M.; Nunzi, E.; Costantini, C.; Bellet, M.M.; Antognelli, C.; Russo, M.A.; Romani, L. Bacteria and fungi of the lung: Allies or enemies? Front. Pharmacol. 2024, 15, 1497173. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Charlson, E.S.; Bittinger, K.; Haas, A.R.; Fitzgerald, A.S.; Frank, I.; Yadav, A.; Bushman, F.D.; Collman, R.G. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am. J. Respir. Crit. Care Med. 2011, 184, 957–963. [Google Scholar] [CrossRef]
- Kohno, A.; Kohno, M.; Ohkoshi, S. Swallowing and aspiration during sleep in patients with obstructive sleep apnea versus control individuals. Sleep 2022, 45, zsac036. [Google Scholar] [CrossRef]
- Mammen, M.J.; Scannapieco, F.A.; Sethi, S. Oral-lung microbiome interactions in lung diseases. Periodontology 2000 2020, 83, 234–241. [Google Scholar] [CrossRef]
- Dong, Z.; Ma, J.; Qiu, J.; Ren, Q.; Shan, Q.; Duan, X.; Li, G.; Zuo, Y.Y.; Qi, Y.; Liu, Y.; et al. VIROLOGY Airborne Fine Particles Drive H1N1 Viruses Deep into the Lower Respiratory Tract and Distant Organs. Sci. Adv. 2023, 9, eadf2165. [Google Scholar] [CrossRef]
- Morris, A.; Beck, J.M.; Schloss, P.D.; Campbell, T.B.; Crothers, K.; Curtis, J.L.; Flores, S.C.; Fontenot, A.P.; Ghedin, E.; Huang, L.; et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am. J. Respir. Crit. Care Med. 2013, 187, 1067–1075. [Google Scholar] [CrossRef]
- Saeedi, P.; Salimian, J.; Ahmadi, A.; Fooladi, A.A.I. The transient but not resident (TBNR) microbiome: A Yin Yang model for lung immune system. Inhal. Toxicol. 2015, 27, 451–461. [Google Scholar] [CrossRef]
- Wu, H.; Kuzmenko, A.; Wan, S.; Schaffer, L.; Weiss, A.; Fisher, J.H.; Kim, K.S.; McCormack, F.X. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J. Clin. Investig. 2003, 111, 1589–1602. [Google Scholar] [CrossRef]
- Abeles, S.R.; Ly, M.; Santiago-Rodriguez, T.M.; Pride, D.T. Effects of long term antibiotic therapy on human oral and fecal viromes. PLoS ONE 2015, 10, e0134941. [Google Scholar] [CrossRef] [PubMed]
- Abeles, S.R.; Pride, D.T. Molecular bases and role of viruses in the human microbiome. J. Mol. Biol. 2014, 426, 3892–3906. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichahn, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-Based Metagenomics Analysis Reveals Markers for Gut Microbiome Composition and Diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frati, F.; Salvatori, C.; Incorvaia, C.; Bellucci, A.; Di Cara, G.; Marcucci, F.; Esposito, S. The role of the microbiome in asthma: The gut–lung axis. Int. J. Mol. Sci. 2018, 20, 123. [Google Scholar] [CrossRef]
- Marsland, B.J.; Trompette, A.; Gollwitzer, E.S. The gut-lung axis in respiratory disease. Ann. Am. Thorac. Soc. 2015, 12, S150–S156. [Google Scholar] [CrossRef]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Vaseghi-Shanjani, M.; Afkhami, S.; Grondin, J.A.; Kang, A.; D’Agostino, M.R.; Yao, Y.; Jain, S.; Zganiacz, A.; Kroezen, Z.; et al. Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut–lung axis. Nat. Immunol. 2022, 23, 1687–1702. [Google Scholar] [CrossRef]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef]
- Segal, L.N.; Rom, W.N.; Weiden, M.D. Lung microbiome for clinicians: New discoveries about bugs in healthy and diseased lungs. Ann. Am. Thorac. Soc. 2014, 11, 108–116. [Google Scholar] [CrossRef]
- Han, G.; Vaishnava, S. Microbial underdogs: Exploring the significance of low-abundance commensals in host-microbe interactions. Exp. Mol. Med. 2023, 55, 2498–2507. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.Y.; Yoon, H.S.; Rho, M.; Sung, J.; Song, Y.-M.; Lee, K.; Ko, G. Analysis of the association between host genetics, smoking, and sputum microbiota in healthy humans. Sci. Rep. 2016, 6, 23745. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E.; Napolitano, M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front. Microbiol. 2015, 6, 1050. [Google Scholar] [CrossRef] [PubMed]
- Cicchinelli, S.; Rosa, F.; Manca, F.; Zanza, C.; Ojetti, V.; Covino, M.; Candelli, M.; Gasbarrini, A.; Franceschi, F.; Piccioni, A. The Impact of Smoking on Microbiota: A Narrative Review. Biomedicines 2023, 11, 1144. [Google Scholar] [CrossRef]
- Du, S.; Shang, L.; Zou, X.; Deng, X.; Sun, A.; Mu, S.; Zhao, J.; Wang, Y.; Feng, X.; Li, B.; et al. Azithromycin Exposure Induces Transient Microbial Composition Shifts and Decreases the Airway Microbiota Resilience from Outdoor PM 2.5 Stress in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Microbiol. Spectr. 2023, 11, e0206622. [Google Scholar] [CrossRef]
- Xue, Y.; Chu, J.; Li, Y.; Kong, X. The influence of air pollution on respiratory microbiome: A link to respiratory disease. Toxicol. Lett. 2020, 334, 14–20. [Google Scholar] [CrossRef]
- Pragman, A.A.; Lyu, T.; Baller, J.A.; Gould, T.J.; Kelly, R.F.; Reilly, C.S.; Isaacson, R.E.; Wendt, C.H. The lung tissue microbiota of mild and moderate chronic obstructive pulmonary disease. Microbiome 2018, 6, 7. [Google Scholar] [CrossRef]
- Turek, E.M.; Cox, M.J.; Hunter, M.; Hui, J.; James, P.; Willis-Owen, S.A.G.; Cuthbertson, L.; James, A.; Musk, A.W.; Moffat, M.F.; et al. Airway microbial communities, smoking and asthma in a general population sample. EBioMedicine 2021, 71, 103538. [Google Scholar] [CrossRef]
- Goto, T. Airway microbiota as a modulator of lung cancer. Int. J. Mol. Sci. 2020, 21, 3044. [Google Scholar] [CrossRef]
- Wu, B.G.; Sulaiman, I.; Tsay, J.-C.J.; Perez, L.; Franca, B.; Li, Y.; Wang, J.; Gonzalez, A.N.; El-Ashmawy, M.; Carpenito, J.; et al. Episodic aspiration with oral commensals induces a MyD88-dependent, pulmonary T-helper cell type 17 response that mitigates susceptibility to streptococcus pneumoniae. Am. J. Respir. Crit. Care Med. 2021, 203, 1099–1111. [Google Scholar] [CrossRef]
- Laiman, V.; Lo, Y.-C.; Chen, H.-C.; Yuan, T.-H.; Hsiao, T.-C.; Chen, J.-K.; Chang, C.-W.; Lin, T.-H.; Li, S.-J.; Chen, Y.-Y.; et al. Effects of antibiotics and metals on lung and intestinal microbiome dysbiosis after sub-chronic lower-level exposure of air pollution in ageing rats. Ecotoxicol. Environ. Saf. 2022, 246, 114164. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, P.; Wu, X.; Gong, Z.; Yang, X.; Zhu, H.; Zhang, J.; Hu, Y.; Li, G.; Sang, N.; et al. Lung injuries induced by ozone exposure in female mice: Potential roles of the gut and lung microbes. Environ. Int. 2024, 183, 108422. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Du, R.; Ren, W.; Lu, Z.; Fu, P. Seasonal variation characteristic of inhalable microbial communities in PM2.5 in Beijing city, China. Sci. Total Environ. 2017, 610–611, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Yasutomi, Y.; Chiba, A.; Haga, K.; Murayama, G.; Makiyama, A.; Kuga, T.; Watanabe, M.; Okamoto, R.; Nagahara, A.; Nagaishi, T.; et al. Activated Mucosal-associated Invariant T Cells Have a Pathogenic Role in a Murine Model of Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 81–93. [Google Scholar] [CrossRef]
- Romani, L.; Zelante, T.; De Luca, A.; Iannitti, R.G.; Moretti, S.; Bartoli, A.; Aversa, F.; Puccetti, P. Microbiota control of a tryptophan-AhR pathway in disease tolerance to fungi. Eur. J. Immunol. 2014, 44, 3192–3200. [Google Scholar] [CrossRef]
- Remot, A.; Descamps, D.; Noordine, M.-L.; Boukadiri, A.; Mathieu, E.; Robert, V.; Riffault, S.; Lambrecht, B.; Langella, P.; Hammad, H.; et al. Bacteria isolated from lung modulate asthma susceptibility in mice. ISME J. 2017, 11, 1061–1074. [Google Scholar] [CrossRef]
- Vicente, E.; Marin, J.M.; Carrizo, S.J.; Osuna, C.S.; Gonzalez, R.; Marin-Oto, M.; Forner, M.; Vicente, P.; Cubero, P.; Gil, A.V.; et al. Upper airway and systemic inflammation in obstructive sleep apnoea. Eur. Respir. J. 2016, 48, 1108–1117. [Google Scholar] [CrossRef]
- Wu, B.G.; Sulaiman, I.; Wang, J.; Shen, N.; Clemente, J.C.; Li, Y.; Laumbach, R.J.; Lu, S.-E.; Udasin, I.; Le-Hoang, O.; et al. Severe obstructive sleep apnea is associated with alterations in the nasal microbiome and an increase in inflammation. Am. J. Respir. Crit. Care Med. 2019, 199, 99–109. [Google Scholar] [CrossRef]
- Hong, S.N.; Jin Kim, K.; Baek, M.-G.; Yi, H.; Hoon Lee, S.; Kim, D.-Y.; Hee Lee, C.; Shin, C.; Rhee, C.-S. Association of obstructive sleep apnea severity with the composition of the upper airway microbiome. J. Clin. Sleep Med. 2022, 18, 505–515. [Google Scholar] [CrossRef]
- Basu, P.; Williams, A.; O’Brien, M.T.; Brouns, M.; Edwards, P. A case of Finegoldia magna (formerly Peptostreptococcus magnus) infection mimicking disseminated malignancy. Int. J. Infect. Dis. 2016, 53, 12–14. [Google Scholar] [CrossRef][Green Version]
- Brüggemann, H.; Jensen, A.; Nazipi, S.; Aslan, H.; Meyer, R.L.; Poehlein, A.; Brzuszkiewicz, E.; Al-Zeer, M.A.; Brinkman, V.; Söderquist, B. Pan-genome analysis of the genus Finegoldia identifies two distinct clades, strain-specific heterogeneity, and putative virulence factors. Sci. Rep. 2018, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Mcdermott, A.J.; Huffnagle, G.B. The microbiome and regulation of mucosal immunity. Immunology 2014, 142, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.V.; Schwartz, A.R. The pathogenesis of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 1358–1372. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Systemic inflammation: A key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax 2009, 64, 631–636. [Google Scholar] [CrossRef]
- Lenk, C.; Messbacher, M.E.; Abel, J.; Mueller, S.K. The influence of obstructive sleep apnea and continuous positive airway pressure on the nasal microbiome. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2605–2618. [Google Scholar]
- Rajilić-Stojanović, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Ivanchenko, O.A.; Karpishchenko, S.A.; Kozlov, R.S.; Krechikova, O.I.; Otvagin, I.V.; Sopko, O.N.; Piskunov, G.Z.; Lopatin, A.S. The microbiome of the maxillary sinus and middle nasal meatus in chronic rhinosinusitis. Rhinology 2016, 54, 68–74. [Google Scholar] [CrossRef]
- Boase, S.; Foreman, A.; Cleland, E.; Tan, L.; Melton-Kreft, R.; Pant, H.; Hu, F.Z.; Ehrlich, G.D.; Wormald, P.-J. The microbiome of chronic rhinosinusitis: Culture, molecular diagnostics and biofilm detection. BMC Infect. Dis. 2013, 13, 210. [Google Scholar] [CrossRef]
- Lu, D.; Yao, X.; Abulimiti, A.; Cai, L.; Zhou, L.; Hong, J.; Li, N. Profiling of lung microbiota in the patients with obstructive sleep apnea. Medicine 2018, 97, e11175. [Google Scholar] [CrossRef]
- El Hage Chehade, N.; Fu, Y.; Ghoneim, S.; Shah, S.; Song, G.; Fass, R. Association between obstructive sleep apnea and gastroesophageal reflux disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2023, 38, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Sahlman, J.; Miettinen, K.; Peuhkurinen, K.; Seppä, J.; Peltonen, M.; Herder, C.; Punnonen, K.; Vanninen, E.; Gylling, H.; Partinen, M.; et al. The activation of the inflammatory cytokines in overweight patients with mild obstructive sleep apnoea: Sleep apnea and inflammation. J. Sleep. Res. 2009, 19, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B.; Lännergren, J.; Westerblad, H. Respiratory and limb muscle weakness induced by tumor necrosis factor-α: Involvement of muscle myofilaments. Am. J. Respir. Crit. Care Med. 2002, 166, 479–484. [Google Scholar] [CrossRef] [PubMed]
- McDonald, F.B.; Williams, R.; Sheehan, D.; O’Halloran, K.D. Early life exposure to chronic intermittent hypoxia causes upper airway dilator muscle weakness, which persists into young adulthood. Exp. Physiol. 2015, 100, 947–966. [Google Scholar] [CrossRef]
- O’Halloran, K.D.; Lewis, P.; McDonald, F. Sex, stress and sleep apnoea: Decreased susceptibility to upper airway muscle dysfunction following intermittent hypoxia in females. Respir. Physiol. Neurobiol. 2017, 245, 76–82. [Google Scholar] [CrossRef]
- Krueger, J.M. The Role of Cytokines in Sleep Regulation. Curr. Pharm. Des. 2008, 14, 3408–3416. [Google Scholar] [CrossRef]
- Griffioen, K.J.S.; Kamendi, H.W.; Gorini, C.J.; Bouairi, E.; Mendelowitz, D. Reactive oxygen species mediate central cardiorespiratory network responses to acute intermittent hypoxia. J. Neurophysiol. 2007, 97, 2059–2066. [Google Scholar] [CrossRef]
- Cofta, S.; Wysocka, E.; Piorunek, T.; Rzymkowska, M.; Batura-Gabryel, H.; Torlinski, L. Oxidative Stress Markers in the Blood of Persons with Different Stages of Obstructive Sleep Apnea Syndrome. J. Physiol. Pharmacol. 2008, 59, 183–190. [Google Scholar]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum inflammatory markers in obstructive sleep apnea: A meta-analysis. J. Clin. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef]
- Khalyfa, A.; Serpero, L.D.; Kheirandish-Gozal, L.; Capdevila, O.S.; Gozal, D. TNF-α gene polymorphisms and excessive daytime sleepiness in pediatric obstructive sleep apnea. J. Pediatr. 2011, 158, 77–82. [Google Scholar] [CrossRef]
- Almpanidou, P.; Hadjigeorgiou, G.; Gourgoulianis, K.; Papadimitriou, A. Association of tumor necrosis factor-α gene polymorphism (-308) and obstructive sleep apnea-hypopnea syndrome. Hippokratia 2012, 16, 217–220. [Google Scholar] [PubMed]
- Vgontzas, A.N.; Zoumakis, E.; Lin, H.M.; Bixler, E.O.; Trakada, G.; Chrousos, G.P. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-α antagonist. J. Clin. Endocrinol. Metab. 2004, 89, 4409–4413. [Google Scholar] [CrossRef] [PubMed]
- Baessler, A.; Nadeem, R.; Harvey, M.; Madbouly, E.; Younus, A.; Sajid, H.; Naseem, J.; Asif, A.; Bawaadam, H. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers—A meta-analysis. J. Inflamm. 2013, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Van Eyck, A.; Van Hoorenbeeck, K.; De Winter, B.Y.; Van Gaal, L.; De Backer, W.; Verhulst, S.L. Sleep-disordered breathing, systemic adipokine secretion, and metabolic dysregulation in overweight and obese children and adolescents. Sleep Med. 2017, 30, 52–56. [Google Scholar] [CrossRef]
- Smith, D.F.; Hossain, M.; Hura, A.; Huang, G.; McConnell, K.; Ishman, S.L.; Amin, R.S. Inflammatory milieu and cardiovascular homeostasis in children with obstructive sleep apnea. Sleep 2017, 40, zsx022. [Google Scholar] [CrossRef]
- Maniaci, A.; Iannella, G.; Cocuzza, S.; Vicini, C.; Magliulo, G.; Ferlito, S.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Nicolai, A.; et al. Oxidative stress and inflammation biomarker expression in obstructive sleep apnea patients. J. Clin. Med. 2021, 10, 277. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Feng, M.; Huang, X.; Li, C.; Han, F.; Zhang, Q.; Gao, X. Altered Salivary Microbiota in Patients with Obstructive Sleep Apnea Comorbid Hypertension. Nat. Sci. Sleep 2022, 14, 593–607. [Google Scholar] [CrossRef]
- Jia, P.; Zou, J.; Yin, S.; Chen, F.; Yi, H.; Zhang, Q. Analysis of the Salivary Microbiome in Obstructive Sleep Apnea Syndrome Patients. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 6682020. [Google Scholar] [CrossRef]
- Téllez Corral, M.A.; Herrera Daza, E.; Cuervo Jimenez, H.K.; Bravo Becerra, M.M.; Villamil, J.C.; Hidalgo Martinez, P.; Roa Molina, N.S.; Otero, L.; Cortés, M.E.; Parra Giraldo, C.M. Cryptic Oral Microbiota: What Is Its Role as Obstructive Sleep Apnea-Related Periodontal Pathogens? Int. J. Environ. Res. Public Health 2023, 20, 1740. [Google Scholar] [CrossRef]
- Kimoff, R.J.; Hamid, Q.; Divangahi, M.; Hussain, S.; Bao, W.; Naor, N.; Payne, R.J.; Ariyarajah, A.; Mulrain, K.; Petrof, B.J. Increased upper airway cytokines and oxidative stress in severe obstructive sleep apnoea. Eur. Respir. J. 2010, 38, 89–97. [Google Scholar] [CrossRef]
- Ryan, C.F.; Lowe, A.A.; Li, D.; Fleetham, J.A. Magnetic Resonance Imaging of the Upper Airway in Obstructive Sleep Apnea before and after Chronic Nasal Continuous Positive Airway Pressure Therapy. Am. Rev. Respir. Dis. 1991, 144, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Li, K.K.; Chen, N.H.; Wang, C.J.; Liao, Y.F.; Wang, P.C. Three-dimensional computed tomography and polysomnography findings after extended uvulopalatal flap surgery for obstructive sleep apnea. Am. J. Otolaryngol.-Head. Neck Med. Surg. 2005, 26, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Jelic, S.; Padeletti, M.; Kawut, S.M.; Higgins, C.; Canfield, S.M.; Onat, D.; Colombo, P.C.; Basner, R.C.; Factor, P.; LeJemtel, T.H. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 2008, 117, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Bilston, L.E.; Gandevia, S.C. Biomechanical properties of the human upper airway and their effect on its behavior during breathing and in obstructive sleep apnea. J. Appl. Physiol. 2014, 116, 314–324. [Google Scholar] [CrossRef]
- Paulsen, F.P.; Steven, P.; Tsokos, M.; Jungmann, K.; Müller, A.; Verse, T.; Pirsig, W. Upper airway epithelial structural changes in obstructive sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 2002, 166, 501–509. [Google Scholar] [CrossRef]
- Boyd, J.H.; Petrof, B.J.; Hamid, Q.; Fraser, R.; Kimoff, R.J. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2004, 170, 541–546. [Google Scholar] [CrossRef]
- Puig, F.; Rico, F.; Almendros, I.; Montserrat, J.M.; Navajas, D.; Farre, R. Vibration Enhances Interleukin-8 Release in a Cell Model of Snoring-Induced Airway Inflammation. Sleep 2005, 10, 1312–1316. [Google Scholar] [CrossRef]
- Kikuchi, R.; Watabe, N.; Konno, T.; Mishina, N.; Sekizawa, K.; Sasaki, H. High Incidence of Silent Aspiration in Elderly Patients with Community-Acquired Pneumonia. Am. J. Respir. Crit. Care Med. 1994, 150, 251–253. [Google Scholar] [CrossRef]
- Sato, K.; Chitose, S.I.; Sato, K.; Sato, F.; Ono, T.; Umeno, H. Recurrent aspiration pneumonia precipitated by obstructive sleep apnea. Auris Nasus Larynx 2021, 48, 659–665. [Google Scholar] [CrossRef]
- Zhang, Q.; Cox, M.; Liang, Z.; Brinkmann, F.; Cardenas, P.A.; Duff, R.; Bhavsar, P.; Cookson, W.; Moffatt, M.; Chung, K.F. Airway microbiota in severe asthma and relationship to asthma severity and phenotypes. PLoS ONE 2016, 11, e0152724. [Google Scholar] [CrossRef]
- Owens, R.L.; Macrea, M.M.; Teodorescu, M. The overlaps of asthma or COPD with OSA: A focused review. Respirology 2017, 22, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Mannion, J.M.; McLoughlin, R.M.; Lalor, S.J. The Airway Microbiome-IL-17 Axis: A Critical Regulator of Chronic Inflammatory Disease. Clin. Rev. Allergy Immunol. 2022, 64, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Green, B.J.; Wiriyachaiporn, S.; Grainge, C.; Rogers, G.B.; Kehagia, V.; Lau, L.; Carroll, M.P.; Bruce, K.D.; Howarth, P.H. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS ONE 2014, 9, e100645. [Google Scholar] [CrossRef]
- Jounio, U.; Juvonen, M.; Bloigu, A.; Silvennoinen-Kassinen, S.; Kaijalainen, T.; Kauma, H.; Peitso, A.; Saukkoriipi, A.; Vainio, O.; Harju, T.; et al. Pneumococcal carriage is more common in asthmatic than in non-asthmatic young men. Clin. Respir. J. 2009, 4, 222–229. [Google Scholar] [CrossRef]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.M.; Muraro, A.; Fleisher, T.A. The microbiome in allergic disease: Current understanding and future opportunities—2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D.L.; Chen, Z.; Shankar, V.; Tyberg, M.; Vicencio, A.; Burk, R. Lower airway microbiota and mycobiota in children with severe asthma. J. Allergy Clin. Immunol. 2018, 141, 808–811.e7. [Google Scholar] [CrossRef]
- Huang, Y.J.; Nelson, C.E.; Brodie, E.L.; DeSantis, T.Z.; Baek, M.S.; Liu, J.; Woyke, T.; Allgaier, M.; Bristow, J.; Wiener-Kronish, J.P.; et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J. Allergy Clin. Immunol. 2011, 127, 372.e3–381.e3. [Google Scholar] [CrossRef]
- Gutierrez, M.J.; Nino, G.; Landeo-Gutierez, J.S.; Weiss, M.R.; Preciado, D.A.; Hong, X.; Wang, X. Lower respiratory tract infections in early life are associated with obstructive sleep apnea diagnosis during childhood in a large birth cohort. Sleep 2021, 44, zsab198. [Google Scholar] [CrossRef]
- Bisgaard, H.; Northman Hermansen, M.; Buchvald, F.; Loland, L.; Brydensholt Halkjaer, L.; Bønnelykke, K.; Brasholt, M.; Heltberg, A.; Vissing, N.H.; Vester Thorsen, S.; et al. Childhood Asthma after Bacterial Colonization of the Airway in Neonates. N. Engl. J. Med. 2007, 357, 1487–1495. [Google Scholar] [CrossRef]
- Larsen, J.M.; Brix, S.; Thysen, A.H.; Birch, S.; Rasmussen, M.A.; Bisgaard, H. Children with asthma by school age display aberrant immune responses to pathogenic airway bacteria as infants. J. Allergy Clin. Immunol. 2014, 133, 1008–1013. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, J.; Wu, Y. Oxidative stress and inflammation mediate the association between elevated oxidative balance scores and improved sleep quality: Evidence from NHANES. Front. Nutr. 2024, 11, 1469779. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karkala, A.; Kotoulas, S.-C.; Tzinas, A.; Massa, E.; Mouloudi, E.; Gkakou, F.; Pataka, A. The Lung Microbiome and Its Impact on Obstructive Sleep Apnea: A Diagnostic Frontier. Diagnostics 2025, 15, 1431. https://doi.org/10.3390/diagnostics15111431
Karkala A, Kotoulas S-C, Tzinas A, Massa E, Mouloudi E, Gkakou F, Pataka A. The Lung Microbiome and Its Impact on Obstructive Sleep Apnea: A Diagnostic Frontier. Diagnostics. 2025; 15(11):1431. https://doi.org/10.3390/diagnostics15111431
Chicago/Turabian StyleKarkala, Aliki, Serafeim-Chrysovalantis Kotoulas, Asterios Tzinas, Eleni Massa, Eleni Mouloudi, Foteini Gkakou, and Athanasia Pataka. 2025. "The Lung Microbiome and Its Impact on Obstructive Sleep Apnea: A Diagnostic Frontier" Diagnostics 15, no. 11: 1431. https://doi.org/10.3390/diagnostics15111431
APA StyleKarkala, A., Kotoulas, S.-C., Tzinas, A., Massa, E., Mouloudi, E., Gkakou, F., & Pataka, A. (2025). The Lung Microbiome and Its Impact on Obstructive Sleep Apnea: A Diagnostic Frontier. Diagnostics, 15(11), 1431. https://doi.org/10.3390/diagnostics15111431






