Abstract
Background/Objectives: Lung-protective ventilation (LPV) reduces postoperative pulmonary complications (PPCs) in obese patients. While the roles of low tidal volume and positive end-expiratory pressure (PEEP) in LPV have been established in patients with healthy lungs, the protective effect of alveolar recruitment strategies (ARSs) remains a subject of debate. This study aims to evaluate the benefit of ARSs in patients with low-to-moderate risk according to the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) score undergoing gynecologic cancer surgery with LPV and low tidal volume intraoperatively. Methods: A total of 88 obese patients were evaluated in this study. They were divided into two groups as the non-ARS group (non-ARS) and the ARS group (ARS). Intraoperative hemodynamics, blood gas analyses, respiratory mechanics, mechanical ventilator parameters, and postoperative outcomes were compared in these obese patients. Results: A total of 40 obese patients undergoing major gynecological cancer surgery were included in this study. Although the non-ARS group presented with higher weight (p < 0.05), body mass indexes were similar to the ARS group. Intraoperative blood gas analysis revealed higher end-tidal carbon dioxide (etCO2) levels in the non-ARS group during the T2 and T3 time intervals (p < 0.05). In the ARS group, peak inspiratory pressure (PIP) at T3 was lower, while drive pressures at T1 and T2 and dynamic compliance at T3 were higher (p < 0.05). Radiologic atelectasis scores were higher in the non-ARS group, indicating more atelectatic lung images (p < 0.05). PPC rates were similar across both groups. Conclusions: Although the ARS demonstrated positive effects on lung mechanics and radiologic atelectasis scores in major open gynecologic cancer surgeries, it did not effectively reduce postoperative pulmonary complications.
1. Introduction
Obesity is a global health issue resulting from physical inactivity and poor nutrition related to lifestyle [1]. Alongside various systemic effects, it imposes significant stress on the respiratory system, reducing respiratory mechanics (vital capacity, inspiratory capacity, expiratory reserve volume, and functional residual capacity), which predisposes individuals to atelectasis [2]. Major gynecologic cancer surgeries are inherently associated with complications due to patient comorbidities and the extent of surgical interventions, complicating perioperative management. Perioperative anesthesia management significantly influences the development of postoperative pulmonary complications (PPCs), subsequently increasing hospital mortality and the length of stay [3,4]. The induction of general anesthesia affects gas exchange and respiratory mechanics, leading to decreased lung volumes and potential atelectasis [5].
The laparotomy approach required for surgeries in obese patients, coupled with lithotomy and Trendelenburg positioning, further complicates respiratory mechanics [6]. With the addition of the Trendelenburg position to lithotomy, airway pressure increases with the upward elevation of the intestinal contents and diaphragm, while functional residual capacity and lung compliance decrease. This makes respiratory mechanics difficult [7]. Therefore, to improve postoperative outcomes associated with general anesthesia, intraoperative lung-protective ventilation (LPV) strategies are increasingly employed [8]. Although the importance of tidal volume and PEEP has been established in LPV, the specific application and significance of alveolar recruitment strategies (ARSs) remain undetermined [9,10]. Our study is designed to emphasize the significance of the ARS in conjunction with intraoperative LPV in obese patients undergoing gynecologic cancer surgery, hypothesizing that it may enhance postoperative pulmonary outcomes.
2. Materials and Methods
After approval by the Local Ethics Committee of Başakşehir Çam and Sakura City Hospital (approval no: 2023-583, approval date 22 November 2023), 68 patients who underwent major open gynecological cancer surgery for endometrial or ovarian cancer between 1 January 2024 and 1 April 2024 were evaluated. This study was registered at clinicaltrials.gov (study registration: NCT06619626, registration date: 10 May 2024). This study was conducted following the Helsinki Declaration and the principles of good clinical practice. Informed consent was obtained from all the participants. Patients with an American Society of Anesthesiologists (ASA) risk score of II or III, a body mass index (BMI) of 30–40 kg/m2, and an ARISCAT risk score of 26–44, who were scheduled for extubation in the operating room, were included. The ARISCAT risk score is a seven-variable model (age, preoperative oxygen saturation (SpO2), respiratory infection within a month prior to surgery, preoperative anemia, surgical incision, duration of surgery, and urgency of the procedure) that classifies patients into low-, moderate-, and high-risk groups [11]. Patients with severe cardiac disease (New York Heart Association class III or IV), respiratory disease (chronic obstructive pulmonary disease, recent respiratory infection, or ARISCAT risk scores > 44), BMI > 40 kg/m2, hypersensitivity or allergic reactions to any medication administered during the procedure, and patients ventilated for reasons other than abdominal surgery (e.g., acute respiratory distress syndrome or acute hypoxemic respiratory failure) were excluded. Respiratory mechanics become more difficult when morbid obesity (BMI > 40 kg/m2) is involved. Since oxygen saturation may be lower in these patients, this may increase the ARISCAT risk score, and higher values for respiratory complications may be obtained. Therefore, these patients were excluded from the study. Patients were randomized into two groups in a 1:1 ratio using the MedCalc 18.2.1 electronic software: No. 1 was designated by the software as non-ARS. No. 2 was designated as the ARS group. Patients were assigned into these groups (Figure 1).
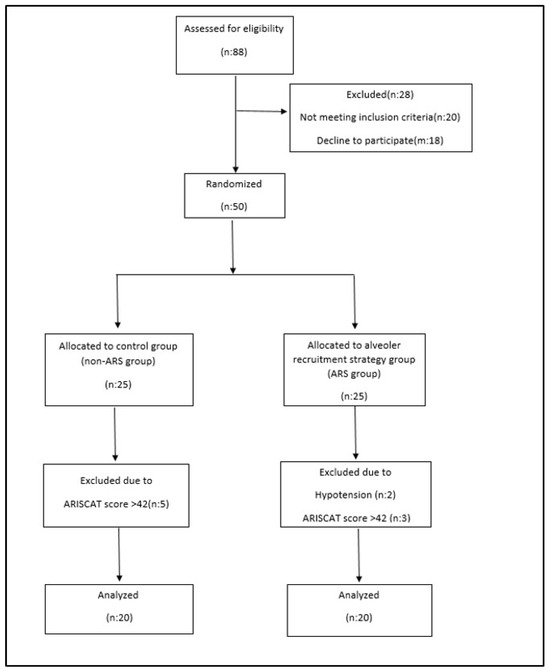
Figure 1.
Consort flow diagram.
All patients underwent standard ASA criteria monitoring (heart rate (HR), noninvasive mean arterial pressure (MAP), peripheral oxygen saturation (SpO2), and temperature), along with near-infrared spectroscopy (NIRS) for cerebral oxygenation and the patient state index (PSI, Masimo Corporation, Radical 7, Irvine, CA, USA) for the depth of anesthesia during the T1 interval. Routine induction of anesthesia (fentanyl 2–3 mcg/kg, propofol 1 mg/kg, and rocuronium 0.6 mg/kg) was followed by tracheal intubation (T2). Anesthesia maintenance was achieved with remifentanil infusion (0.05–0.2 mcg/kg/min) and sevoflurane inhalation (minimum alveolar concentration (MAC) of (0.6–0.9)). The depth of anesthesia was monitored via PSI within the 25–50 range. After induction, continuous arterial blood pressure monitoring and arterial blood sampling were conducted using a radial artery catheter. The ideal body weight was calculated using defined formulas (The predicted body weight of female patients was calculated as equal to 45.5 + 0.91 (centimeters of height—152.4)). Mechanical ventilation was initiated in volume-controlled ventilation (VCV) mode with tidal volumes adjusted to 6–8 mL/kg based on the ideal body weight, 8 cmH2O PEEP, and respiration rates were adjusted to maintain an end-tidal carbon dioxide (etCO2) range of 35–45 mmHg. All patients received low-flow anesthesia (flow: 0.25–1 L). To protect patients from hypoxia, inspired oxygen pressure alarm limits were set at >30 mmHg; FiO2 and flow parameters in low-flow anesthesia were adjusted according to arterial blood gas PaO2 values and NIRS readings.
Mechanical ventilator parameters (TV, FiO2, flow, peak inspiratory pressure (PIP), plateau pressure (Pplato), etCO2, and dynamic compliance (Cdyn)) and hemodynamic, cerebral oxygenation, and arterial blood gas parameters (pH, partial carbon dioxide pressure (pCO2), partial oxygen pressure (pO2), ctO2C, and lactate) were recorded after orotracheal intubation (T2) and ten minutes post ventilation (T3). After ten minutes of ventilation, patients assigned to undergo ARS were switched to pressure-controlled ventilation (PCV) mode, and ARS commenced. PIP was escalated from 30 cmH2O, and PEEP increased in increments of 2 until it reached 20 cmH2O, with ventilation maintained for 2 min at each PEEP change. Respiratory rates were adjusted to maintain PaCO2 at 35–45 mmHg. If the mean arterial pressure dropped by more than 20% or if systolic blood pressure fell below 90 mmHg, ARS was discontinued, and those patients were excluded from the study. Once 20 cmH2O PEEP was reached, PEEP was reduced back to 8 cmH2O, and mechanical ventilation resumed in VCV mode. All patients were positioned in lithotomy for ease of surgical access, followed by a 20° Trendelenburg position after surgical incision. Mechanical ventilation parameters, blood gas parameters, and hemodynamic parameters were recorded following Trendelenburg positioning (T4) and after correcting the position (T5). Intraoperative fluid management included targeted fluid therapy using non-invasive advanced hemodynamic monitoring. Hemodynamic parameters of extubated patients were recorded post-extubation (T6) (Figure 2). Fluid administration, pain management, vasoactive medications, and blood transfusions were managed according to routine protocols. Patients with a modified Aldrete score of > 9 were discharged to the ward. On postoperative day 1, routine X-ray imaging was performed and evaluated according to the radiological atelectasis score (0, clear lung field; 1, plate-like atelectasis or slight infiltration; 2, partial atelectasis; 3, lobar atelectasis; and 4, bilateral lobar atelectasis) [12]. Postoperative pulmonary complications were determined using a sliding scale from 1 to 4, with increasing levels representing the severity of the worsening complication (grade 1: microatelectasis; grade 2: bronchospasm, hypoxemia, and atelectasis; grade 3: pleural effusion, pneumonia, and pneumothorax; and grade 4: respiratory failure) [13]. Postoperative assessments recorded pulmonary and non-pulmonary complications (surgical complications (anastomotic leakage and need for surgical re-intervention), systemic inflammatory response syndrome (SIRS) and sepsis, wound infections, hepatic complications, and renal complications), patients requiring hospitalization, mechanical ventilation status, and the length of stay. One anesthetist performed intraoperative management, while another assessed postoperative lung images, scores, and outcomes. The anesthetist assessing postoperative outcomes was blinded to the study groups.
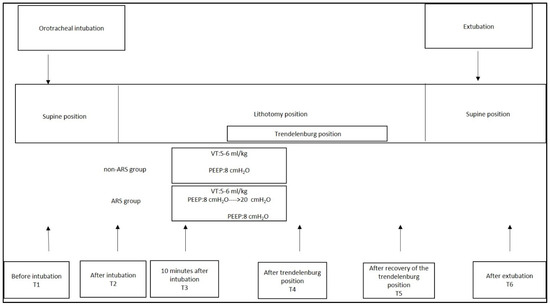
Figure 2.
Study protocol of the groups.
2.1. Sample Size Calculation
Based on power analysis predicting the effect of the primary objective on postoperative pulmonary complication scores, the minimum sample size was set at 20 for each group, with an effect size of 1.2, a 5% margin of error, and 95% power.
2.2. Statistical Analysis
Statistical analyses were performed using NCSS 11 (Number Cruncher Statistical System, 2017 Statistical Software). Frequency and percentage values were presented for categorical variables. Continuous variables were reported as the mean ± standard deviation and the median (IQR). The normality of continuous variables was assessed using the Kolmogorov–Smirnov test. Chi-square tests were employed to evaluate relationships between categorical variables. Fisher’s exact test and Fisher–Freeman–Halton tests were utilized as appropriate. Independent sample t-tests were conducted to compare means between two independent groups with normally distributed continuous variables. The Mann–Whitney U test was employed for comparisons between two independent groups that did not meet normal distribution assumptions. The analysis of variance test (post hoc: Bonferroni correction) was used for the group comparison of the normally distributed variables, and the Kruskal–Wallis H test (post hoc: Dunn’s correction) was used for the intergroup comparison of the non-normally distributed variables. The chi-square and Fisher exact tests were used for the intergroup comparison of the categorical variables. The significance level was set at α = 0.05. p < 0.05 was determined as statistically significant.
3. Results
In this study that evaluated a total of 40 patients undergoing gynecologic cancer surgery—20 in the non-ARS group and 20 in the ARS group—the mean ages of the patients were 56.050 ± 9.411 and 55.200 ± 12.614, respectively (p > 0.05). While the weights of patients in the non-ARS group were statistically significantly higher (p < 0.05), the body mass indices (BMIs) were comparable between both groups. When ARISCAT scores were assessed, the median (IQR) values for the non-ARS and ARS groups were 41 (37.25–41) and 36 (34–41), respectively, demonstrating similarity. In the non-ARS group, 70% was classified as ASA II, while 90% of the ARS group was classified as ASA II (p < 0.05). Lengths of hospital stay and rates of admission to intensive care were comparable between both groups (p > 0.05) (Table 1).

Table 1.
Basic characteristics of the non-ARS and ARS group patients.
The evaluated blood gas parameters, including pH, pO2, ctO2c, and lactate, showed no significant differences between the groups during T2, T3, T4, T5, and T6. However, etCO2 values were higher in the non-ARS group compared to the ARS group during T3 and T4 (Figure 3).
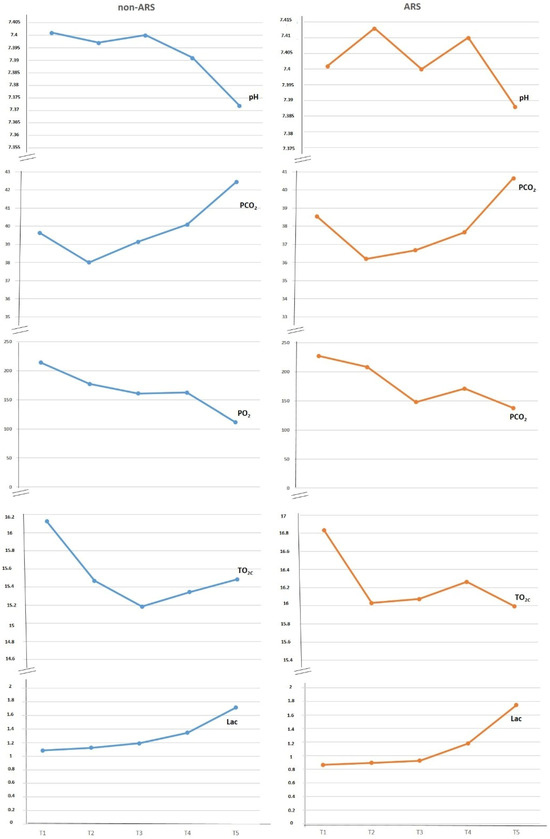
Figure 3.
Evaluation of blood gases between groups.
Hemodynamic parameters and cerebral oxygenation values for both the non-ARS and ARS groups are detailed in Table 2. The patients’ KTA, MAP, and SpO2 values at time points T1, T2, T3, T4, T5, and T6 were found to be similar across both groups. When central oxygenation values (NIRSright and NIRSleft) were analyzed, no statistically significant difference was observed between the two groups.

Table 2.
Evaluation of hemodynamics and cerebral oxygenation between groups.
Mechanical ventilator parameters are summarized in Table 3. Tidal volume, frequency, FiO2, and flow rates were similar in both groups. Assessing PIP revealed lower pressures in the ARS group during T4 (p < 0.05). Although plateau pressures were higher in the ARS group post-intubation (p < 0.05), following the application of ARS and the Trendelenburg position, pressures were similar to those in the non-ARS group during these intervals. Driving pressures (DPs) were higher in the ARS group during the T2 and T3 time intervals (p < 0.05). However, since lung-protective ventilation was maintained during all time intervals, driving pressures remained below 15 cmH2O. Dynamic compliance (Cdyn) was higher in the ARS group compared to the non-ARS group at T4 (p < 0.05) (Table 3).

Table 3.
Comparison of respiratory mechanics and mechanical ventilator parameters.
When examined for radiologic atelectasis scores, atelectasis was not observed in 25% of the non-ARS group and 50% of the ARS group. In the non-ARS group, Grade 1 was identified in nine patients, Grade 2 in two patients, Grade 3 in two patients, and Grade 4 in two patients. In contrast, the ARS group exhibited Grade 1 in nine patients, with one patient showing Grade 4. No patients in the ARS group presented with Grade 2 or 3 (p < 0.05). PPC rates were comparable between both groups (Table 4).

Table 4.
Comparison of radiological atelectasis score and pulmonary and extrapulmonary complications between groups. PPC: postoperative pulmonary complication.
Extrapulmonary complications were similar among both groups, with SIRS and sepsis at 0.1, surgical complications at 1.0, surgical site infections at 0.1, and renal complications at 1.2 in the non-ARS and ARS groups, respectively (Table 4).
4. Discussion
Our findings indicate that within the intraoperative lung-protective ventilation strategy for obese patients, the inclusion of alveolar recruitment strategies resulted in lower peak inspiratory pressures and improved dynamic compliance, leading to reduced radiological atelectasis scores, with 50% of patients experiencing no atelectasis. However, this did not translate into a reduction in clinically significant postoperative pulmonary complications as reflected by the graded scales. Despite higher pCO2 levels observed in the control group compared to the ARS group during T2 and T3, these values remained within normal ranges for both groups, indicating no clinical significance. Abdominal surgeries carry a high and unavoidable risk of postoperative pulmonary complications [3,14,15]. Additionally, prolonged surgical duration exceeding three hours is associated with deteriorations in postoperative pulmonary function [16]. The development of atelectasis due to general anesthesia adversely affects mechanical ventilation dynamics and can contribute to lung injury [17]. Strategies employing low tidal volumes, moderate PEEP, and recruitment maneuvers in lung-protective ventilation may enhance postoperative pulmonary outcomes in high-risk abdominal surgeries [18]. While the implementation of low tidal volume and PEEP has shown survival benefits, the impact of intraoperative LPV in patients without underlying lung disease is still debated. The exacerbating factor of obesity complicates the situation, as increased adipose tissue leads to reduced total lung capacity and functional residual capacity, resulting in impaired respiratory mechanics. It does this by decreasing lung compliance and tidal volumes and impairing alveolar oxygenation due to increased abdominal and intrathoracic pressure in obese patients and the effects of general anesthesia [19]. Even the ventilation strategy and postoperative pulmonary complications have been associated with the length of hospital stay and mortality in obese patients [20]. It is known that the level of PEEP is influenced by chest wall compliance and the body mass index, with individual optimal PEEP values varying [21]. To mitigate this, LPV is implemented with low tidal volumes and moderate PEEP levels (5–8 cmH2O) to prevent atelectasis while minimizing hemodynamic instability and fluid infusion needs [22,23]. In a meta-analysis, it was shown that there were large differences in intraoperative ventilation strategies in preventing perioperative pulmonary complications in obese patients [24]. One study even showed that high PEEP (12 cmH2O) did not reduce postoperative complications compared to low PEEP (0–2 cmH2O) in abdominal operations, leaving the role of PEEP in postoperative outcomes unclear [23]. Furthermore, driving pressures below 15 cmH2O are essential for establishing lung-protective ventilation alongside low tidal volumes and moderate PEEP [25]. Dynamic compliancy provides alveolar stability and improves intraoperative oxygenation by decreasing the end-expiratory respiratory volume closer to the functional residual capacity by decreasing the drive pressure with PEEP titration [26]. In our patients, tidal volume was determined based on the ideal body weight and delivered with moderate PEEP while maintaining driving pressures below 15 cmH2O. Despite the improved alveolar recruitment with ARS, the overall clinical significance in postoperative hospital and intensive care unit settings remained minimal [27].
Numerous ARS methods have been proposed, yet a gradual recruitment maneuver is preferred to avoid excessive alveolar distension [28]. Although ARS has been applied through continuous high inspiratory pressures or incremental PEEP increases, an optimal technique remains unattained, and the timing and methods of intraoperative application are not standardized. Despite evidence demonstrating improvements in intraoperative oxygenation associated with alveolar recruitment maneuvers, the recurrence of atelectasis within 40 min post-maneuver and the lack of long-term effect have been noted [29,30]. Therefore, ARS appears to be more effective when integrated within LPV frameworks [31]. In a meta-analysis evaluating ventilation strategies in obese patients, the combination of PEEP and ARS was found to possess the highest potential for reducing postoperative atelectasis in volume-controlled modes [32]. High PEEP and ARS reduce postoperative atelectasis, reduce drive pressure, homogenize ventilation, and improve postoperative pulmonary outcomes but may increase inflammation and impair hemodynamics and lymphatic drainage [33].
Nonetheless, ARS can result in increased intrathoracic pressure and a decrease in left ventricular diastolic end pressure and cardiac output, leading to hypotension [23]. Thus, we advocate for a carefully controlled hemodynamic approach for the incorporation of recruitment maneuvers as part of lung-protective ventilation, given their potential to improve intraoperative lung mechanics and reduce postoperative atelectasis through the maintenance of low driving pressures.
The lack of impact from ARS on alveolar oxygenation, alongside diverse results ranging from no effect to hemodynamic instability, has been observed [34]. According to systematic reviews, intraoperative pO2 values were consistently higher in groups undergoing ARS compared to the control [35]. In obese patients, meta-analysis results indicate that ARS improves intraoperative arterial oxygenation [24]. However, in our study, comparable arterial oxygenation levels may be attributed to adjustments in low-flow anesthesia based on arterial blood gas results rather than fixed flow rates.
The limitations of this study include the inability to generalize results to laparoscopic cases. Additionally, patients with a BMI exceeding 40 kg/m2, who are more susceptible to respiratory impairments, were excluded. Furthermore, postoperative complications were evaluated only in the short term by chest radiography and PPC grading scales. For the postoperative pulmonary evaluation, results can be evaluated with studies that can be combined with tomography or lung ultrasonography. Long-term outcomes were not evaluated.
5. Conclusions
The application of intraoperative lung-protective ventilation in obese patients remains uncertain. Although its application in the intraoperative period is somewhat controversial, the feasibility of lung-protective ventilation strategies in obese patients (BMI: 30–40 kg/m2) in gynecological open cancer surgeries and the potential enhancement of dynamic compliance and PIP through the use of intraoperative ARS have been demonstrated. However, additional studies are warranted to reinforce the beneficial effects of ventilation strategies, identify optimal PEEP levels, determine the best alveolar recruitment methods, and explore their impacts on clinical outcomes.
Author Contributions
Conceptualization, D.A. and F.G.Ö.; methodology, D.A. and F.G.Ö.; software, D.A. and F.G.Ö.; validation, D.A.; formal analysis, D.A.; investigation, D.A. and F.G.Ö.; resources, D.A. and F.G.Ö.; data curation, D.A.; writing—original draft, D.A. and F.G.Ö.; writing—review and editing, D.A. and F.G.Ö.; visualization, D.A. and F.G.Ö.; supervision, D.A. and F.G.Ö.; project administration, D.A. and F.G.Ö.; funding acquisition, D.A. and F.G.Ö. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Ethics Review Board of Basaksehir Çam and Sakura City Hospital (approval no: 2023-583, approval date 22 November 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Supporting data will be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Salome, C.M.; King, G.G.; Berend, N. Physiology of obesity and effects on lung function. J. Appl. Physiol. 2010, 108, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.L.; Ball, L.; Rocco, P.; Pelosi, P. Physiological and pathophysiological consequences of mechanical ventilation. Semin. Respir. Crit. Care Med. 2022, 43, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bustamante, A.; Frendl, G.; Sprung, J.; Kor, D.J.; Subramaniam, B.; Martinez Ruiz, R.; Lee, J.-W.; Henderson, W.G.; Moss, A.; Mehdiratta, N.; et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: Amulticenter study by the perioperative research network investigators. JAMA Surg. 2017, 152, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Odor, P.M.; Bampoe, S.; Gilhooly, D.; Creagh-Brown, B.; Moonesinghe, S.R. Perioperative interventions for prevention of postoperative pulmonary complications: Systematic review and meta-analysis. BMJ 2020, 368, m540. [Google Scholar] [CrossRef]
- Bigatello, L.; Pesenti, A. Respiratory Physiology for the Anesthesiologist. Anesthesiology 2019, 130, 1064–1077. [Google Scholar] [CrossRef]
- Weller, W.E.; Rosati, C. Comparing outcomes of laparoscopic versus open bariatric surgery. Ann. Surg. 2008, 248, 10–15. [Google Scholar] [CrossRef]
- O’Malley, C.; Cunningham, A.J. Physiologic changes during laparoscopy. Anesthesiol. Clin. N. Am. 2001, 19, 1–19. [Google Scholar] [CrossRef]
- Ruszkai, Z.; Kiss, E.; László, I.; Bokrétás, G.P.; Vizserálek, D.; Vámossy, I.; Surány, E.; Buzogány, I.; Bajory, Z.; Molnár, Z. Effects of Intraoperative Positive End-Expiratory Pressure Optimization on Respiratory Mechanics and the Inflammatory Response: A Randomized Controlled Trial. J. Clin. Monit. Comput. 2020, 35, 469–482. [Google Scholar] [CrossRef]
- Leme, A.C.; Hajjar, L.A.; Volpe, M.S.; Fukushima, J.T.; Santis Santiago, R.R.; Osawa, E.A.; de Almeida, J.P.; Gerent, A.M.; Franco, R.A.; Feltrim, M.I.Z.; et al. Effect of Intensive vs Moderate Alveolar Recruitment Strategies Added to Lung-Protective Ventilation on Postoperative Pulmonary Complications: A Randomized Clinical Trial. JAMA 2017, 317, 1422–1432. [Google Scholar] [CrossRef]
- Kim, J.K. Importance of alveolar recruitment strategy revisited. Korean J. Anesthesiol. 2014, 67, 75–76. [Google Scholar] [CrossRef][Green Version]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Vallès, J.; Castillo, J.; Sabaté, S.; Mazo, V.; Briones, Z.; Sanchis, J. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Baneton, S.; Dauvergne, J.E.; Gouillet, C.; Cartron, E.; Volteau, C.; Nicolet, J.; Corne, F.; Rozec, B. Effect of Active Physiotherapy with Positive Airway Pressure on Pulmonary Atelectasis After Cardiac Surgery: A Randomized Controlled Study. J. Cardiothorac. Vasc. Anesth. 2023, 37, 1668–1676. [Google Scholar] [CrossRef]
- Zhou, Z.; Fang, J.; Chem, L.; Wang, H.; Yu, Y.; Wang, W.; Chen, J.; Zhang, M.; Hu, S. Effects of intraoperative PEEP on postoperative pulmonary complications in patients undergoing robot-assisted laparoscopic radical resection for bladder cancer or prostate cancer: Study protocol for a randomized controlled. Trial 2019, 20, 304. [Google Scholar] [CrossRef] [PubMed]
- Karalapillai, D.; Weinberg, L.; Peyton, P.; Ellard, L.; Hu, R.; Pearce, B.; Tan, C.O.; Story, D.; O’Donnell, M.; Hamilton, P.; et al. Effect of Intraoperative Low Tidal Volume vs Conventional Tidal Volume on Postoperative Pulmonary Complications in Patients Undergoing Major Surgery: A Randomized Clinical Trial. JAMA 2020, 324, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, Z.N.; Zhang, N.R.; Guo, J.; Wang, K.; Wang, W.; Li, L.; Jin, J.; Tang, J.; Liao, Y.; et al. Intra-operative open-lung ventilatory strategy reduces postoperative complications after laparoscopic colorectal cancer resection: A randomised controlled trial. Eur. J. Anaesthesiol. 2021, 38, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Mazo, V.; Sabate, S.; Canet, J.; Gallart, L.; de Abreu, M.G.; Belda, J.; Langeron, O.; Hoeft, A.; Pelosi, P. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 2014, 121, 219–231. [Google Scholar] [CrossRef]
- Cressoni, M.; Chiumello, D.; Algieri, I.; Brioni, M.; Chiurazzi, C.; Colombo, A.; Crimella, F.; Guanziroli, M.; Tomic, I.; Tonetti, T.; et al. Opening pressures and atelectrauma in acute respiratory distress syndrome. Intensive Care Med. 2017, 43, 603–611. [Google Scholar] [CrossRef]
- Park, S. Perioperative lung-protective ventilation strategy reduces postoperative pulmonary complications in patients undergoing thoracic and major abdominal surgery. Korean J. Anesthesiol. 2016, 69, 3–7. [Google Scholar] [CrossRef]
- Littleton, S.W. Impact of obesity on respiratory function. Respirology 2012, 17, 43–49. [Google Scholar] [CrossRef]
- Ball, L.; Hemmes, S.; Neto, A.S.; Bluth, T.; Canet, J.; Hiesmayr, M.; Hollmann, M.; Mills, G.; Melo, M.V.; Putensen, C.; et al. Intraoperative ventilation settings and their associations with postoperative pulmonary complications in obese patients. Br. J. Anaesth. 2018, 121, 899–908. [Google Scholar] [CrossRef]
- Ferrando, C.; Soro, M.; Unzueta, C.; Suarez-Sipmann, F.; Canet, J.; Librero, J.; Pozo, N.; Peiró, S.; Llombart, A.; León, I.; et al. Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): A randomised controlled trial. Lancet Respir. Med. 2018, 6, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ladha, K.; Vidal Melo, M.F.; McLean, D.J.; Wanderer, J.P.; Grabitz, S.D.; Kurth, T.; Eikermann, M. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: Hospital based registry study. BMJ 2015, 351, h3646. [Google Scholar] [CrossRef] [PubMed]
- Hemmes, S.N.; Gama de Abreu, M.; Pelosi, P.; Schultz, M.J. PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology: High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): A multicentre randomised controlled trial. Lancet 2014, 384, 495–503. [Google Scholar] [PubMed]
- Aldenkortt, M.; Lysakowski, C.; Elia, N.; Brochard, L.; Tramer, M.R. Ventilation strategies in obese patients undergoing surgery: A quantitative systematic review and meta-analysis. Br. J. Anaesth. 2012, 109, 493–502. [Google Scholar] [CrossRef]
- Neto, A.S.; Hemmes, S.N.T.; Barbas, C.S.V.; Beiderlinden, M.; Fernandez-Bustamante, A.; Futier, E.; Gajic, O.; El-Tahan, M.R.; A Al Ghamdi, A.; Günay, E.; et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: A metaanalysis of individual patient data. Lancet Respir. Med. 2016, 4, 272–280. [Google Scholar] [CrossRef]
- Ferrando, C.; Suarez-Sipmann, F.; Tusman, G.; León, I.; Romero, E.; Gracia, E.; Mugarra, A.; Arocas, B.; Pozo, N.; Soro, M.; et al. Open lung approach versus standard protective strategies: Effects on driving pressure and ventilatory efficiency during anesthesia—A pilot, randomized controlled trial. PLoS ONE 2017, 12, e0177399. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Soni, K.D.; Maitra, S. Recruitment maneuver does not provide any mortality benefit over lung protective strategy ventilation in adult patients with acute respiratory distress syndrome: A meta-analysis and systematic review of the randomized controlled trials. J. Intensive Care 2018, 6, 35. [Google Scholar] [CrossRef]
- Hess, D.R. Recruitment maneuvers and PEEP titration. Respir. Care 2015, 60, 1688–1704. [Google Scholar] [CrossRef]
- Nestler, C.; Simon, P.; Petroff, D.; Hammermüller, S.; Kamrath, D.; Wolf, S.; Dietrich, A.; Camilo, L.M.; Beda, A.; Carvalho, A.R.; et al. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: A randomized controlled clinical trial using electrical impedance tomography. Br. J. Anaesth. 2017, 119, 1194–1205. [Google Scholar] [CrossRef]
- Lagier, D.; Zeng, C.; Fernandez-Bustamante, A.; Melo, M.F.V. Perioperative Pulmonary Atelectasis—Part II: Clinical Implications. Anesthesiology 2021, 136, 206–236. [Google Scholar] [CrossRef]
- Park, S.; Kim, B.; Oh, A.; Han, S.; Han, H.; Ryu, J. Effects of intraoperative protective lung ventilation on postoperative pulmonary complications in patients with laparoscopic surgery: Prospective, randomized and controlled trial. Surg. Endosc. 2016, 30, 4598–4606. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zeng, J.; Zhang, C.; Zheng, W.; Huang, X.; Zhao, N.; Duan, G.; Yu, C. Optimized ventilation strategy for surgery on patients with obesity from the perspective of lung protection: A network meta-analysis. Front. Immunol. 2022, 13, 1032783. [Google Scholar] [CrossRef] [PubMed]
- Bluth, T.; Teichmann, R.; Kiss, T.; PROBESE Investigators; PROtective VEntilation Network (PROVEnet); Clinical Trial Network of the European Society of Anaesthesiology (ESA). Protective intraoperative ventilation with higher versus lower levels of Positive End-Expiratory Pressure in Obese Patients (PROBESE): Study protocol for a randomized controlled trial. Trials 2017, 18, 202. [Google Scholar] [CrossRef]
- Paulus, F.; Binnekade, J.M.; Vroom, M.B.; Schultz, M.J. Benefits and risks of manual hyperinflation in intubated and mechanically ventilated intensive care unit subjects: A systematic review. Crit. Care 2012, 16, R145. [Google Scholar] [CrossRef] [PubMed]
- Hartland, B.L.; Newell, T.J.; Damico, N. Alveolar recruitment maneuvers under general anesthesia: A systematic review of the literature. Respir. Care 2015, 60, 609–620. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).