The Value of the Naples Prognostic Score and the Systemic Immune-Inflammation Index in Predicting Ischemia on Myocardial Perfusion Scintigraphy
Abstract
1. Introduction
2. Methods
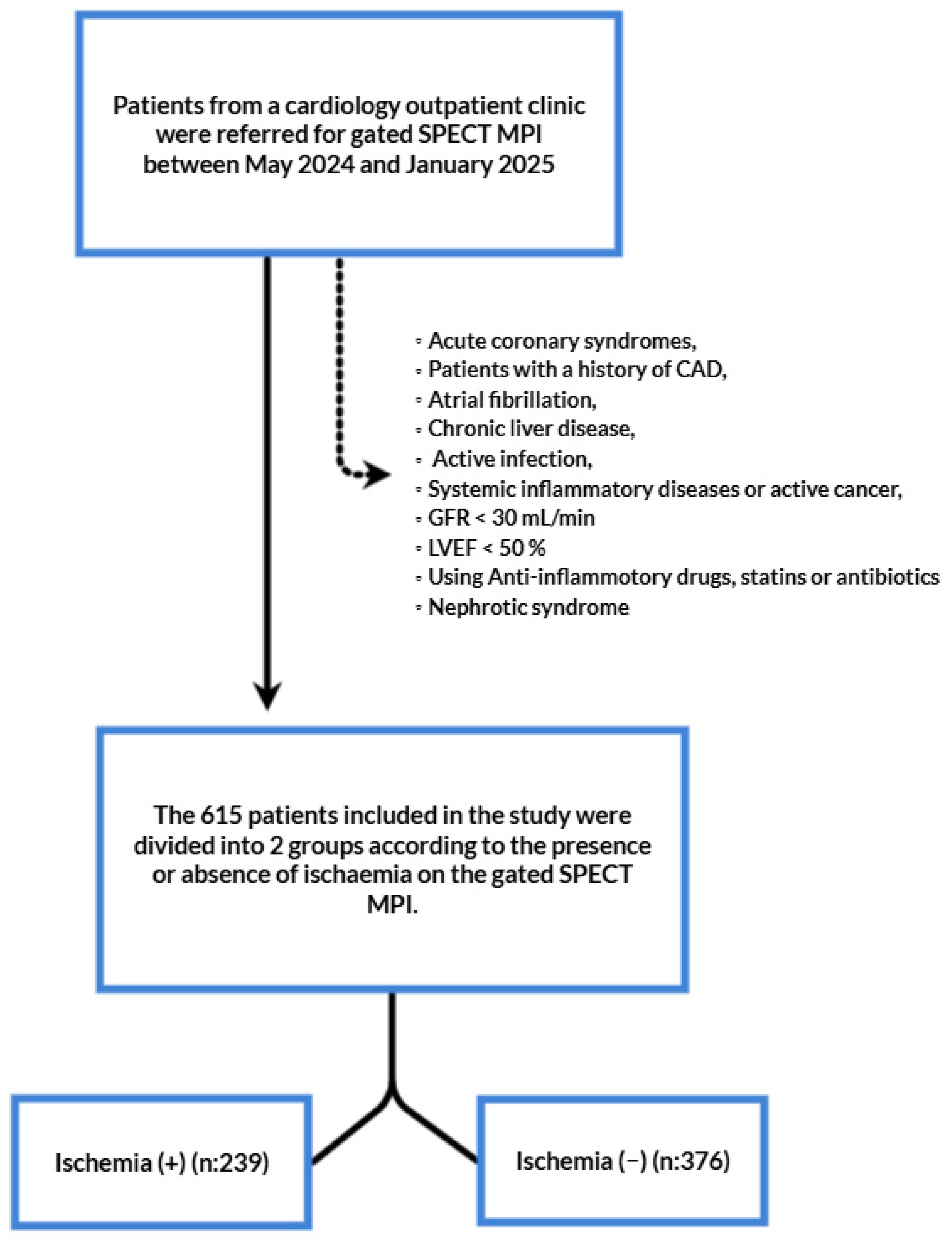
2.1. Patient Population and Study Design
2.2. Data Collection and Analysis
2.3. Laboratory Measurements
2.4. Myocardial Perfusion Imaging
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: A narrative review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef]
- Montecucco, F.; Liberale, L.; Bonaventura, A.; Vecchiè, A.; Dallegri, F.; Carbone, F. The role of inflammation in cardiovascular outcome. Curr. Atheroscler. Rep. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Ateş, A.H.; Aytemir, K.; Koçyiğit, D.; Yalcin, M.U.; Gürses, K.M.; Yorgun, H.; Canpolat, U.; Hazırolan, T.; Özer, N. Association of neutrophil-to-lymphocyte ratio with the severity and morphology of coronary atherosclerotic plaques detected by multidetector computerized tomography. Acta Cardiol. Sin. 2016, 32, 676. [Google Scholar] [PubMed]
- Hansson, G.K.; Libby, P.; Tabas, I. Inflammation and plaque vulnerability. J. Intern. Med. 2015, 278, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Davì, G.; Patrono, C. Platelet activation and atherothrombosis. N. Engl. J. Med. 2007, 357, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef]
- Chien, S.-C.; Chen, C.-Y.; Leu, H.-B.; Su, C.-H.; Yin, W.-H.; Tseng, W.-K.; Wu, Y.-W.; Lin, T.-H.; Chang, K.-C.; Wang, J.-H.; et al. Association of low serum albumin concentration and adverse cardiovascular events in stable coronary heart disease. Int. J. Cardiol. 2017, 241, 1–5. [Google Scholar] [CrossRef]
- Suzuki, S.; Hashizume, N.; Kanzaki, Y.; Maruyama, T.; Kozuka, A.; Yahikozawa, K. Prognostic significance of serum albumin in patients with stable coronary artery disease treated by percutaneous coronary intervention. PLoS ONE 2019, 14, e0219044. [Google Scholar] [CrossRef]
- Galizia, G.; Lieto, E.; Auricchio, A.; Cardella, F.; Mabilia, A.; Podzemny, V.; Castellano, P.; Orditura, M.; Napolitano, V. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Dis. Colon Rectum 2017, 60, 1273–1284. [Google Scholar] [CrossRef]
- Elia, S.; Patirelis, A.; Hardavella, G.; Santone, A.; Carlea, F.; Pompeo, E. The Naples prognostic score is a useful tool to assess surgical treatment in non-small cell lung cancer. Diagnostics 2023, 13, 3641. [Google Scholar] [CrossRef]
- Chen, F.; Xie, C.; Ren, K.; Xu, X. Prognostic value of the Naples prognostic score in patients with gastrointestinal cancers: A meta-analysis. Nutr. Cancer 2023, 75, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Çetin, Z.G.; Balun, A.; Çiçekçioğlu, H.; Demirtaş, B.; Yiğitbaşı, M.M.; Özbek, K.; Çetin, M. A novel score to predict one-year mortality after transcatheter aortic valve replacement, Naples prognostic score. Medicina 2023, 59, 1666. [Google Scholar] [CrossRef] [PubMed]
- Gitmez, M.; Ekingen, E.; Zaman, S. Predictive Value of the Naples Prognostic Score for One-Year Mortality in NSTEMI Patients Undergoing Selective PCI. Diagnostics 2025, 15, 640. [Google Scholar] [CrossRef]
- Kılıç, O.; Suygun, H.; Mustu, M.; Karadeniz, F.O.; Ozer, S.F.; Senol, H.; Kaya, D.; Buber, I.; Karakurt, A. Is the Naples prognostic score useful for predicting heart failure mortality. Kardiologiia 2023, 63, 61–65. [Google Scholar] [CrossRef]
- Erdogan, A.; Genc, O.; Ozkan, E.; Goksu, M.M.; Ibisoglu, E.; Bilen, M.N.; Guler, A.; Karagoz, A. Impact of Naples prognostic score at admission on in-hospital and follow-up outcomes among patients with ST-segment elevation myocardial infarction. Angiology 2023, 74, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, E.; Erdogan, A.; Karagoz, A.; Tanboğa, I.H. Comparison of systemic immune-inflammation index and Naples prognostic score for prediction coronary artery severity patients undergoing coronary computed tomographic angiography. Angiology 2024, 75, 62–71. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J.; et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur. J. Clin. Investig. 2020, 50, e13230. [Google Scholar]
- Dziedzic, E.A.; Gąsior, J.S.; Tuzimek, A.; Dąbrowski, M.; Jankowski, P. The Association between Serum Vitamin D concentration and new inflammatory biomarkers—Systemic inflammatory index (SII) and systemic inflammatory response (SIRI)—In patients with ischemic heart disease. Nutrients 2022, 14, 4212. [Google Scholar] [CrossRef]
- Özen, Y.; Erdöl, A.; Özbay, M.B.; Erdoğan, M. The Prognostic Role of the Systemic Inflammatory Index (SII) in Heart Failure Patients: Systemic Inflammatory Index and Heart Failure. Int. J. Curr. Med. Biol. Sci. 2023, 3, 45–50. [Google Scholar]
- Dziedzic, E.A.; Gąsior, J.S.; Tuzimek, A.; Paleczny, J.; Junka, A.; Dąbrowski, M.; Jankowski, P. Investigation of the associations of novel inflammatory biomarkers—Systemic inflammatory index (SII) and systemic inflammatory response index (SIRI)—With the severity of coronary artery disease and acute coronary syndrome occurrence. Int. J. Mol. Sci. 2022, 23, 9553. [Google Scholar] [CrossRef]
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low diagnostic yield of elective coronary angiography. N. Engl. J. Med. 2010, 362, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A. 2024 ESC guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar]
- American Diabetes Association Professional Practice Committee. Diagnosis and classification of diabetes: Standards of care in diabetes—2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension: Developed by the task force on the management of elevated blood pressure and hypertension of the European Society of Cardiology (ESC) and endorsed by the European Society of Endocrinology (ESE) and the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar]
- Visseren, F.L.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [PubMed]
- Toldo, S.; Mauro, A.G.; Cutter, Z.; Abbate, A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H1553–H1568. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Tamhane, U.U.; Aneja, S.; Montgomery, D.; Rogers, E.-K.; Eagle, K.A.; Gurm, H.S. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am. J. Cardiol. 2008, 102, 653–657. [Google Scholar] [CrossRef] [PubMed]
- González-Pacheco, H.; Amezcua-Guerra, L.M.; Sandoval, J.; Martínez-Sánchez, C.; Ortiz-León, X.A.; Peña-Cabral, M.A.; Bojalil, R. Prognostic implications of serum albumin levels in patients with acute coronary syndromes. Am. J. Cardiol. 2017, 119, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Azab, B.; Zaher, M.; Weiserbs, K.F.; Torbey, E.; Lacossiere, K.; Gaddam, S.; Gobunsuy, R.; Jadonath, S.; Baldari, D.; McCord, D.; et al. Usefulness of neutrophil to lymphocyte ratio in predicting short-and long-term mortality after non–ST-elevation myocardial infarction. Am. J. Cardiol. 2010, 106, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Jang, H.-J.; Oh, I.-Y.; Yoon, C.-H.; Suh, J.-W.; Cho, Y.-S.; Youn, T.-J.; Cho, G.-Y.; Chae, I.-H.; Choi, D.-J. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am. J. Cardiol. 2013, 111, 636–642. [Google Scholar] [CrossRef]
- Arbel, Y.; Finkelstein, A.; Halkin, A.; Birati, E.Y.; Revivo, M.; Zuzut, M.; Shevach, A.; Berliner, S.; Herz, I.; Keren, G. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis 2012, 225, 456–460. [Google Scholar] [CrossRef]
- Arefnia, M.; Bayat, M.; Hosseinzadeh, E.; Basiri, E.A.; Ghodsirad, M.; Naghshineh, R.; Zamani, H. The predictive value of CRP/albumin ratio (CAR) in the diagnosis of ischemia in myocardial perfusion scintigraphy. Hipertens. Riesgo Vasc. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Sabanoglu, C.; Inanc, I. C-reactive protein to albumin ratio predicts for severity of coronary artery disease and ischemia. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7623–7631. [Google Scholar]
- Ozdemir, S.; Barutcu, A.; Gazi, E.; Tan, Y.; Turkon, H. The relationship between some complete blood count parameters and myocardial perfusion: A scintigraphic approach. World J. Nucl. Med. 2015, 14, 197–201. [Google Scholar] [CrossRef]
- Unkun, T.; Fidan, S.; Derebey, S.T.; Şengör, B.G.; Aytürk, M.; Sarı, M.; Efe, S.Ç.; Alıcı, G.; Özkan, B.; Karagöz, A. The Value of Naples Prognostic Score in Predicting Ischemia on Myocardial Perfusion Scintigraphy. Koşuyolu Heart J. 2024, 27, 63–69. [Google Scholar] [CrossRef]
- Efe, S.Ç.; Candan, Ö.Ö.; Gündoğan, C.; Öz, A.; Yüksel, Y.; Ayca, B.; Çermik, T.F. Value of C-reactive protein/albumin ratio for predicting ischemia in myocardial perfusion scintigraphy. Mol. Imaging Radionucl. Ther. 2020, 29, 112. [Google Scholar] [CrossRef]
- Don, B.R.; Kaysen, G. Poor nutritional status and inflammation: Serum albumin: Relationship to inflammation and nutrition. In Seminars in Dialysis; Blackwell Science Inc.: Oxford, UK, 2004; pp. 432–437. [Google Scholar]
- Lai, G.; Zhao, Y.; Yang, C.; Zheng, Y.; Sun, J.; Zhao, Y.; Ding, M. Association of Naples prognostic score with cardiovascular disease risk and its longitudinal prognostic impact on mortality in cardiovascular disease patients: Evidence from NHANES. Nutrition, Metabolism and Cardiovascular Diseases. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103840. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Sun, T.; Huang, X.; Ma, M.; Yang, S.; Zhou, Y. Prognostic value of systemic immune-inflammation index in CAD patients: Systematic review and meta-analyses. Eur. J. Clin. Investig. 2024, 54, e14100. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, M.; Erdöl, M.A.; Öztürk, S.; Durmaz, T. Systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark. Med. 2020, 14, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Schutt, R.C.; Hannawi, B.; DeLao, T.; Barker, C.M.; Kleiman, N.S. Association of immature platelets with adverse cardiovascular outcomes. J. Am. Coll. Cardiol. 2014, 64, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Coppinger, J.A.; Cagney, G.; Toomey, S.; Kislinger, T.; Belton, O.; McRedmond, J.P.; Cahill, D.J.; Emili, A.; Fitzgerald, D.J.; Maguire, P.B. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 2004, 103, 2096–2104. [Google Scholar] [CrossRef]
- Pitsilos, S.; Hunt, J.; Mohler, E.; Prabhakar, A.; Poncz, M.; Dawicki, J.; Khalapyan, T.; Wolfe, M.L.; Fairman, R.; Mitchell, M.; et al. Platelet factor 4 localization in carotid atherosclerotic plaques: Correlation with clinical parameters. Thromb. Haemost. 2003, 90, 1112–1120. [Google Scholar] [CrossRef]
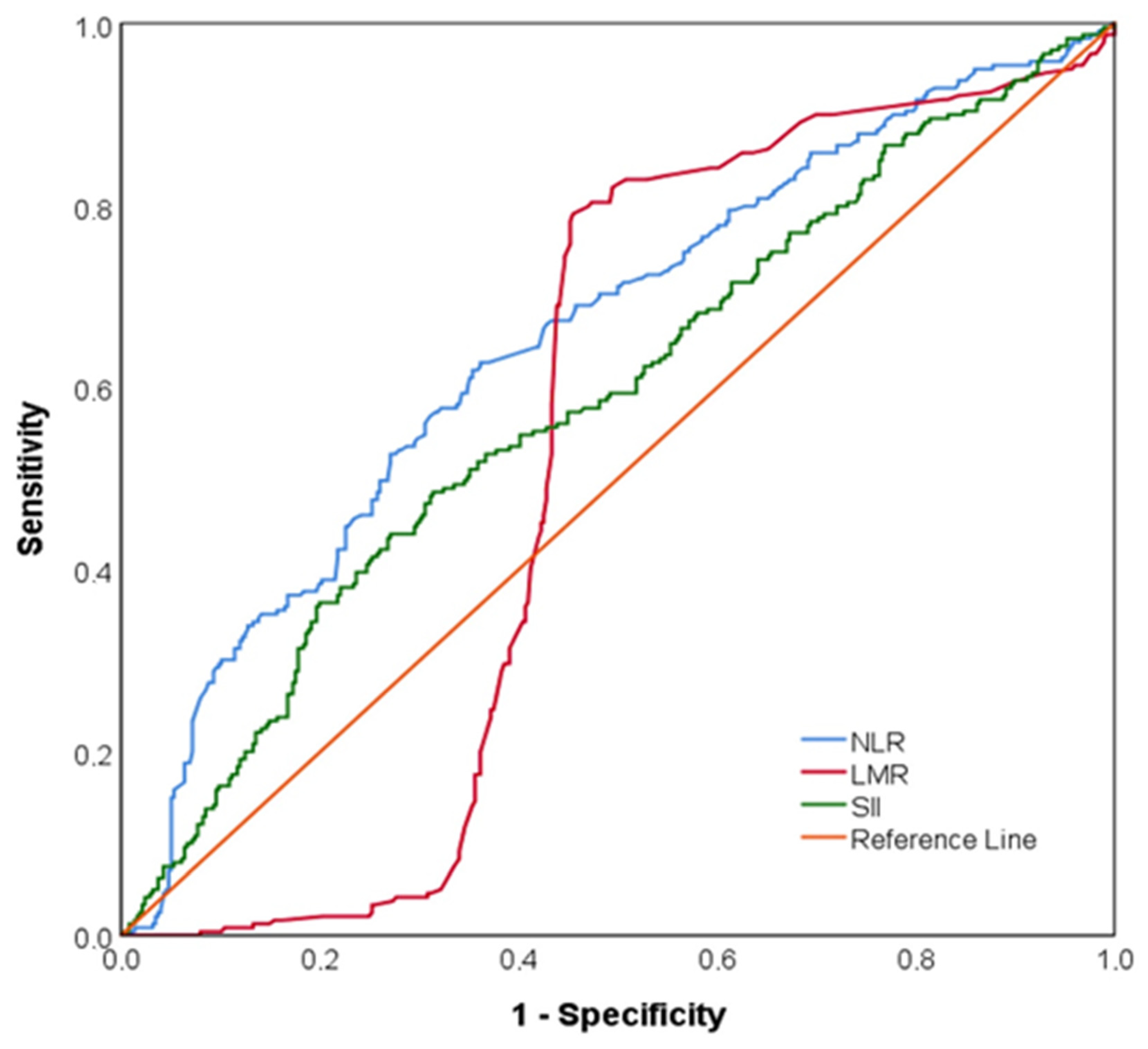
| Overall (n = 615) | No Ischemia (n = 376) | Ischemia (n = 239) | p-Value | |
|---|---|---|---|---|
| Age (years) | 61.6 ± 9.5 | 61.1 ± 9.8 | 62.3 ± 9.0 | 0.130 A |
| Gender | <0.001 B | |||
| Female | 249 (40.5%) | 210 (55.9%) | 39 (16.3%) | |
| Male | 366 (59.5%) | 166 (44.1%) | 200 (83.7%) | |
| BMI (kg/m2) | 25.5 ± 2.2 | 25.4 ± 2.2 | 25.6 ± 2.1 | 0.250 A |
| HT | 359 (58.4%) | 217 (57.7%) | 142 (59.4%) | 0.677 B |
| DM | 153 (24.9%) | 92 (24.5%) | 61 (25.5%) | 0.768 B |
| HL | 113 (18.4%) | 67 (17.8%) | 46 (19.2%) | 0.656 B |
| Smoking | 141 (22.9%) | 77 (20.5%) | 64 (26.8%) | 0.070 B |
| LVEF | 60.0 (55.0–62.0) | 60.0 (56.0–62.0) | 60.0 (55.0–62.0) | 0.183 C |
| Hemoglobin | 14.2 ± 1.49 | 14.1 ± 1.47 | 14.3 ± 1.53 | 0.111 A |
| Hematocrit | 41.7 ± 4.1 | 41.5 ± 4.0 | 42.1 ± 4.2 | 0.051 A |
| LDL | 131.0 (113.0–149.0) | 131.0 (107.2–148.0) | 131.0 (118.0–151.0) | 0.100 C |
| HDL | 48.0 (42.0–55.0) | 48.0 (41.0–55.0) | 48.0 (42.0–55.0) | 0.852 C |
| Triglyceride | 132.0 (100.0–183.0) | 130.0 (100.0–175.7) | 133.0 (100.0–190.0) | 0.550 C |
| Total cholesterol | 196.0 (171.0–244.0) | 178.0 (165.2–250.0) | 206.0 (184.0–236.0) | <0.001 C |
| CRP | 0.70 (0.50–1.50) | 0.60 (0.50–1.20) | 1.20 (0.60–2.10) | <0.001 C |
| Creatinine | 0.87 (0.72–1.00) | 0.84 (0.70–1.00) | 0.90 (0.75–1.01) | 0.074 C |
| Albumin | 4.23 (3.93–4.43) | 4.25 (4.12–4.40) | 4.10 (3.68–4.50) | <0.001 C |
| WBC | 7.1 (6.0–8.5) | 7.0 (6.0–8.4) | 7.4 (6.3–8.8) | 0.005 C |
| PLT | 230.0 (194.0–270.0) | 235.5 (197.0–273.0) | 220.0 (188.0–260.0) | 0.021 C |
| Neutrophile | 4.6 (3.8–5.6) | 4.2 (3.5–5.1) | 5.1 (4.5–5.8) | <0.001 C |
| Lymphocyte | 2.3 (2.0–2.6) | 2.3 (1.9–2.7) | 2.3 (2.0–2.5) | 0.508 C |
| Monocyte | 0.60 (0.50–0.80) | 0.60 (0.50–0.90) | 0.60 (0.60–0.80) | 0.432 C |
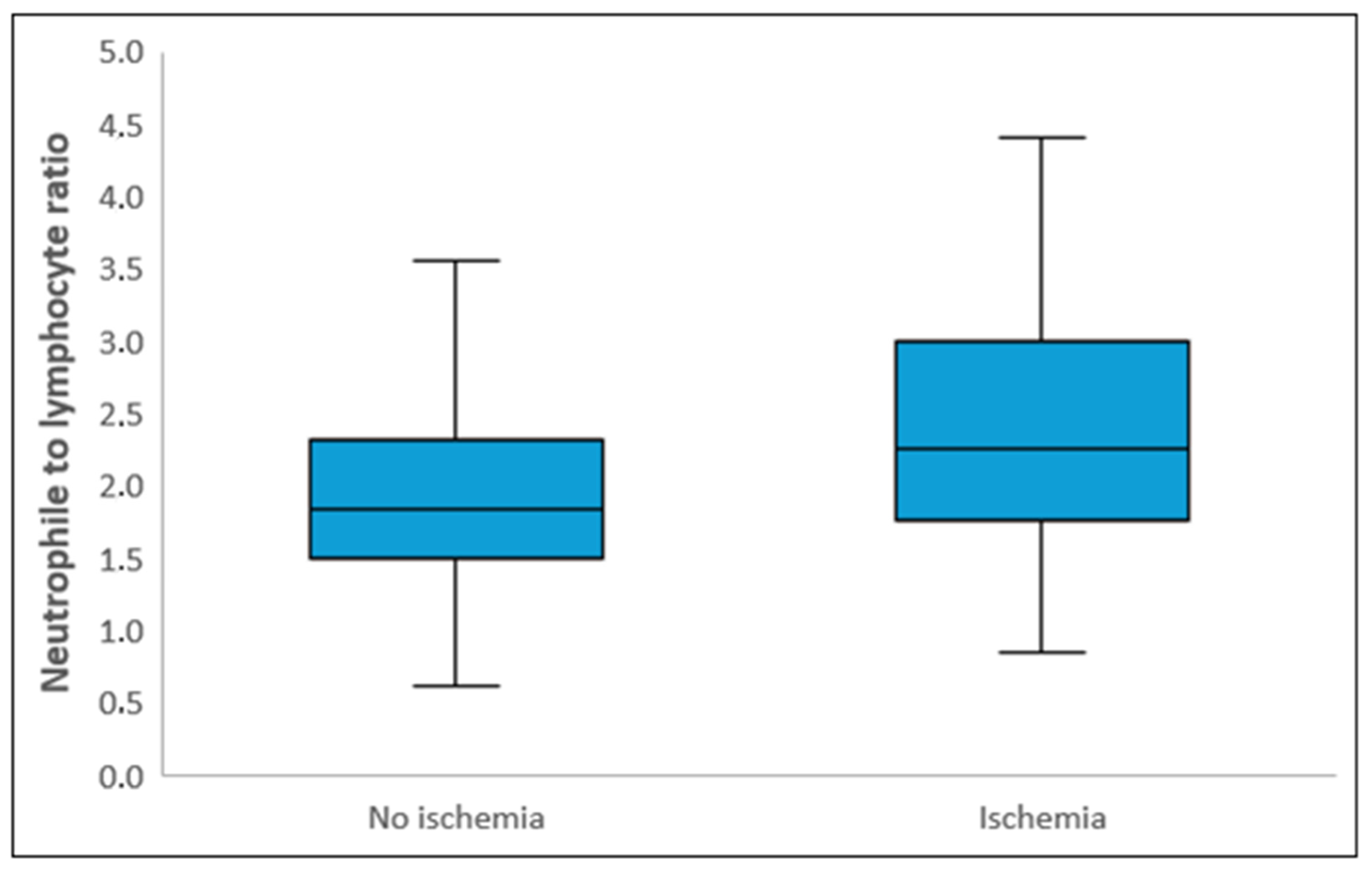
| NLR | 2.00 (1.59–2.50) | 1.84 (1.50–2.32) | 2.26 (1.76–3.00) | <0.001 C |
| LMR | 3.8 (2.6–4.8) | 4.5 (2.1–5.0) | 3.7 (3.0–4.2) | 0.161 C |
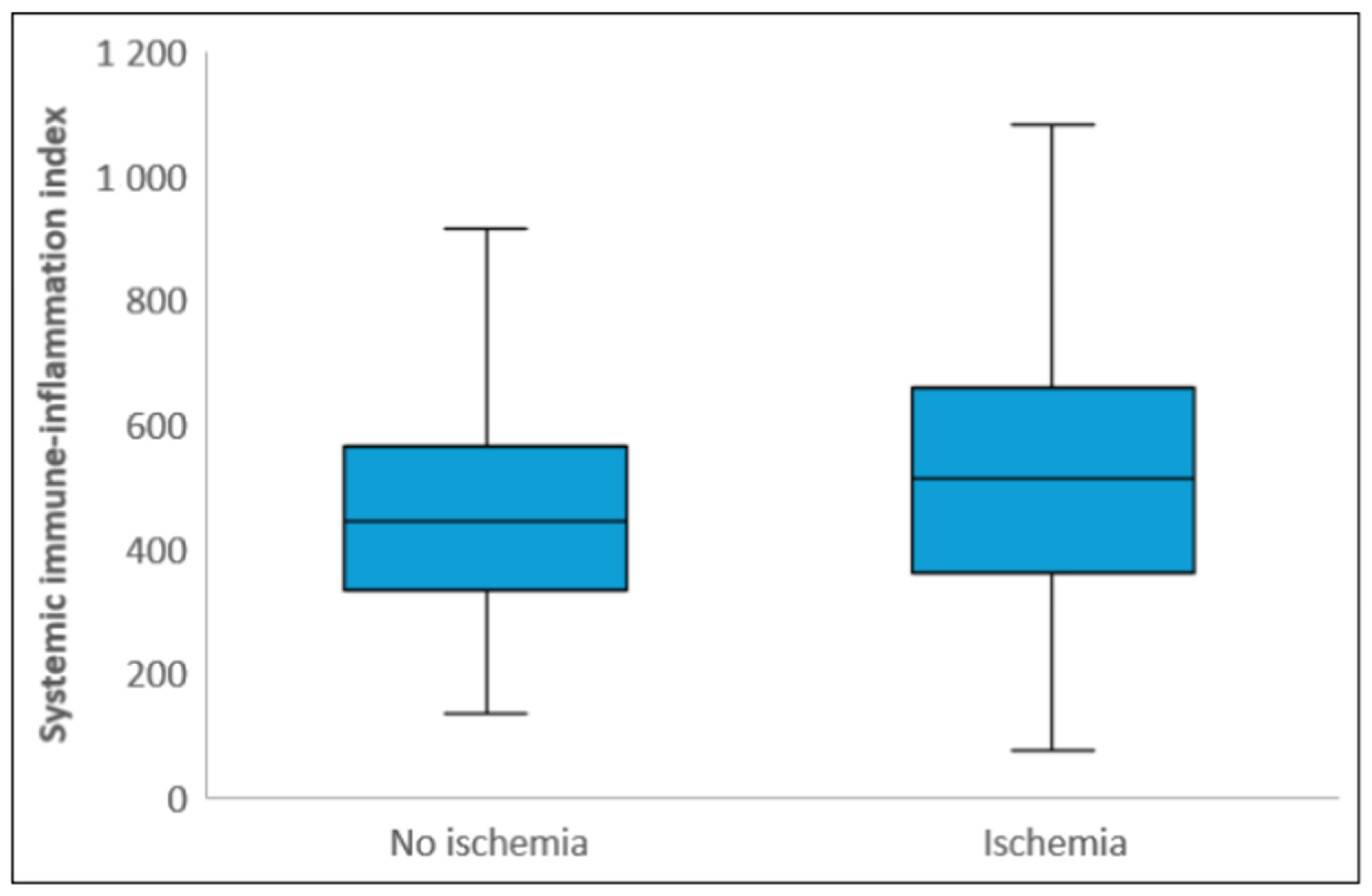
| SII | 459.5 (341.7–617.1) | 445.3 (332.0–566.7) | 513.0 (361.2–659.7) | <0.001 C |
| Naples | 2 (1–2) | 1 (0–2) | 2 (2–3) | <0.001 C |
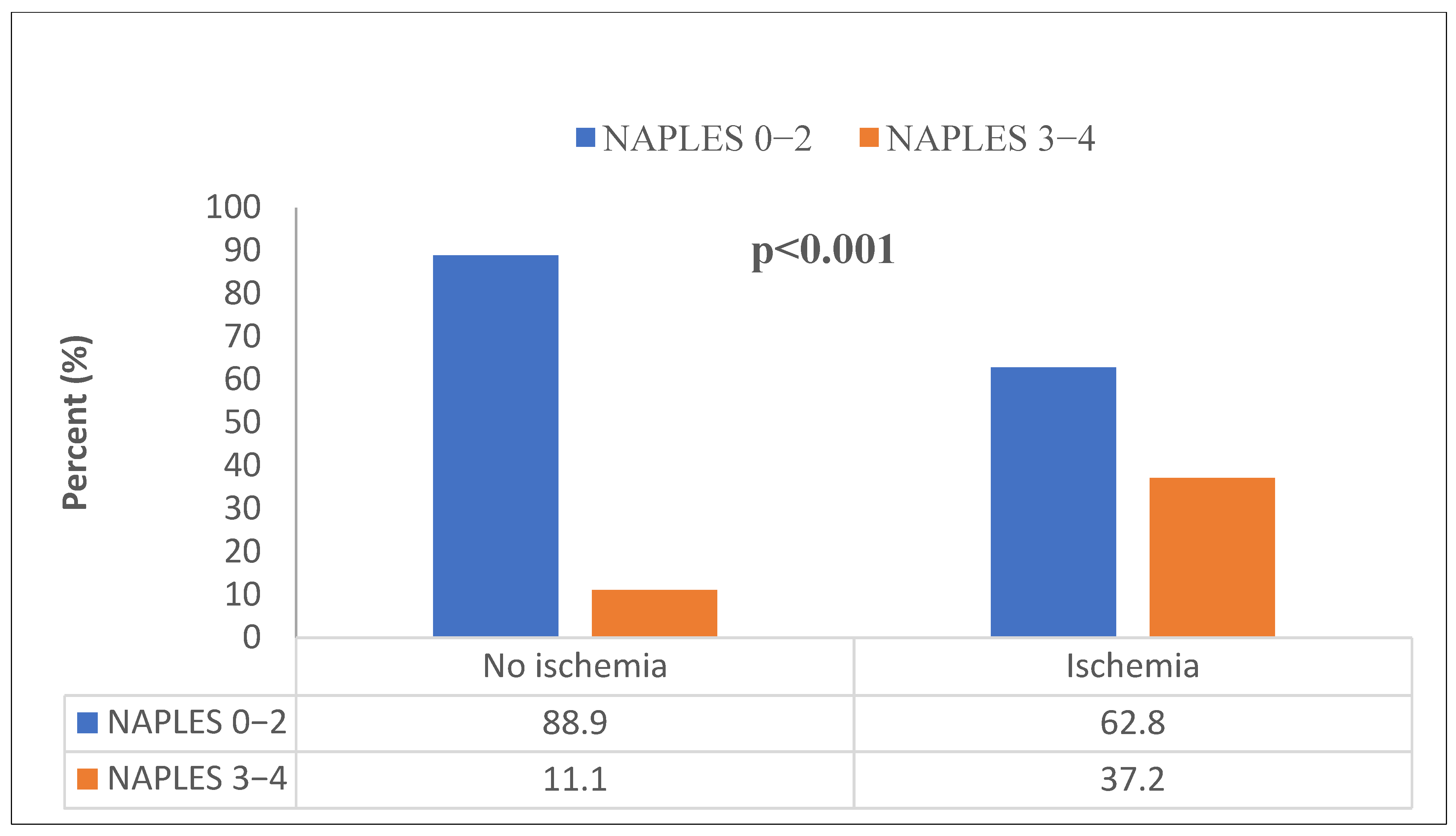
| Naples | <0.001 B | |||
| Low (0–2) | 484 (78.7%) | 334 (88.9%) | 150 (62.8%) | |
| High (3–4) | 131 (21.3%) | 42 (11.1%) | 89 (37.2%) |
| Odds Ratio | 95% Confidence Interval | Wald | p-Value | |
|---|---|---|---|---|
| Age | 1.014 | 0.993–1.036 | 1.654 | 0.198 |
| Male factor | 6.792 | 4.168–11.068 | 59.131 | <0.001 |
| Total cholesterol | 1.003 | 0.999–1.007 | 2.047 | 0.152 |
| CRP | 1.181 | 1.046–1.333 | 7.216 | 0.007 |
| Albumin | 0.682 | 0.394–1.182 | 1.860 | 0.173 |
| WBC | 1.061 | 0.980–1.148 | 2.123 | 0.145 |
| Naples > 2 | 4.427 | 2.642–7.923 | 19.764 | <0.001 |
| NLR > 2.04 | 1.580 | 1.028–2.429 | 4.346 | 0.037 |
| Odds Ratio | 95% Confidence Interval | Wald | p-Value | |
|---|---|---|---|---|
| Age | 1.017 | 0.995–1.039 | 2.370 | 0.124 |
| Male factor | 7.250 | 4.430–11.865 | 62.110 | <0.001 |
| Total cholesterol | 1.003 | 0.999–1.007 | 1.973 | 0.160 |
| CRP | 1.191 | 1.053–1.348 | 7.731 | 0.005 |
| Albumin | 0.693 | 0.401–1.199 | 1.718 | 0.190 |
| WBC | 1.051 | 0.968–1.140 | 1.416 | 0.234 |
| Naples > 2 | 4.945 | 2.913–8.767 | 20.912 | <0.001 |
| SII > 528.27 | 1.676 | 1.072–2.621 | 5.136 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Süygün, H.; Yalçınkaya Öner, D.; Karakulak, U.N. The Value of the Naples Prognostic Score and the Systemic Immune-Inflammation Index in Predicting Ischemia on Myocardial Perfusion Scintigraphy. Diagnostics 2025, 15, 1372. https://doi.org/10.3390/diagnostics15111372
Süygün H, Yalçınkaya Öner D, Karakulak UN. The Value of the Naples Prognostic Score and the Systemic Immune-Inflammation Index in Predicting Ischemia on Myocardial Perfusion Scintigraphy. Diagnostics. 2025; 15(11):1372. https://doi.org/10.3390/diagnostics15111372
Chicago/Turabian StyleSüygün, Hakan, Damla Yalçınkaya Öner, and Ugur Nadir Karakulak. 2025. "The Value of the Naples Prognostic Score and the Systemic Immune-Inflammation Index in Predicting Ischemia on Myocardial Perfusion Scintigraphy" Diagnostics 15, no. 11: 1372. https://doi.org/10.3390/diagnostics15111372
APA StyleSüygün, H., Yalçınkaya Öner, D., & Karakulak, U. N. (2025). The Value of the Naples Prognostic Score and the Systemic Immune-Inflammation Index in Predicting Ischemia on Myocardial Perfusion Scintigraphy. Diagnostics, 15(11), 1372. https://doi.org/10.3390/diagnostics15111372






