Comparison of Aqueous Depth Changes Following Cataract Surgery in Vitrectomized and Non-Vitrectomized Fellow Eyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Eligibility
2.3. Methods
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scharwey, K.; Pavlovic, S.; Jacobi, K.W. Combined Clear Corneal Phacoemulsification, Vitreoretinal Surgery, and Intraocular Lens Implantation. J. Cataract Refract Surg. 1999, 25, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Demetriades, A.M.; Gottsch, J.D.; Thomsen, R.; Azab, A.; Stark, W.J.; Campochiaro, P.A.; De Juan, E.; Haller, J.A. Combined Phacoemulsification, Intraocular Lens Implantation, and Vitrectomy for Eyes with Coexisting Cataract and Vitreoretinal Pathology. Am. J. Ophthalmol. 2003, 135, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Do, D.V.; Gichuhi, S.; Vedula, S.S.; Hawkins, B.S. Surgery for Postvitrectomy Cataract. Cochrane Database Syst. Rev. 2018, 1, CD006366. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, A.; Han, X.; Chen, Z.; Diao, C.; Zhang, Y.; Liu, X.; Xu, F.; Liu, J.; Qiu, X.; et al. The LISA-PPV Formula: An Ensemble Artificial Intelligence-Based Thick Intraocular Lens Calculation Formula for Vitrectomized Eyes. Am. J. Ophthalmol. 2024, 262, 237–245. [Google Scholar] [CrossRef]
- Shiraki, N.; Wakabayashi, T.; Sakaguchi, H.; Nishida, K. Effect of Gas Tamponade on the Intraocular Lens Position and Refractive Error after Phacovitrectomy: A Swept-Source Anterior Segment OCT Analysis. Ophthalmology 2020, 127, 511–515. [Google Scholar] [CrossRef]
- Patel, D.; Rahman, R.; Kumarasamy, M. Accuracy of Intraocular Lens Power Estimation in Eyes Having Phacovitrectomy for Macular Holes. J. Cataract Refract Surg. 2007, 33, 1760–1762. [Google Scholar] [CrossRef] [PubMed]
- Hamoudi, H.; La Cour, M. Refractive Changes after Vitrectomy and Phacovitrectomy for Macular Hole and Epiretinal Membrane. J. Cataract Refract Surg. 2013, 39, 942–947. [Google Scholar] [CrossRef]
- Jeoung, J.W.; Chung, H.; Yu, H.G. Factors Influencing Refractive Outcomes after Combined Phacoemulsification and Pars Plana Vitrectomy: Results of a Prospective Study. J. Cataract Refract Surg. 2007, 33, 108–114. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, J.; Fils, J.F.; Jansen, J.; Hua, M.T.; Stalmans, P. The Role of the Vitreous Body in Effective IOL Positioning. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1517–1520. [Google Scholar] [CrossRef]
- Falkner-Radler, C.I.; Benesch, T.; Binder, S. Accuracy of Preoperative Biometry in Vitrectomy Combined with Cataract Surgery for Patients with Epiretinal Membranes and Macular Holes: Results of a Prospective Controlled Clinical Trial. J. Cataract Refract Surg. 2008, 34, 1754–1760. [Google Scholar] [CrossRef]
- Govetto, A.; Lalane, R.A.; Sarraf, D.; Figueroa, M.S.; Hubschman, J.P. Insights into Epiretinal Membranes: Presence of Ectopic Inner Foveal Layers and a New Optical Coherence Tomography Staging Scheme. Am. J. Ophthalmol. 2017, 175, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. No Adjustments Are Needed for Multiple Comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Norrby, S. Sources of Error in Intraocular Lens Power Calculation. J. Cataract Refract Surg. 2008, 34, 368–376. [Google Scholar] [CrossRef]
- Byrne, S.; Ng, J.; Hildreth, A.; Danjoux, J.P.; Steel, D.H.W. Refractive Change Following Pseudophakic Vitrectomy. BMC Ophthalmol. 2008, 8, 19. [Google Scholar] [CrossRef]
- Gao, Q.; Chen, X.; Ge, J.; Liu, Y.; Jiang, Z.; Lin, Z.; Liu, Y. Refractive Shifts in Four Selected Artificial Vitreous Substitutes Based on Gullstrand-Emsley and Liou-Brennan Schematic Eyes. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3529–3534. [Google Scholar] [CrossRef]
- Cruysberg, L.P.J.; Doors, M.; Verbakel, F.; Berendschot, T.T.J.M.; De Brabander, J.; Nuijts, R.M.M.A. Evaluation of the Lenstar LS 900 Non-Contact Biometer. Br. J. Ophthalmol. 2010, 94, 106–110. [Google Scholar] [CrossRef]
- Olsen, T. Calculation of Intraocular Lens Power: A Review. Acta Ophthalmol. Scand. 2007, 85, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Xanthaki, C.F.; Hirnschall, N.; Gabriel, M.; Großpötzl, M.; Wallisch, F.; Findl, O.; Haas, A. Influence of Combined Phacovitrectomy without Tamponade on Intraocular Lens Displacement and Postoperative Refraction. Acta Ophthalmol. 2022, 100, 15192. [Google Scholar] [CrossRef]
- Chatzimichail, E.; Wertheimer, C.; Kilani, A.; König, S.; Gatzioufas, Z.; Wolf, A.; Vounotrypidis, E. Influence of Endotamponade on Anterior Chamber Depth and Refractive Outcome after Combined Phacovitrectomy: Case-Control Study. J. Cataract Refract Surg. 2023, 49, 1228. [Google Scholar] [CrossRef]
- Khodabande, A.; Mohammadi, M.; Riazi-Esfahani, H.; Karami, S.; Mirghorbani, M.; Modjtahedi, B.S. Changes in Anterior Segment Optical Coherence Tomography Following Pars Plana Vitrectomy without Tamponade. Int. J. Retina Vitreous 2021, 7, 15. [Google Scholar] [CrossRef]
- Crincoli, E.; Savastano, A.; Ferrara, S.; Caporossi, T.; Miere, A.; Souied, E.H.; Savastano, M.C.; Kilian, R.; Rizzo, C.; Faraldi, F.; et al. Refractive Outcome in Combined Phacovitrectomy: Anterior Segment Changes and Corrective Factor for IOL Power Calculation Improvement. Eur. J. Ophthalmol. 2024, 34, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Shioya, M.; Ogino, N.; Shinjo, U. Change in postoperative refractive error when vitrectomy is added to intraocular lens implantation. J. Cataract Refract. Surg. 1997, 23, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Senn, P.; Schipper, I.; Perren, B. Combined pars plana vitrectomy, phacoemulsification, and intraocular lens implantation in the capsular bag: A comparison to vitrectomy and subsequent cataract surgery as a two-step procedure. Ophthalmic Surg. Lasers 1995, 26, 420–428. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sakuraba, T.; Mizutani, H.; Matsuhashi, H.; Nakazawa, M. Postoperative refractive error after simultaneous vitrectomy and cataract surgery. Ophthalmic Surg. Lasers 2000, 31, 271–275. [Google Scholar] [CrossRef]
- Kovács, I.; Ferencz, M.; Nemes, J.; Somfai, G.; Salacz, G.; Récsán, Z. Intraocular lens power calculation for combined cataract surgery, vitrectomy and peeling of epiretinal membranes for macular oedema. Acta Ophthalmol. Scand. 2007, 85, 88–91. [Google Scholar] [CrossRef]
- Schweitzer, K.D.; García, R. Myopic shift after combined phacoemulsification and vitrectomy with gas tamponade. Can. J. Ophthalmol. 2008, 43, 581–583. [Google Scholar] [CrossRef]
- Manvikar, S.R.; Allen, D.; Steel, D.H.W. Optical biometry in combined phacovitrectomy. J. Cataract Refract. Surg. 2009, 35, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Jee, D. Effects of the intraocular lens type on refractive error following phacovitrectomy with gas tamponade. Curr. Eye Res. 2011, 36, 1148–1152. [Google Scholar] [CrossRef]
- Sun, H.J.; Choi, K.S. Improving intraocular lens power prediction in combined phacoemulsification and vitrectomy in eyes with macular oedema. Acta Ophthalmol. 2011, 89, 575–578. [Google Scholar] [CrossRef]
- Tranos, P.G.; Allan, B.; Balidis, M.; Vakalis, A.; Asteriades, S.; Anogeianakis, G.; Triantafilla, M.; Kozeis, N.; Stavrakas, P. Comparison of postoperative refractive outcome in eyes undergoing combined phacovitrectomy vs cataract surgery following vitrectomy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 987–993. [Google Scholar] [CrossRef]
- Graziano, F.; Valsecchi, M.G.; Rebora, P. Sampling Strategies to Evaluate the Prognostic Value of a New Biomarker on a Time-to-Event End-Point. BMC Med. Res. Methodol. 2021, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Farag, C.S.; Gouda, J.; Maher, S.; El-Fayoumi, D.; Elhilali, H. Incidence and Predisposing Factors of Intraocular Lens Tilt Following Secondary Ciliary Sulcus Implantation in Children: An Ultrasound Biomicroscopic Study. Eur. J. Ophthalmol. 2024, 34, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.P.; Jandewerth, T.; Bucur, J.; Kohnen, T.; Lwowski, C. Axial Length Adjustment in Eyes with Silicone Oil Endotamponade Reduces Overestimation by a Swept-Source Optical Coherence Tomography-Based Biometer. Clin. Exp. Ophthalmol. 2024, 52, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Toklu, E.; Altinisik, M.; Elbay, A.; Koytak, A. Comparison of Postoperative Anterior Segment Changes Associated with Pars Plana Vitrectomy with and without Vitreous Base Shaving. Int. J. Ophthalmol. 2020, 13, 1745. [Google Scholar] [CrossRef]
- Szala, K.; Sirek, S.; Wygledowska-Promienska, D. Navigating Retinal Complications and Refractive Outcomes in High Myopia: A Case Report with Multi-Surgical Interventions. Cureus 2025, 17, e78850. [Google Scholar] [CrossRef]
- Devireddy, N.; Borkhetaria, R.; Cannon, N.; Bowie, E.; Pantanelli, S.M. Cataract Outcomes Following Scleral Buckle Surgery for Retinal Detachment. Clin. Ophthalmol. 2024, 18, 1225–1233. [Google Scholar] [CrossRef]
- Sugita, T.; Aomatsu, M.; Yoshida, M.; Kaneko, T.; Hasegawa, Y.; Oshika, T. Clinical and Laboratory Studies on the Effects of Capsular Tension Ring on Surgical Outcomes of Trifocal Intraocular Lens Implantation. J. Cataract Refract Surg. 2023, 49, 1111. [Google Scholar] [CrossRef]
- Chang-Sotomayor, M.; Gϋell, J.L.; de Silva, M.V.R.; Corretger, X.; Bandeira, F.; Mendez-Mourelle, A.; Veillet, L.Z.; Adán, A.; Figueras-Roca, M. Comparison of Intraocular Lens Tilt after Capsular Sutured Scleral Fixation with Capsular Segments versus Uneventful Cataract Surgery. Eur. J. Ophthalmol. 2024, 34, 1450–1457. [Google Scholar] [CrossRef]
- Shousha, M.A.; Yoo, S.H. Cataract Surgery after Pars Plana Vitrectomy. Curr. Opin. Ophthalmol. 2010, 21, 45–49. [Google Scholar] [CrossRef]
| Characteristics | Vitrectomized | Non-Vitrectomized | p-Value |
|---|---|---|---|
| Sample size, eyes | 20 | 20 | N/A |
| Age, years | 71.6 (5.8) | N/A | |
| Sex, female | 11 (55.0%) | N/A | |
| Right eye | 10 (50%) | 10 (50%) | 1.00 |
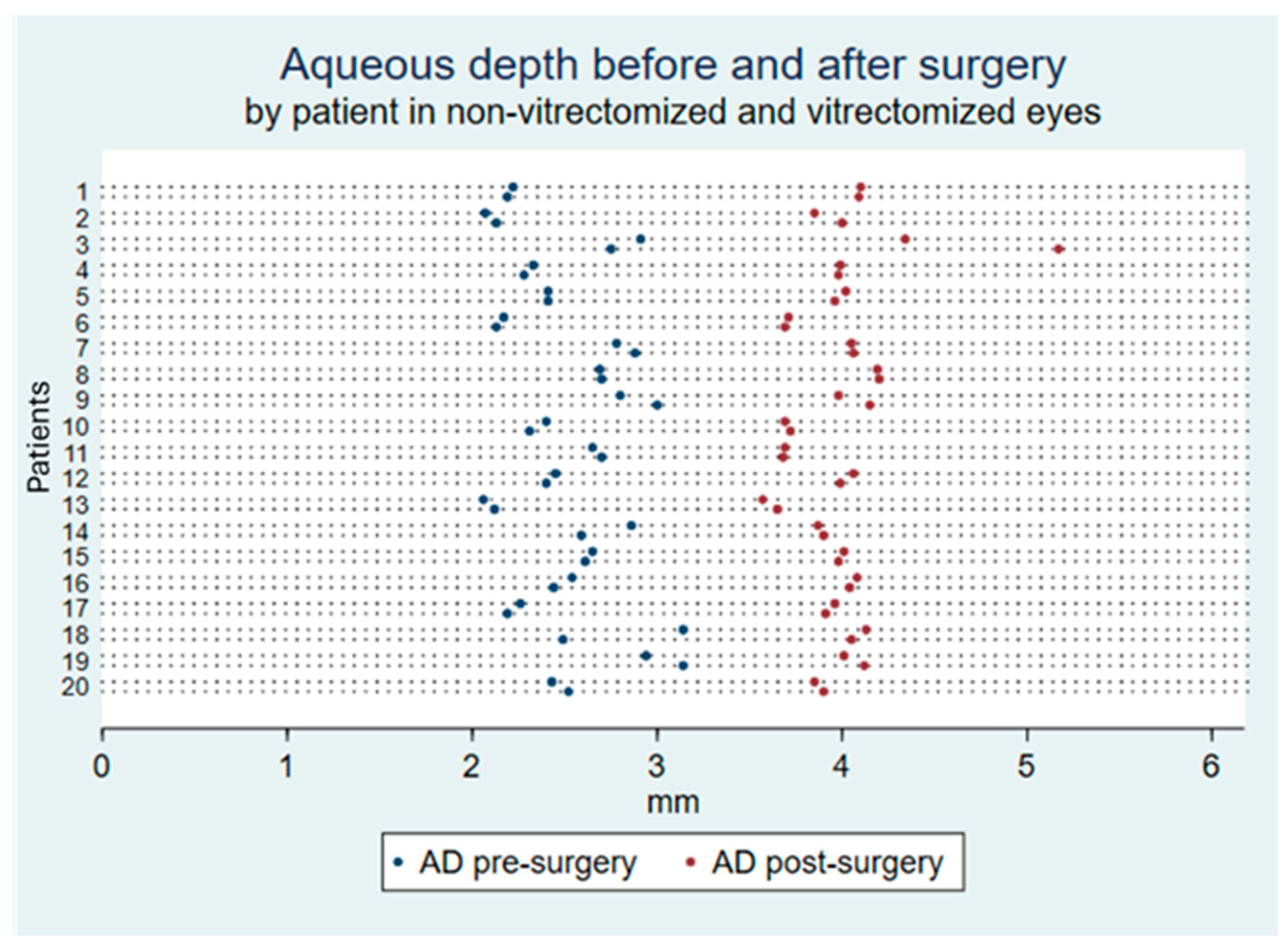
| AD, mm | 2.50 (0.30) | 2.54 (0.31) | 0.69 |
| ACD, mm | 3.04 (0.29) | 3.08 (0.31) | 0.67 |
| Pachymetry, μm | 540.6 (36.3) | 542.6 (37.3) | 0.86 |
| K1, D | 43.23 (1.62) | 43.37 (1.58) | 0.78 |
| K2, D | 43.96 (1.55) | 44.11 (1.59) | 0.76 |
| Mean K, D | 43.67 (1.50) | 43.95 (1.62) | 0.57 |
| Lens thickness, mm | 4.58 (0.38) | 4.52 (0.42) | 0.65 |
| Axial length, mm | 23.42 (0.73) | 23.36 (0.73) | 0.81 |
| Spherical equivalent, D | +1.04 (1.69) | +1.01 (1.49) | 0.94 |
| Characteristics | Vitrectomized | Non-Vitrectomized | p-Value |
|---|---|---|---|
| AD change, mm | +1.51 (0.33) | +1.42 (0.26) | 0.33 |
| AD change, % | +62.1% (17.1) | +57.7% (16.4) | 0.41 |
| ACD change, mm | +1.52 (0.33) | +1.42 (0.26) | 0.31 |
| ACD change, % | +50.8% (13.5) | +47.2% (12.6) | 0.38 |
| Characteristics | Coefficient (β), SE | 95% CI | p-Value |
|---|---|---|---|
| Dependent variable: AD | |||
| Vitrec vs. non-vitrect | 0.07 (0.05) | −0.02 to 0.16 | 0.11 |
| Baseline AD, mm | −0.63 (0.14) | −0.90 to −0.36 | <0.001 * |
| Axial length, mm | −0.07 (0.06) | −0.20 to 0.05 | 0.25 |
| Dependent variable: ACD | |||
| Vitrec vs. non-vitrect | 0.08 (0.05) | −0.01 to 0.17 | 0.10 |
| Baseline ACD, mm | −0.63 (0.14) | −0.91 to 0.36 | <0.001 * |
| Axial length, mm | −0.07 (0.06) | −0.19 to 0.06 | 0.28 |
| Study | N Eyes | Major Indication | Study Design | Measurement Method | Results (Refractive Error or Change in ACD) |
|---|---|---|---|---|---|
| Shioya, J., et al., 1997 * [22] | 36 | MH | Case series | Ultrasound (Alpha 20/20, Storz, Tuttlingen, Germany) | −0.55 D |
| Senn, P., et al., 2000 [23] | 26 | DR, ERM, uveitis | Prospective case series | N/R | −0.18 D |
| Suzuki, Y., et al., 2000 [24] | 206 | DR, MH, RD | Case series | N/R | −0.05 D |
| Jeoung, J.W., et al., 2007 [8] | 154 | DR, ERM, MH | Prospective case series | Ultrasound (A-scan 820, Zeiss, Jena, Germany) | −0.06 D |
| Kovács, I., et al., 2007 [25] | 12 | ERM, DR | Prospective case series | Ultrasound (Ultrascan, Alcon) | −0.79 D |
| Patel, D., et al., 2007 [6] | 40 | MH | Retrospective case series | Ultrasound (EchoScan US-1800, Nidek, Gamagori, Japan) | −0.39 D |
| Byrne, S., et al., 2008 * [14] | 87 | DR, ERM, MH, RD, miscellaneous | Retrospective case series | Optical (IOL Master, Zeiss) | −0.65 D |
| Falkner-Radler, C.I., et al., 2008 * [10] | 40 | ERM, MH | Clinical trial | Optical (IOL Master, Zeiss) | −0.52 D (−0.20 D with gas) |
| Schweitzer, K.D., et al., 2008 * [26] | 54 | DR, ERM, MH | Consecutive case series | Optical (IOL Master, Zeiss) | +0.16 D (−0.30 D with gas) |
| Manvikar, S.R., et al., 2009 * [27] | 59 | DR, ERM, MH, RD | Retrospective case series | Optical (IOL Master, Zeiss) | −0.10 D (+0.03 D with gas) |
| Hwang, H.S., et al., 2011 * [28] | 40 | MH | Prospective case series | Ultrasound (Ecograph axis II, Quantel Medical, Clermont-Ferrand, France) | −0.61 D |
| Sun, H.J., et al., 2011 * [29] | 23 | ERM, MH | Retrospective case series | Ultrasound (A/B scan Workstation, Paradigm MI, Salt Lake City, UT, USA) | −0.46 D |
| Mijnsbrugge, J.V., et al., 2018 [9] | 40 | ERM, floaters, VMT | Prospective case series | Optical (IOL Master 700, Zeiss) | Phacoemulsification: change SE −0.05 D; change in ACD 1.99 mm Phacovitrectomy: change in SE −0.18 D (p = 0.18); change in ACD 2.12 mm (p = 0.044) |
| Shiraki, N., et al., 2020 * [5] | 76 | ERM, MH and RD | Retrospective case series | Optical (IOL Master 500, Zeiss) | Group A: phacoemulsification: +0.08 D Group B: phacovitrectomy w/o gas: −0.07 D Group C: phacovitrectomy w/gas: −0.82 D, p < 0.001 vs. A and B |
| Tranos, P.G., et al., 2020 * [30] | 109 | ERM, MH | Retrospective case series | Optical (Lenstar 9000, Haag-Streit, Köniz, Switzerland) | Phacovitrectomy: +0.59 D Phacoemulsification after PPV: +0.35 D, p = 0.01 |
| Mayer-Xanthaki, C.G., et al., 2022 [18] | 40 | ERM | Prospective case series | Optical (IOL Master 700, Zeiss) | Phacoemulsification: change in ACD −0.10 mm Phacovitrectomy: change in ACD −0.12 mm, p = 0.36 |
| Chatzimichail, E., et al., 2023 [19] | 160 | ERM, MH, RD | Retrospective case–control | Optical (IOL Master 700, Zeiss) | No difference in ACD between groups defined by endotamponade: BSS (4.60 mm), air (4.52 mm) or gas (4.56 mm; p = 0.40). The refractive prediction error was slightly higher in the gas than in the phacoemulsification group (p ≤ 0.012) |
| Crincoli, E., et al., 2024 [21] | 219 | Prospective case series | Biometry | Phacovitrectomy: −0.29 D Phacoemulsification: −0.03 D, p = 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarro, M.; Sararols, L.; López, E.; Vázquez, M.; Ruiz, S.; Biarnés, M. Comparison of Aqueous Depth Changes Following Cataract Surgery in Vitrectomized and Non-Vitrectomized Fellow Eyes. Diagnostics 2025, 15, 1429. https://doi.org/10.3390/diagnostics15111429
Guarro M, Sararols L, López E, Vázquez M, Ruiz S, Biarnés M. Comparison of Aqueous Depth Changes Following Cataract Surgery in Vitrectomized and Non-Vitrectomized Fellow Eyes. Diagnostics. 2025; 15(11):1429. https://doi.org/10.3390/diagnostics15111429
Chicago/Turabian StyleGuarro, Mercè, Laura Sararols, Elena López, Meritxell Vázquez, Sergi Ruiz, and Marc Biarnés. 2025. "Comparison of Aqueous Depth Changes Following Cataract Surgery in Vitrectomized and Non-Vitrectomized Fellow Eyes" Diagnostics 15, no. 11: 1429. https://doi.org/10.3390/diagnostics15111429
APA StyleGuarro, M., Sararols, L., López, E., Vázquez, M., Ruiz, S., & Biarnés, M. (2025). Comparison of Aqueous Depth Changes Following Cataract Surgery in Vitrectomized and Non-Vitrectomized Fellow Eyes. Diagnostics, 15(11), 1429. https://doi.org/10.3390/diagnostics15111429






