Imaging Features and Clinical Characteristics of Granular Cell Tumors: A Single-Center Investigation
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Examination
2.3. Analysis of the Images
3. Results
3.1. Clinical Findings
3.2. Imaging Findings
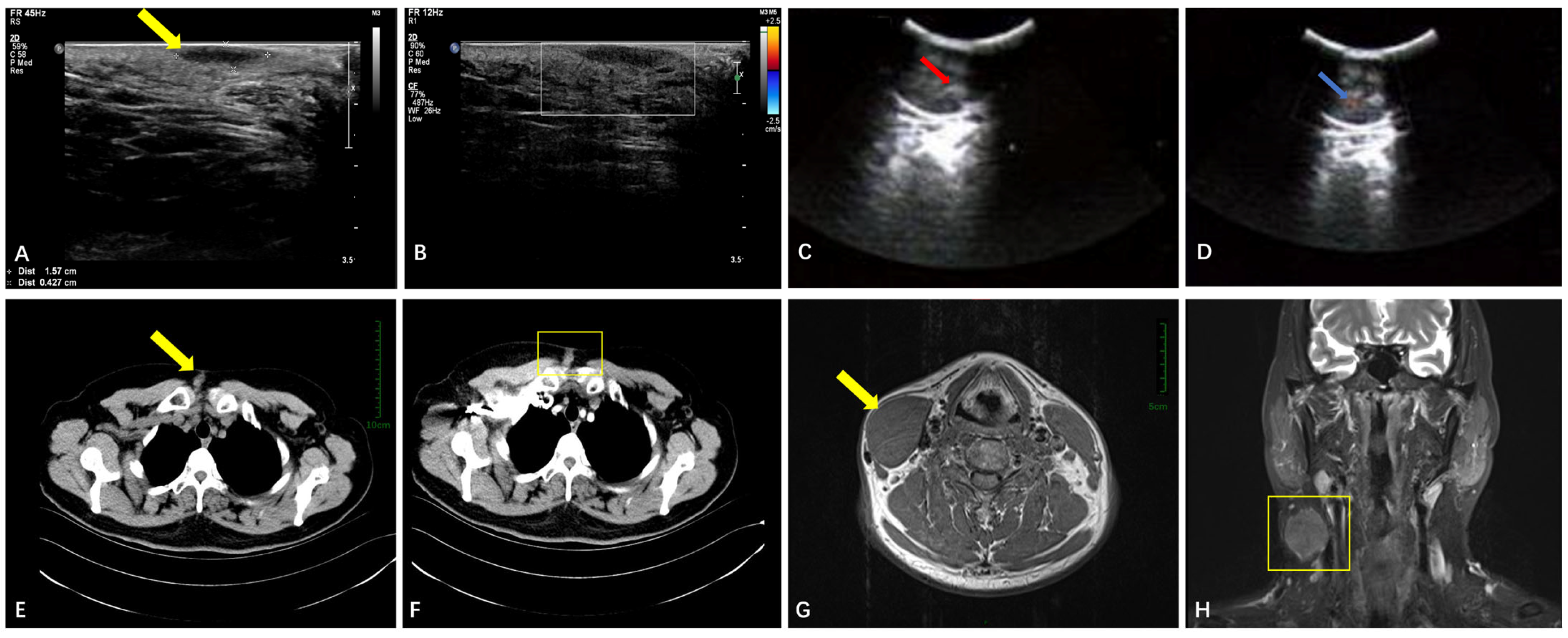
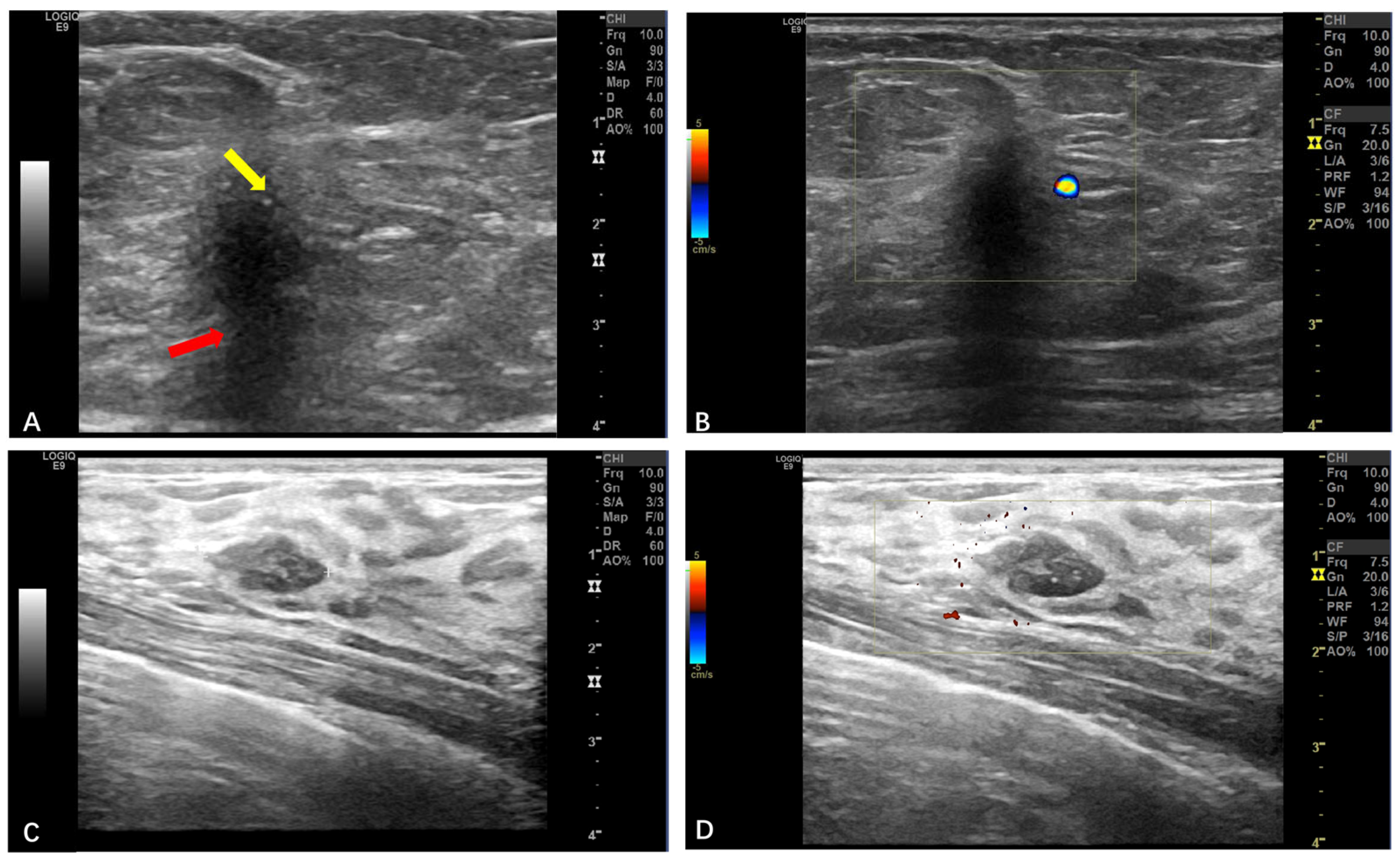
3.2.1. Soft Tissue
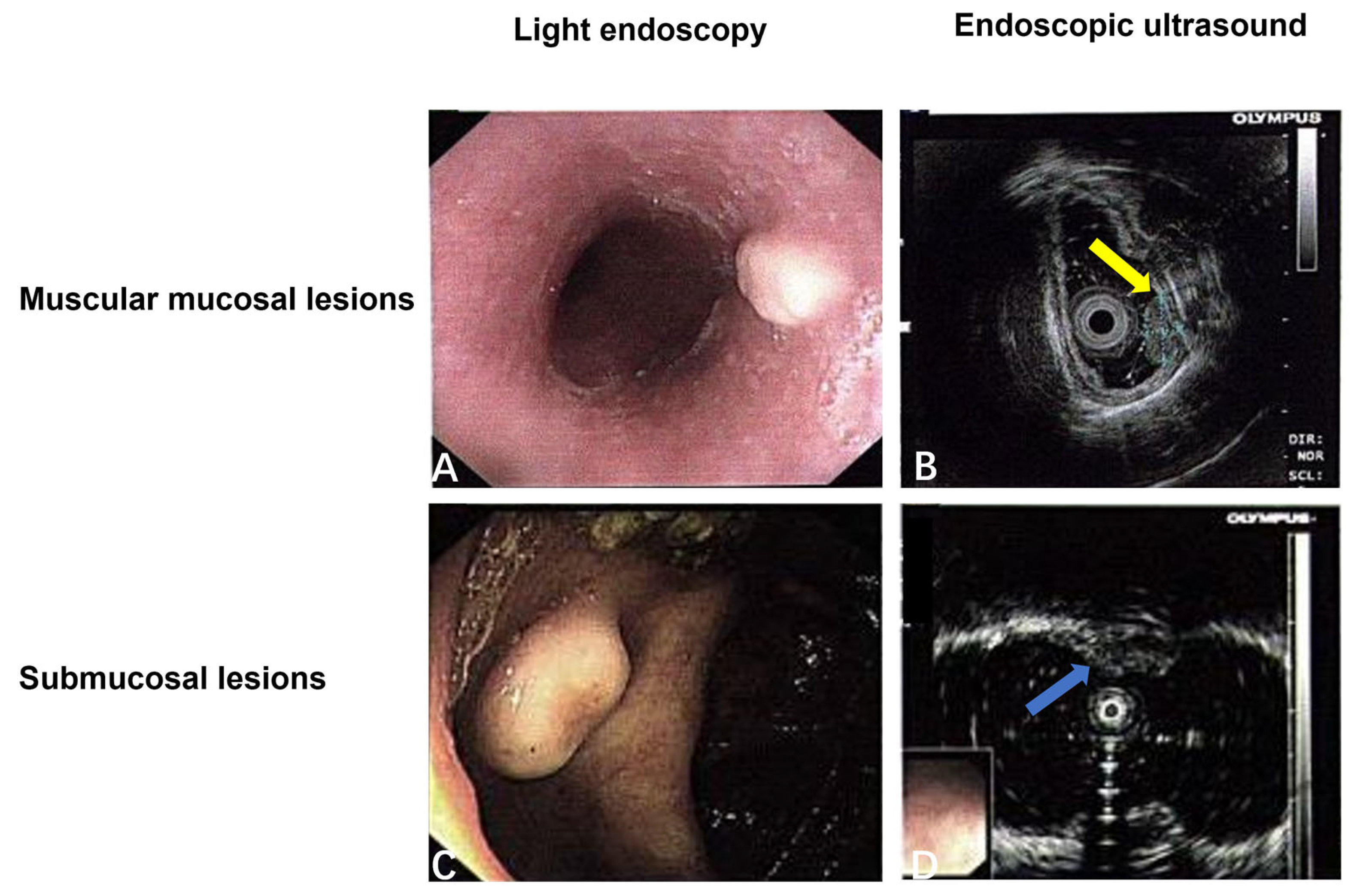
3.2.2. The Digestive System
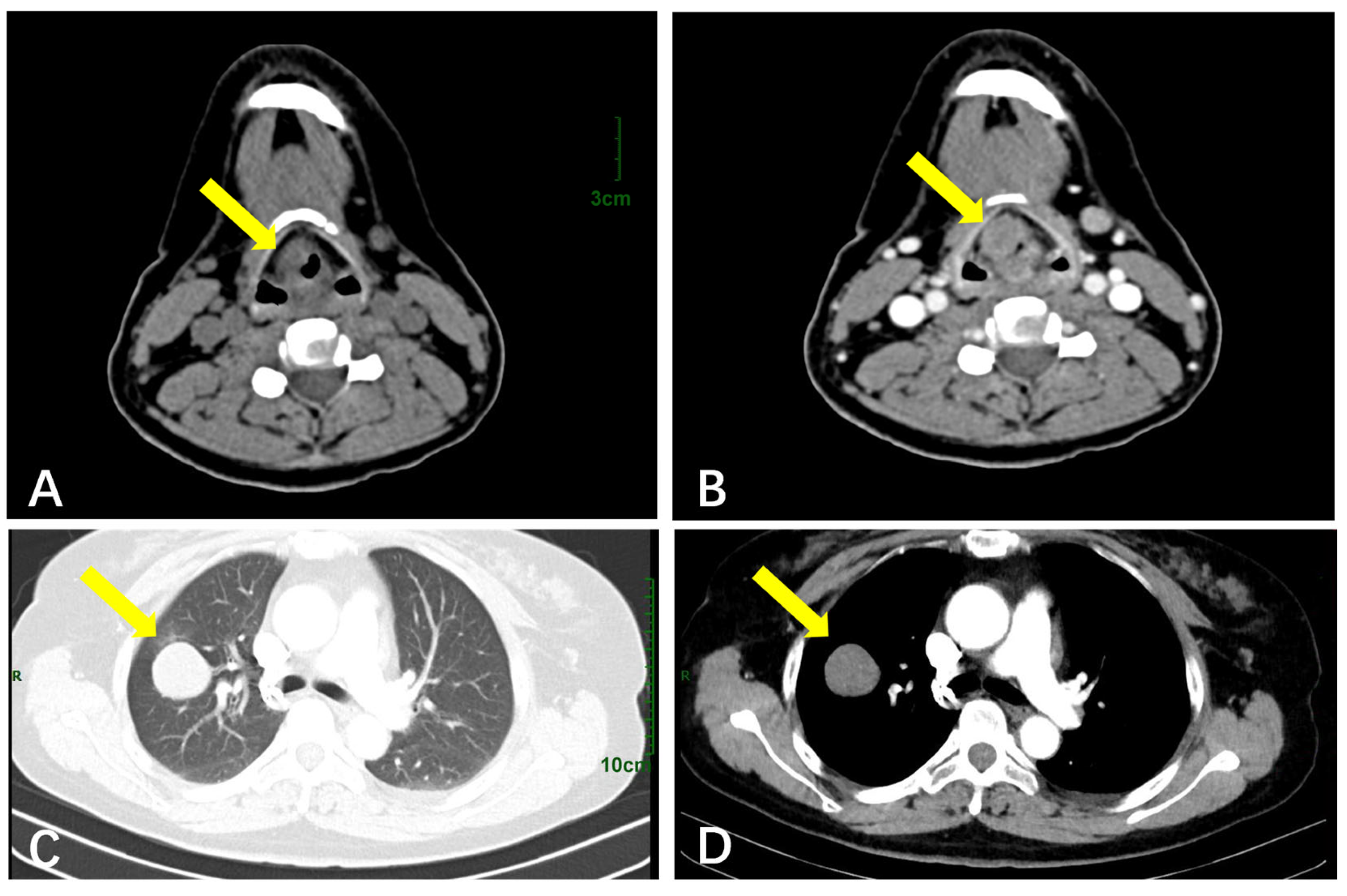
3.2.3. The Respiratory System
3.2.4. Breast GCTs
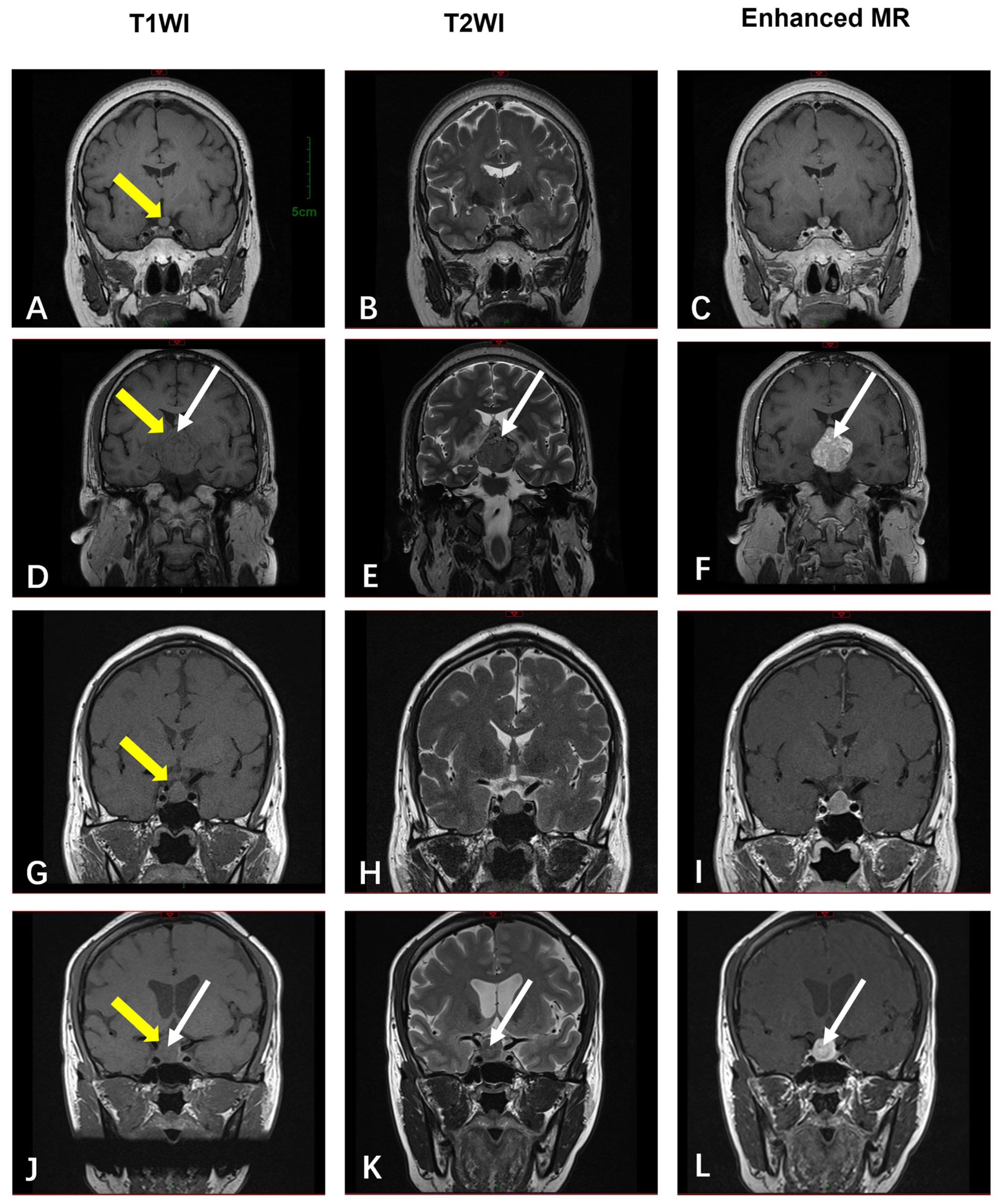
3.2.5. The Sellar Region
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fanburg-Smith, J.C.; Meis-Kindblom, J.M.; Fante, R.; Kindblom, L.G. Malignant granular cell tumor of soft tissue: Diagnostic criteria and clinicopathologic correlation. Am. J. Surg. Pathol. 1998, 22, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.; Cruz, J.; Lavernia, J.; Llombart-Bosch, A. Solitary, multiple, benign, atypical, or malignant: The “Granular Cell Tumor” puzzle. Virchows Arch. 2016, 468, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Nasser, H.; Ahmed, Y.; Szpunar, S.M.; Kowalski, P.J. Malignant granular cell tumor: A look into the diagnostic criteria. Pathol. Res. Pract. 2011, 207, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.N.; Tuggle, C.T.; Zogg, C.K.; Mirza, H.N.; Narayan, D. Epidemiology of malignant cutaneous granular cell tumors: A US population-based cohort analysis using the Surveillance, Epidemiology, and End Results (SEER) database. J. Am. Acad. Dermatol. 2018, 78, 490–497.e1. [Google Scholar] [CrossRef]
- Akahane, K.; Kato, K.; Ogiso, S.; Sakaguchi, K.; Hashimoto, M.; Ishikawa, A.; Kato, T.; Fuwa, Y.; Takahashi, A.; Kobayashi, K. Malignant granular cell tumor of the breast: Case report and literature review. Breast Cancer 2015, 22, 317–323. [Google Scholar] [CrossRef]
- Lack, E.E.; Worsham, G.F.; Callihan, M.D.; Crawford, B.E.; Klappenbach, S.; Rowden, G.; Chun, B. Granular cell tumor: A clinicopathologic study of 110 patients. J. Surg. Oncol. 1980, 13, 301–316. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhou, J.; Yu, H.L. Esophageal granular cell tumor: A case report. World J. Clin. Cases 2022, 10, 6543–6547. [Google Scholar] [CrossRef]
- Famà, F.; Pino, A.; Cavallari, V.; Fadda, G.; Ieni, A.; Dionigi, G. Granular cell tumor of the trachea mimicking an infiltrating thyroid cancer. A case report. Int. J. Surg. Case Rep. 2022, 94, 107031. [Google Scholar] [CrossRef]
- Houcine, Y.; Mlika, M.; Moussa, C.; Rouis, H.; Brahem, E.; Ismail, O.; Maȃlej, S.; El Mezni, F. Granular cell tumor of the lung and tracheobronchial tree: Two case-presentation with a review of the literature. Rare Tumors 2023, 15, 20363613231187822. [Google Scholar] [CrossRef]
- Hwang, M.J.; Lee, Y.P.; Jin, Y.T.; Chiang, C.P. Granular cell tumor of the tongue: Case report. J. Dent. Sci. 2023, 18, 1431–1432. [Google Scholar] [CrossRef]
- Lafuente Ibáñez de Mendoza, I.; López Ortega, K.; Trierveiler, M.; Oliveira Alves, M.G.; Dias Almeida, J.; Gándara Vila, P.; Aguirre Urízar, J.M.; Pérez Sayans, M. Oral granular cell tumour: A multicentric study of 56 cases and a systematic review. Oral. Dis. 2020, 26, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Mansour, W.A.; El Saloussy, Y.; Wahdan, W. Forearm Granular Cell Tumor: A Rare Entity. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5485. [Google Scholar] [CrossRef] [PubMed]
- Mobarki, M.; Dumollard, J.M.; Dal Col, P.; Camy, F.; Peoc’h, M.; Karpathiou, G. Granular cell tumor a study of 42 cases and systemic review of the literature. Pathol. Res. Pract. 2020, 216, 152865. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, K.J.; Lee, J. Retinal granular cell tumor: A case report. BMC Ophthalmol. 2021, 21, 453. [Google Scholar] [CrossRef]
- Shi, Y.; Xueyan, Z.; Zhou, Y.; Zhang, X. Intramuscular granular cell tumor in the sternocleidomastoid muscle: A case report and literature review. Ear Nose Throat J. 2022, 104, 307S–312S. [Google Scholar] [CrossRef]
- Stemm, M.; Suster, D.; Wakely, P.E., Jr.; Suster, S. Typical and Atypical Granular Cell Tumors of Soft Tissue: A Clinicopathologic Study of 50 Patients. Am. J. Clin. Pathol. 2017, 148, 161–166. [Google Scholar] [CrossRef]
- Andalib, A.; Heidary, M.; Sajadieh-Khajouei, S. Granular cell tumor presenting as a large leg mass. Arch. Bone Jt. Surg. 2014, 2, 260–267. [Google Scholar]
- Tsuchida, T.; Okada, K.; Itoi, E.; Sato, T.; Sato, K. Intramuscular malignant granular cell tumor. Skeletal Radiol. 1997, 26, 116–121. [Google Scholar] [CrossRef]
- Marcoval, J.; Bauer-Alonso, A.; Llobera-Ris, C.; Moreno-Vilchez, C.; Penín, R.M.; Bermejo, J. Granular Cell Tumor: A Clinical Study of 81 Patients. Actas Dermosifiliogr. (Engl. Ed.) 2021, 112, 441–446. [Google Scholar] [CrossRef]
- Yu-Fong Chang, J.; Hwang, M.J.; Sun, A.; Chiang, C.P. Granular cell tumor: Case report. J. Dent. Sci. 2021, 16, 1018–1019. [Google Scholar] [CrossRef]
- Nasser, H.; Danforth, R.D., Jr.; Sunbuli, M.; Dimitrijevic, O. Malignant granular cell tumor: Case report with a novel karyotype and review of the literature. Ann. Diagn. Pathol. 2010, 14, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Liu, L.; Wen, Q.; Hu, L.; Zhong, L.; Zhou, Y. Imaging features of granular cell tumor in the breast: Case report. Medicine 2020, 99, e23264. [Google Scholar] [CrossRef] [PubMed]
- Meani, F.; Di Lascio, S.; Wandschneider, W.; Montagna, G.; Vitale, V.; Zehbe, S.; Harder, Y.; Parvex, S.L.; Spina, P.; Canonica, C.; et al. Granular cell tumor of the breast: A multidisciplinary challenge. Crit. Rev. Oncol. Hematol. 2019, 144, 102828. [Google Scholar] [CrossRef]
- Abreu, N.; Filipe, J.; André, S.; Marques, J.C. Granular cell tumor of the breast: Correlations between imaging and pathology findings. Radiol. Bras. 2020, 53, 105–111. [Google Scholar] [CrossRef]
- Adeniran, A.; Al-Ahmadie, H.; Mahoney, M.C.; Robinson-Smith, T.M. Granular cell tumor of the breast: A series of 17 cases and review of the literature. Breast J. 2004, 10, 528–531. [Google Scholar] [CrossRef]
- Ssi-Yan-Kai, G.; Barthier, S.; Trichot, C.; Prevot, S.; De Laveaucoupet, J. Granular cell breast cancer: A rare misleading lesion. Diagn. Interv. Imaging 2015, 96, 287–289. [Google Scholar] [CrossRef]
- Han, F.; Gao, L.; Wang, Y.; Jin, Y.; Lv, Y.; Yao, Z.; Zhang, J. Clinical and imaging features of granular cell tumor of the neurohypophysis: A retrospective analysis. Medicine 2018, 97, e9745. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.Y.; Wu, C.Y.; Huang, H.J.; Lin, Y.M. Granular cell tumor of the lung. Lung 2010, 188, 355–357. [Google Scholar] [CrossRef]
- Venur, V.A.; Zhang, G.; Farver, C.; Mukhopadhyay, S.; Raymond, D.P.; Velcheti, V. Coexistent pulmonary granular cell tumor and adenocarcinoma of the lung. Transl. Lung Cancer Res. 2014, 3, 262–264. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Zheng, Y.; Zhang, H. Gastric granular cell tumor: A case report and literature review. Oncol. Lett. 2024, 28, 403. [Google Scholar] [CrossRef]
| Age | Years |
| range | 7–69 |
| median, mean ± SD | 45, 42 ± 12 |
| Gender | |
| female | 48 (72.7%) |
| male | 18 (27.3%) |
| Lesion Location | Presenting Symptoms |
| soft tissue | palpable painless masses |
| orbit | decreased vision, pain, and eye movement disorders |
| digestive system | choking, abdominal pain, bloating, diarrhea |
| respiratory system | hoarseness, coughing, sputum production, chest tightness, or choking after eating |
| breast | palpable lumps |
| sellar region | decreased vision, dizziness or headache, emaciation, and fatigue |
| Pathological Nature | Number of Cases, (%) |
| benign | 58 (87.9%) |
| atypical | 2 (3.0%) |
| malignant | 6 (9.1%) |
| Lesion Location | Number of Cases, (%) |
|---|---|
| soft tissue | 32 (48.4%) |
| upper limbs | 2 (3.0%) |
| lower limbs | 4 (6.1%) |
| neck | 2 (3.0%) |
| chest wall | 4 (6.1%) |
| abdominal wall | 8 (12.1%) |
| back | 5 (7.6%) |
| crissum | 1 (1.5%) |
| axilla | 1 (1.5%) |
| orbit | 3 (4.5%) |
| umbilicus | 1 (1.5%) |
| groin | 1 (1.5%) |
| digestive system | (20, 30.3%) |
| tongue | 5 (7.6%) |
| esophagus | 10 (15.2%) |
| stomach | 2 (3.0%) |
| colon | 3 (4.5%) |
| respiratory system | (5, 7.6%) |
| larynx | 1 (1.5%) |
| vocal cords | 1 (1.5%) |
| lung | 3 (4.5%) |
| breasts | (5, 7.6%) |
| sellar region | (4, 6.1%) |
| Location | Imaging Modality | Key Features | Malignant Indicators |
|---|---|---|---|
| Soft Tissue | Ultrasound (n = 26) | Well-circumscribed hypoechoic masses (n = 25, 96.1%); sparse blood flow. | Larger size (mean: 93 mm); rare irregular margins. |
| CT (n = 9) | Well-defined hypodense nodules (n = 9, 100%); moderate enhancement | N/A | |
| MRI (n = 4) | T1-hypointense (n = 3, 75%) or T1-isointense (n = 1, 25%); variable T2-signals (hyper-/mixed). | N/A (all benign) | |
| Digestive System | Endoscopy/EUS (n = 15) | Submucosal/muscularis hypoechoic nodules; smooth surface (n = 15, 100%). | N/A (all benign) |
| Respiratory System | CT (n = 5) | Round/oval hypodense masses; lobulated contours; mild–moderate enhancement | A larger size (mean: 50 mm); no pathognomonic signs. |
| Breasts | Ultrasound (n = 5) | Irregular margins; a non-parallel orientation; posterior shadowing (BI-RADS 4B) (n = 4, 80%) | N/A (all benign) |
| Sellar Region | MRI (n = 4) | T1-isointense; T2-variable (“star-like crack signs” in 50%); heterogeneous enhancement in 50% | N/A (all benign) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, H.; Yu, L.; Wu, Y. Imaging Features and Clinical Characteristics of Granular Cell Tumors: A Single-Center Investigation. Diagnostics 2025, 15, 1336. https://doi.org/10.3390/diagnostics15111336
Gu H, Yu L, Wu Y. Imaging Features and Clinical Characteristics of Granular Cell Tumors: A Single-Center Investigation. Diagnostics. 2025; 15(11):1336. https://doi.org/10.3390/diagnostics15111336
Chicago/Turabian StyleGu, Hui, Lan Yu, and Yu Wu. 2025. "Imaging Features and Clinical Characteristics of Granular Cell Tumors: A Single-Center Investigation" Diagnostics 15, no. 11: 1336. https://doi.org/10.3390/diagnostics15111336
APA StyleGu, H., Yu, L., & Wu, Y. (2025). Imaging Features and Clinical Characteristics of Granular Cell Tumors: A Single-Center Investigation. Diagnostics, 15(11), 1336. https://doi.org/10.3390/diagnostics15111336





