Validation of Takotsubo Syndrome Scoring System
Abstract
1. Introduction
2. Methods
2.1. Diagnoses
2.2. TS Scoring System
2.3. Study Groups
2.4. Data Collection
2.5. Ethics
2.6. Statistics
3. Results
3.1. Patients’ Characteristics
3.2. Clinical Characteristics
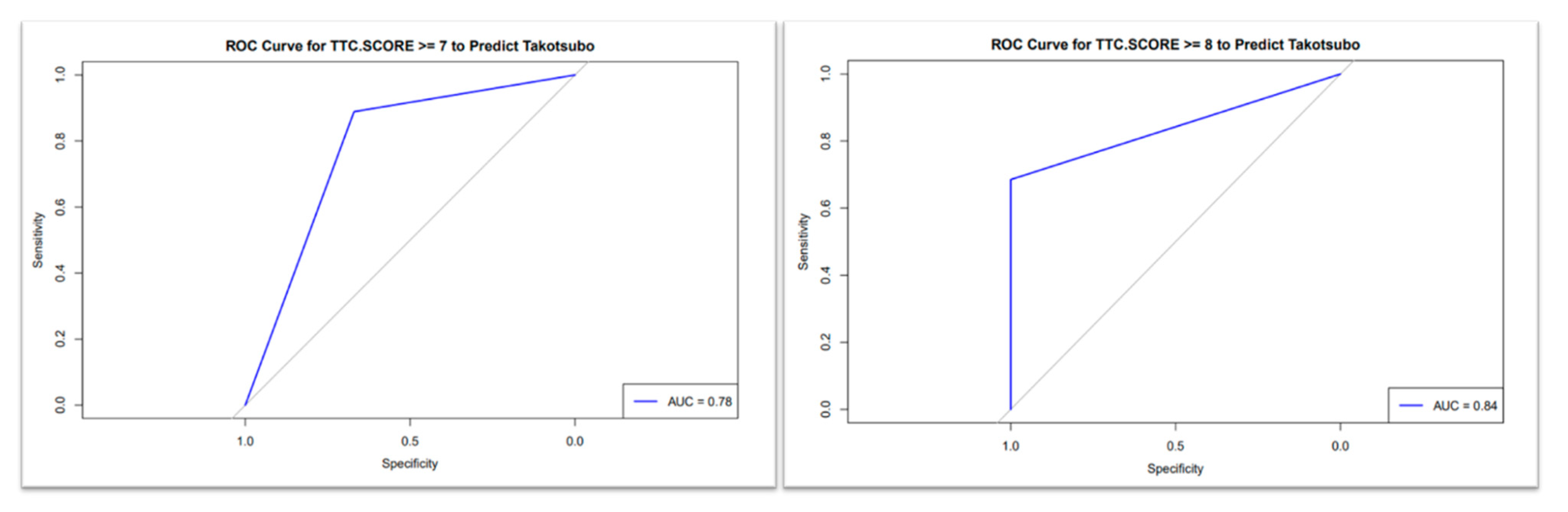
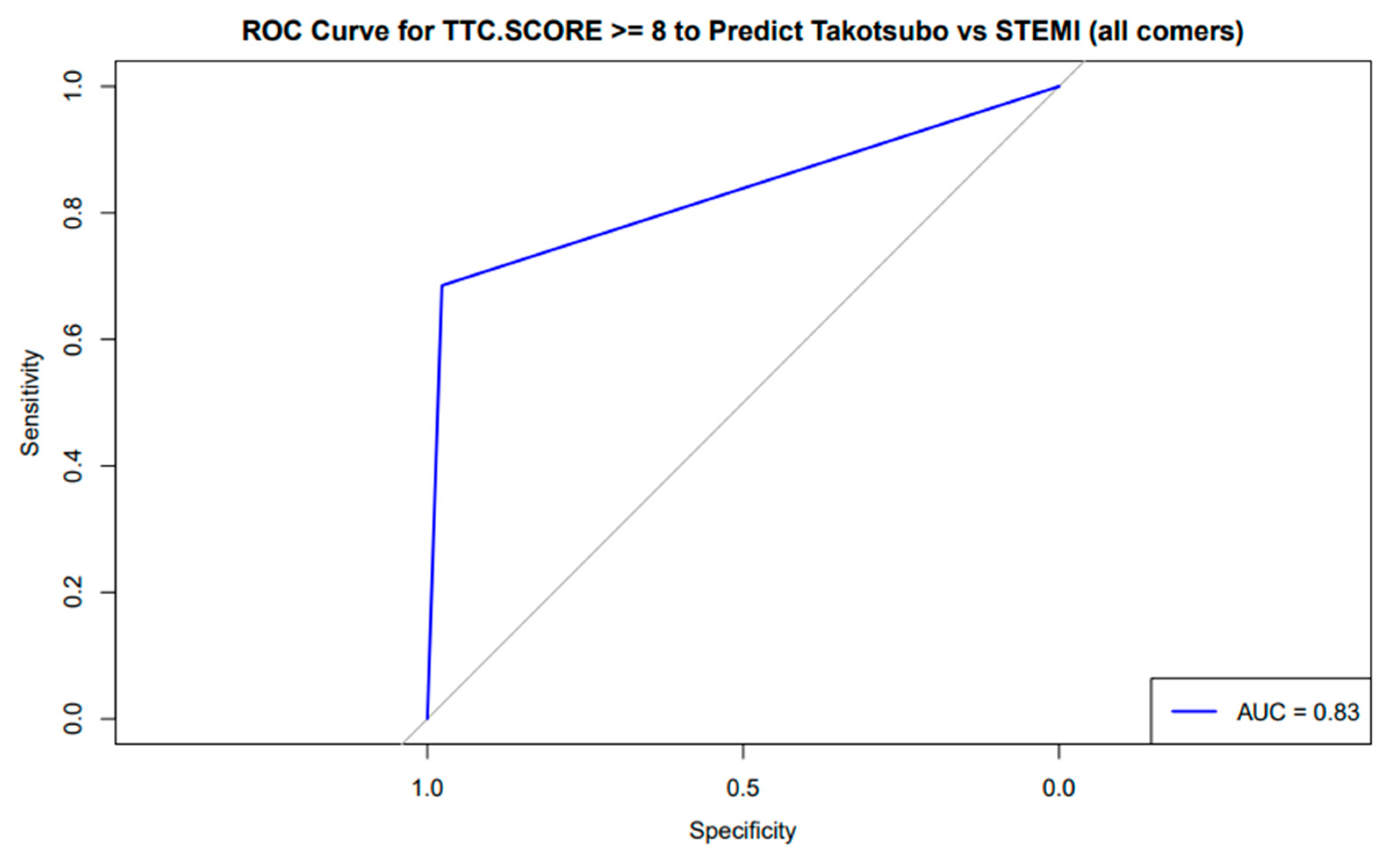
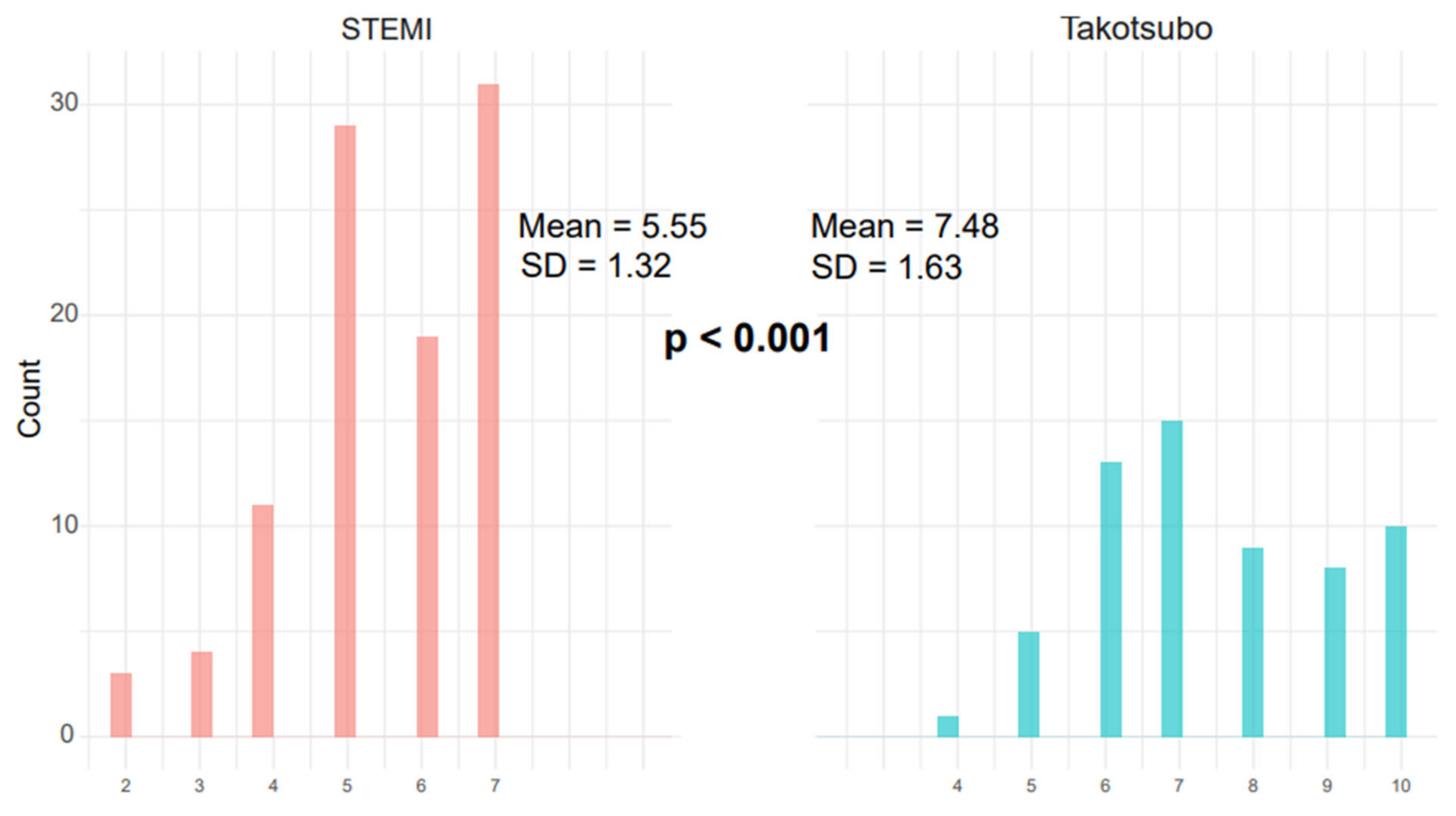
3.3. TS Scoring System
3.4. Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dias, A.; Franco, E.; Mercedes, A.; Hebert, K.; Messina, D.; Quevedo, H.C. Clinical features of takotsubo cardiomyopathy—A single-center experience. Cardiology 2013, 126, 126–130. [Google Scholar] [CrossRef]
- Matta, A.; Delmas, C.; Campelo-Parada, F.; Lhermusier, T.; Bouisset, F.; Elbaz, M.; Nader, V.; Blanco, S.; Roncalli, J.; Carrié, D. Takotsubo cardiomyopathy. Rev. Cardiovasc. Med. 2022, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Sattar, Y.; Siew, K.S.W.; Connerney, M.; Ullah, W.; Alraies, C. Management of Takotsubo Syndrome: A Comprehensive Review. Cureus 2020, 12, e6556. [Google Scholar] [CrossRef] [PubMed]
- Assad, J.; Femia, G.; Pender, P.; Badie, T.; Rajaratnam, R. Takotsubo Syndrome: A Review of Presentation, Diagnosis and Management. Clin. Med. Insights Cardiol. 2022, 16, 11795468211065782. [Google Scholar] [CrossRef]
- Murakami, T.; Komiyama, T.; Kobayashi, H.; Ikari, Y. Gender Differences in Takotsubo Syndrome. Biology 2022, 11, 653. [Google Scholar] [CrossRef]
- Von Mackensen, J.K.R.; Zwaans, V.I.T.; El Shazly, A.; Van Praet, K.M.; Heck, R.; Starck, C.T.; Schoenrath, F.; Potapov, E.V.; Kempfert, J.; Jacobs, S.; et al. Mechanical Circulatory Support Strategies in Takotsubo Syndrome with Cardiogenic Shock: A Systematic Review. J. Clin. Med. 2024, 13, 473. [Google Scholar] [CrossRef]
- Escobar, J.A.P.; Aung, M.; Amin, S.; Gulraiz, A.; Gandhi, F.R.; Malik, B.H. Pathogenesis of Ventricular Arrhythmias and Its Effect on Long-Term Prognosis in Patients with Takotsubo Cardiomyopathy. Cureus 2020, 12, e11171. [Google Scholar] [CrossRef]
- Y-Hassan, S.; Holmin, S.; Abdula, G.; Böhm, F. Thrombo-embolic complications in takotsubo syndrome: Review and demonstration of an illustrative case. Clin. Cardiol. 2019, 42, 312–319. [Google Scholar] [CrossRef]
- Lüscher, T.F.; Templin, C. Is takotsubo syndrome a microvascular acute coronary syndrome? Towards of a new definition. Eur. Heart J. 2016, 37, 2816–2820. [Google Scholar] [CrossRef]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.C.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champio, H.C. Neurohumoral Features of Myocardial Stunning Due to Sudden Emotional Stress. N. Engl. J. Med. 2005, 352, 539–548. Available online: www.nejm.org (accessed on 10 February 2005). [CrossRef]
- Matta, A.G.; Carrié, D. Epidemiology, Pathophysiology, Diagnosis, and Principles of Management of Takotsubo Cardiomyopathy: A Review. Med. Sci. Monit. 2023, 29, e939020. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, Y. The Electrocardiogram in ST Elevation Acute Myocardial Infarction: Correlation with Coronary Anatomy and Prognosis. 2003. Available online: https://academic.oup.com/pmj/article/79/935/490/7045807 (accessed on 14 August 2002).
- Sharkey, S.W.; Lesser, J.R.; Menon, M.; Parpart, M.; Maron, M.S.; Maron, B.J. Spectrum and significance of electrocardiographic patterns, troponin levels, and thrombolysis in myocardial infarction frame count in patients with stress (tako-tsubo) cardiomyopathy and comparison to those in patients with ST-elevation anterior wall myocardial infarction. Am. J. Cardiol. 2008, 101, 1723–1728. [Google Scholar]
- Frangieh, A.H.; Obeid, S.; Ghadri, J.-R.; Imori, Y.; D’Ascenzo, F.; Kovac, M.; Ruschitzka, F.; Lüscher, T.F.; Duru, F.; Templin, C.; et al. ECG criteria to differentiate between Takotsubo (stress) cardiomyopathy and myocardial infarction. J. Am. Heart Assoc. 2016, 5, e003418. [Google Scholar] [CrossRef]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Cammann, V.L.; Jurisic, S.; Seifert, B.; Napp, L.C.; Diekmann, J.; Bataiosu, D.R.; D’Ascenzo, F.; Ding, K.J.; Sarcon, A.; et al. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: Results from the International Takotsubo Registry. Eur. J. Heart Fail. 2017, 19, 1036–1042. [Google Scholar] [CrossRef]
- Looi, J.L.; Poppe, K.; Lee, M.; Gilmore, J.; Webster, M.; To, A.; Kerr, A.J. A Score to differentiate Takotsubo syndrome from non-ST-elevation myocardial nfarction in women at the bedside. Open Heart 2020, 7, e001197. [Google Scholar] [CrossRef]
- Isaak, A.; Bratz, J.; Kravchenko, D.; Mesropyan, N.; Eckardt, I.; Bischoff, L.M.; Weinhold, L.; Kuetting, D.; Pieper, C.C.; Attenberger, U.; et al. A novel and simple cardiac magnetic resonance score (PE2RT) predicts outcome in takotsubo syndrome. Eur. Radiol. 2023, 33, 5498–5508. [Google Scholar] [CrossRef]
- Agrawal, A.; Bhagat, U.; Yesilyaprak, A.; Bayat, A.; Sawhney, A.; Arockiam, A.D.; Haroun, E.; Faulx, M.; Desai, M.Y.; Jabe, W.; et al. Contemporary characteristics, outcomes and novel risk score for Takotsubo cardiomyopathy: A national inpatient sample analysis. Open Heart 2024, 11, e002922. [Google Scholar] [CrossRef]
- Santoro, F.; Gil, I.J.N.; Stiermaier, T.; El-Battrawy, I.; Guerra, F.; Novo, G.; Guastafierro, F.; Tarantino, N.; Novo, S.; Mariano, E.; et al. Assessment of the German and Italian Stress Cardiomyopathy Score for Risk Stratification for In-hospital Complications in Patients with Takotsubo Syndrome. JAMA Cardiol. 2019, 4, 892–899. [Google Scholar] [CrossRef]
- Asher, E.; Odeh, Q.; Sabbag, A.; Goldkorn, R.; Elian, D.; Ben Zekry, S.; Peled, Y.; Abu-Much, A.; Mazin, I.; Beigel, R.; et al. Differentiating Takotsubo cardiomyopathy from ST-segment elevation myocardial infarction. Hong Kong J. Emerg. Med. 2019, 26, 203–208. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; Bax, J.J. Helmut Baumgartner (Germany). Eur. Heart J. 2012, 126, 2020–2035. [Google Scholar] [CrossRef]
- Solberg, O.G.; Aaberge, L.; Bosse, G.; Ueland, T.; Gullestad, L.; Aukrust, P.; Stavem, K. Microvascular function and inflammatory activation in Takotsubo cardiomyopathy. ESC Heart Fail. 2023, 10, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Citro, R.; Schneider, B.; Morel, O.; Ghadri, J.R.; Templin, C.; Omerovic, E. Pathophysiology of Takotsubo Syndrome: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 902–921. [Google Scholar] [CrossRef]
- Jaguszewski, M.; Osipova, J.; Ghadri, J.R.; Napp, L.C.; Widera, C.; Franke, J.; Fijalkowski, M.; Nowak, R.; Fijalkowska, M.; Volkmann, I. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur. Heart J. 2014, 35, 999–1006. [Google Scholar] [CrossRef]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef]
- Salamanca, J.; García-Guimaraes, M.; Sabaté, M.; Sanz-Ruiz, R.; Macaya, F.; Roura, G.; Jimenez-Kockar, M.; Nogales, J.M.; Tizón-Marcos, H.; Velazquez, M.; et al. Non-atherosclerotic acute cardiac syndromes: Spontaneous coronary artery dissection and Takotsubo syndrome. Comparison of long-term clinical outcomes. Coron. Artery Dis. 2024, 35, 50–58. [Google Scholar] [CrossRef]
- Faucher, L.; Matsushita, K.; Kikuchi, S.; Tatarcheh, T.; Marchandot, B.; Granier, A.; Amissi, S.; Trimaille, A.; Jesel, L.; Ohlmann, P.; et al. Mortality risk stratification for Takotsubo syndrome: Evaluating CRP measurement alongside the InterTAK prognostic score. ESC Heart Fail. 2025, 12, 1427–1436. [Google Scholar] [CrossRef]
- Matsushita, K.; Lachmet-Thébaud, L.; Marchandot, B.; Trimaille, A.; Sato, C.; Dagrenat, C.; Greciano, S.; De Poli, F.; Leddet, P.; Peillex, M.; et al. Incomplete Recovery from Takotsubo Syndrome Is a Major Determinant of Cardiovascular Mortality. Circ. J. 2021, 85, 1823–1831. [Google Scholar] [CrossRef]
- Citro, R.; Okura, H.; Ghadri, J.R.; Izumi, C.; Meimoun, P.; Izumo, M.; Dawson, D.; Kaji, S.; Eitel, I.; Kagiyama, N.; et al. Multimodality imaging in takotsubo syndrome: A joint consensus document of the European Association of Cardiovascular Imaging (EACVI) and the Japanese Society of Echocardiography (JSE). Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1184–1207. [Google Scholar] [CrossRef]
- Kanaji, Y.; Ozcan, I.; Tryon, D.N.; Ahmad, A.; Sara, J.D.S.; Lewis, B.; Friedman, P.; Noseworthy, P.A.; Lerman, L.O.; Kakuta, T.; et al. Predictive Value of Artificial Intelligence-Enabled Electrocardiography in Patients with Takotsubo Cardiomyopathy. J. Am. Heart Assoc. 2024, 13, e031859. [Google Scholar] [CrossRef]
- Laumer, F.; Di Vece, D.; Cammann, V.L.; Würdinger, M.; Petkova, V.; Schönberger, M.; Schönberger, A.; Mercier, J.C.; Niederseer, D.; Seifert, B.; et al. Assessment of Artificial Intelligence in Echocardiography Diagnostics in Differentiating Takotsubo Syndrome from Myocardial Infarction. JAMA Cardiol. 2022, 7, 494–503. [Google Scholar] [CrossRef]

| Female Anterior STEMI (N = 97) | Takotsubo Syndrome (N = 54) | All-Comer STEMIs (N = 999) | Overall (N = 1150) | p-Value | |
|---|---|---|---|---|---|
| Age | |||||
| Median (Q1–Q3) | 71.0 (64.0–83.0) | 68.0 (61.3–78.8) | 72.0 | 70.0 (38–106) | 0.041 |
| Mean (SD) | 71.2 (12.9) | 69.0 (12.1) | 72.55 (11.36) | 70.97 (12.21) | |
| Gender | |||||
| F | 97 (100%) | 53 (98.1%) | 171 (17.1%) | 321 (27.91%) | 0.001 |
| M | 0 (0%) | 1 (1.9%) | 828 (82.9%) | 829 (72.0%) | |
| Height | |||||
| Median (Q1–Q3) | 1.60 (1.57–1.64) | 1.60 (1.55–1.65) | 1.7 (1.37–1.9) | 1.69 | 0.001 |
| Mean (SD) | 1.61 (0.0595) | 1.60 (0.0685) | 1.7 (0.09) | 1.68 (0.011) | |
| Missing | 2 (2.1%) | 0 (0%) | 4 (0.4%) | 6 (0.52%) | |
| Weight | |||||
| Median (Q1–Q3) | 68.0 (60.0–77.5) | 70.0 (56.3–80.0) | 80 (45–160) | 79.0 | 0.001 |
| Mean (SD) | 70.0 (13.6) | 67.9 (15.7) | 80.08 (18.02) | 78.3 (17.6) | |
| Missing | 2 (2.1%) | 0 (0%) | 4 (0.4%) | 6 (0.52%) | |
| BMI | |||||
| Median (Q1–Q3) | 26.4 (24.1–29.8) | 26.7 (22.2–31.1) | 26.67 (14.86–51.65) | 26.7 | 0.012 |
| Mean (SD) | 27.1 (4.54) | 26.4 (6.09) | 27.63 (5.31) | 27.4 (5.3) | |
| Missing | 2 (2.1%) | 0 (0%) | 4 (0.4%) | 6 (0.5%) | |
| Ethnicity | |||||
| 1 | 83 (85.6%) | 43 (79.6%) | 888 (88.9%) | 1014 (88.2%) | 0.001 |
| 2 | 12 (12.4%) | 10 (18.5%) | 105 (10.51%) | 127 (11.0%) | |
| 3 | 2 (2.1%) | 1 (1.9%) | 6 (0.6%) | 9 (0.78%) | |
| Smoking | |||||
| no | 83 (85.6%) | 48 (88.9%) | 463 (46.3%) | 594 (51.65%) | 0.001 |
| yes | 14 (14.4%) | 6 (11.1%) | 536 (53.7%) | 556 (48.34%) | |
| Stressful event | |||||
| no | 95 (97.9%) | 17 (31.5%) | 916 (91.7%) | 1028 (89.39%) | <0.001 |
| yes | 2 (2.1%) | 37 (68.5%) | 83 (8.3%) | 122 (10.61%) | |
| DM | |||||
| no | 55 (56.7%) | 43 (79.6%) | 335 (33.5%) | 433 (37.65%) | 0.00001 |
| yes | 42 (43.3%) | 11 (20.4%) | 664 (66.5%) | 717 (62.35%) | |
| LVEF < 40% | |||||
| no | 31 (32.0%) | 14 (25.9%) | 648 (64.9%) | 693 (60.26%) | 0.001 |
| yes | 66 (68.0%) | 40 (74.1%) | 350 (35.0%) | 456 (39.65%) | |
| Positive troponin on admission | |||||
| no | 3 (3.1%) | 0 (0%) | 66 (6.6%) | 69 (6.0%) | 0.023 |
| yes | 94 (96.9%) | 54 (100%) | 933 (93.4%) | 1081 (94.0%) | |
| TS SCORE | |||||
| Median (Q1–Q3) | 6.00 (5.00–7.00) | 8.00 (7.00–9.75) | 3.83 | 4.00 | <0.001 |
| Mean (SD) | 5.7 (1.16) | 8.3 (1.39) | 3.83 (1.53) | 3.92 (1.73) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deeb, D.; Loutati, R.; Taha, L.; Karmi, M.; Brin, A.; Rabi, O.; Levi, N.; Fink, N.; Sabouret, P.; Manassra, M.; et al. Validation of Takotsubo Syndrome Scoring System. Diagnostics 2025, 15, 1314. https://doi.org/10.3390/diagnostics15111314
Deeb D, Loutati R, Taha L, Karmi M, Brin A, Rabi O, Levi N, Fink N, Sabouret P, Manassra M, et al. Validation of Takotsubo Syndrome Scoring System. Diagnostics. 2025; 15(11):1314. https://doi.org/10.3390/diagnostics15111314
Chicago/Turabian StyleDeeb, Dana, Ranel Loutati, Louay Taha, Mohammad Karmi, Akiva Brin, Ofir Rabi, Nir Levi, Noam Fink, Pierre Sabouret, Mohammed Manassra, and et al. 2025. "Validation of Takotsubo Syndrome Scoring System" Diagnostics 15, no. 11: 1314. https://doi.org/10.3390/diagnostics15111314
APA StyleDeeb, D., Loutati, R., Taha, L., Karmi, M., Brin, A., Rabi, O., Levi, N., Fink, N., Sabouret, P., Manassra, M., Qadan, A., Amro, M., Khalev, B., Glikson, M., & Asher, E. (2025). Validation of Takotsubo Syndrome Scoring System. Diagnostics, 15(11), 1314. https://doi.org/10.3390/diagnostics15111314








