Intraepidermal Nerve Fiber Density as an Indicator of Neuropathy Predisposition: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Statistical Analysis
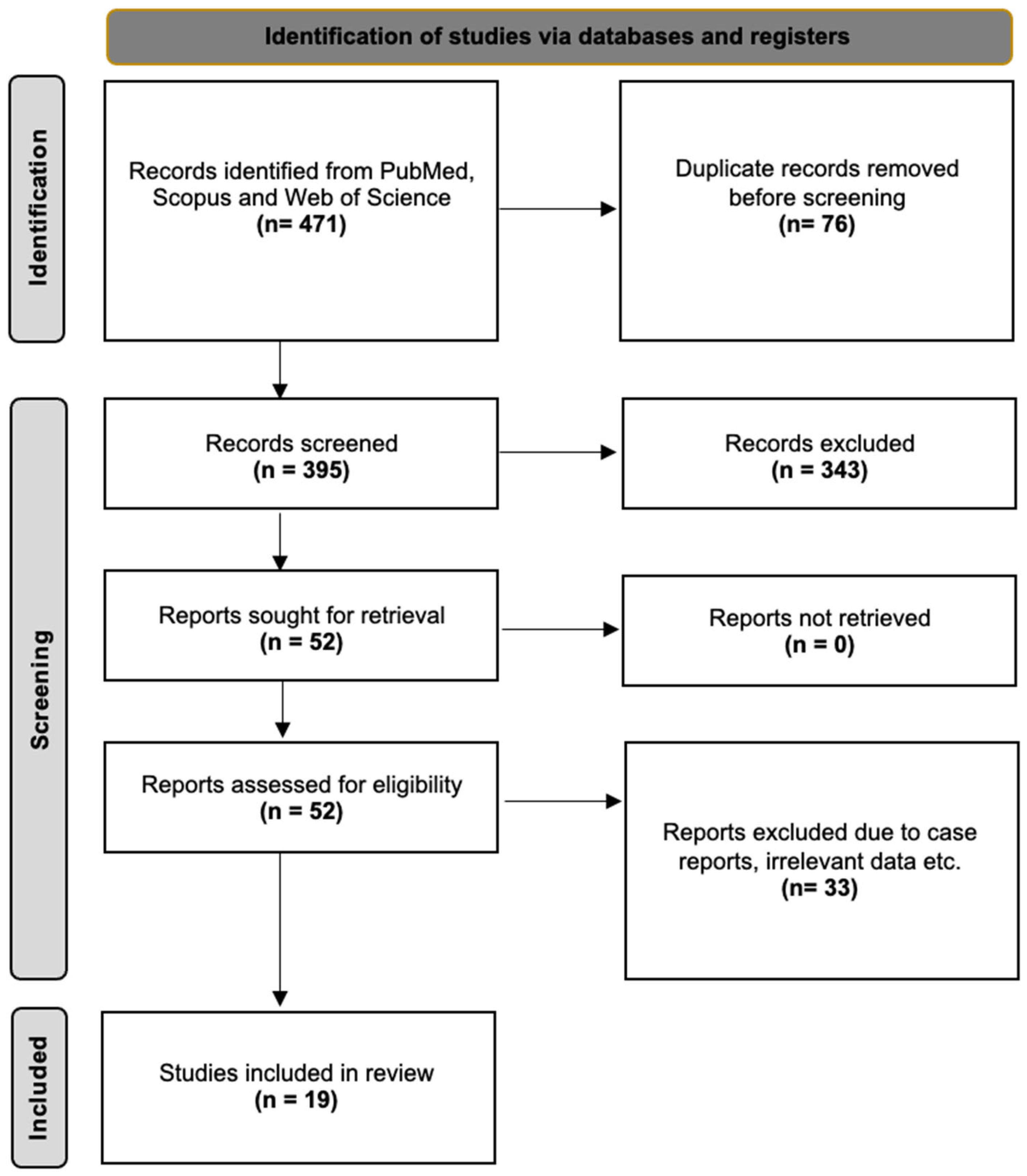
2.4. Study Identification
3. Results
3.1. Study Population
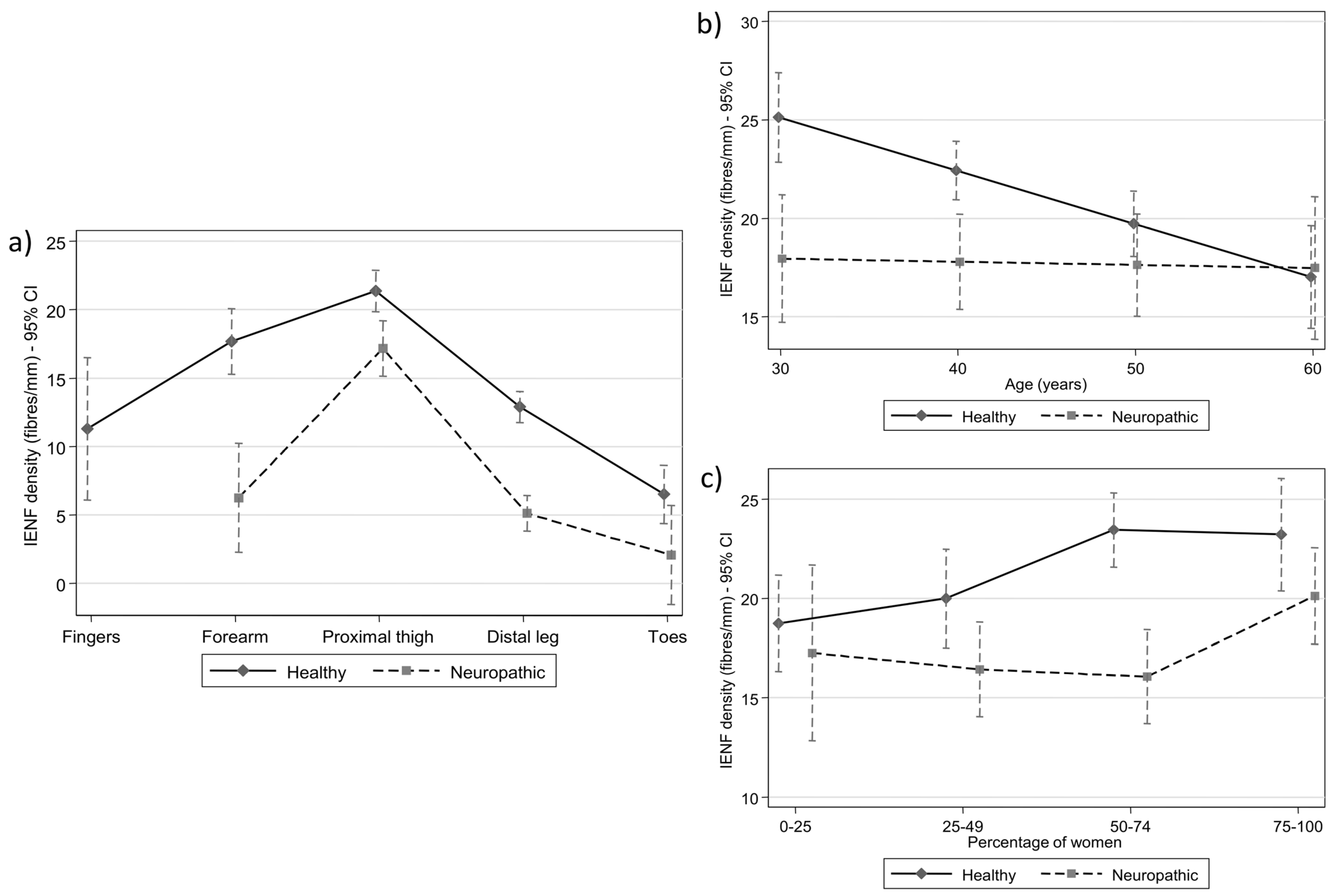
3.2. Multilevel Meta-Regression Results: Anatomical Region
3.3. Multilevel Meta-Regression Results: Age
3.4. Multilevel Meta-Regression Results: Gender
4. Discussion
4.1. IENFD Distribution Among Studies and Anatomical Regions from Proximal to Distal
4.2. IENFD Values and Gender Impact
4.3. IENFD Values and Age Impact
4.4. IENFD Values and Neuropathy Impact
4.5. IENFD Values Variability and Heterogeneity—Contributing Factors and Limitations
4.6. IENFD Decrease Values and the Relationship Among Healthy Status and Neuropathy Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauria, G.; Lombardi, R. Small Fiber Neuropathy: Is Skin Biopsy the Holy Grail? Curr. Diabetes Rep. 2012, 12, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Nolano, M.; Tozza, S.; Caporaso, G.; Provitera, V. Contribution of Skin Biopsy in Peripheral Neuropathies. Brain Sci. 2020, 10, 989. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Morbin, M.; Lombardi, R.; Borgna, M.; Mazzoleni, G.; Sghirlanzoni, A.; Pareyson, D. Axonal Swellings Predict the Degeneration of Epidermal Nerve Fibers in Painful Neuropathies. Neurology 2003, 61, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Piscosquito, G.; Provitera, V.; Mozzillo, S.; Caporaso, G.; Borreca, I.; Stancanelli, A.; Manganelli, F.; Santoro, L.; Nolano, M. The Analysis of Epidermal Nerve Fibre Spatial Distribution Improves the Diagnostic Yield of Skin Biopsy. Neuropathol. Appl. Neurobiol. 2021, 47, 210–217. [Google Scholar] [CrossRef]
- Provitera, V.; Gibbons, C.H.; Wendelschafer-Crabb, G.; Donadio, V.; Vitale, D.F.; Loavenbruck, A.; Stancanelli, A.; Caporaso, G.; Liguori, R.; Wang, N.; et al. The Role of Skin Biopsy in Differentiating Small-fiber Neuropathy from Ganglionopathy. Eur. J. Neurol. 2018, 25, 848–853. [Google Scholar] [CrossRef]
- Wang, L.; Hilliges, M.; Jernberg, T.; Wiegleb-Edström, D.; Johansson, O. Protein Gene Product 9.5-Immunoreactive Nerve Fibres and Cells in Human Skin. Cell Tissue Res. 1990, 261, 25–33. [Google Scholar] [CrossRef]
- Lacomis, D. Small-fiber Neuropathy. Muscle Nerve 2002, 26, 173–188. [Google Scholar] [CrossRef]
- Kim, D.H.; Na, J.E.; Lee, S.J.; Sun, W.; Ahn, H.H.; Kim, B.; Rhyu, I.J. Quantification of Intraepidermal Nerve Fiber Density Using Three-dimensional Microscopy. Microsc. Res. Tech. 2019, 82, 47–52. [Google Scholar] [CrossRef]
- Provitera, V.; Nolano, M.; Stancanelli, A.; Caporaso, G.; Vitale, D.F.; Santoro, L. Intraepidermal Nerve Fiber Analysis Using Immunofluorescence with and without Confocal Microscopy. Muscle Nerve 2015, 51, 501–504. [Google Scholar] [CrossRef]
- Kennedy, W.R.; Wendelschafer-Crabb, G. The Innervation of Human Epidermis. J. Neurol. Sci. 1993, 115, 184–190. [Google Scholar] [CrossRef]
- McCarthy, B.G.; Hsieh, S.-T.; Stocks, A.; Hauer, P.; Macko, C.; Cornblath, D.R.; Griffin, J.W.; McArthur, J.C. Cutaneous Innervation in Sensory Neuropathies. Neurology 1995, 45, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, W.R.; Wendelschafer-Crabb, G.; Johnson, T. Quantitation of Epidermal Nerves in Diabetic Neuropathy. Neurology 1996, 47, 1042–1048. [Google Scholar] [CrossRef]
- Holland, N.R.; Stocks, A.; Hauer, P.; Cornblath, D.R.; Griffin, J.W.; McArthur, J.C. Intraepidermal Nerve Fiber Density in Patients with Painful Sensory Neuropathy. Neurology 1997, 48, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.R.; Crawford, T.O.; Hauer, P.; Cornblath, D.R.; Griffin, J.W.; McArthur, J.C. Small-fiber Sensory Neuropathies: Clinical Course and Neuropathology of Idiopathic Cases. Ann. Neurol. 1998, 44, 47–59. [Google Scholar] [CrossRef]
- Lauria, G.; Holland, N.; Hauer, P.; Cornblath, D.R.; Griffin, J.W.; McArthur, J.C. Epidermal Innervation: Changes with Aging, Topographic Location, and in Sensory Neuropathy. J. Neurol. Sci. 1999, 164, 172–178. [Google Scholar] [CrossRef]
- Periquet, M.I.; Novak, V.; Collins, M.P.; Nagaraja, H.N.; Erdem, S.; Nash, S.M.; Freimer, M.L.; Sahenk, Z.; Kissel, J.T.; Mendell, J.R. Painful Sensory Neuropathy. Neurology 1999, 53, 1641–1647. [Google Scholar] [CrossRef]
- Scott, L.J.C.; Griffin, J.W.; Luciano, C.; Barton, N.W.; Banerjee, T.; Crawford, T.; McArthur, J.C.; Tournay, A.; Schiffmann, R. Quantitative Analysis of Epidermal Innervation in Fabry Disease. Neurology 1999, 52, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Nolano, M.; Provitera, V.; Crisci, C.; Saltalamacchia, A.M.; Wendelschafer-Crabb, G.; Kennedy, W.R.; Filla, A.; Santoro, L.; Caruso, G. Small Fibers Involvement in Friedreich’s Ataxia. Ann. Neurol. 2001, 50, 17–25. [Google Scholar] [CrossRef]
- Pan, C.-L.; Lin, Y.-H.; Lin, W.-M.; Tai, T.-Y.; Hsieh, S.-T. Degeneration of Nociceptive Nerve Terminals in Human Peripheral Neuropathy. Neuroreport 2001, 12, 787–792. [Google Scholar] [CrossRef]
- Smith, A.G.; Ramachandran, P.; Tripp, S.; Singleton, J.R. Epidermal Nerve Innervation in Impaired Glucose Tolerance and Diabetes-Associated Neuropathy. Neurology 2001, 57, 1701–1704. [Google Scholar] [CrossRef]
- Lauria, G.; Sghirlanzoni, A.; Lombardi, R.; Pareyson, D. Epidermal Nerve Fiber Density in Sensory Ganglionopathies: Clinical and Neurophysiologic Correlations. Muscle Nerve 2001, 24, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-F.; Tseng, T.-J.; Lin, W.-M.; Yang, C.-C.; Chang, Y.-C.; Chen, R.-C.; Hsieh, S.-T. Quantitative Pathology of Cutaneous Nerve Terminal Degeneration in the Human Skin. Acta Neuropathol. 2001, 102, 455–461. [Google Scholar] [CrossRef]
- Omdal, R.; Mellgren, S.I.; Gøransson, L.; Skjesol, A.; Lindal, S.; Koldingsnes, W.; Husby, G. Small Nerve Fiber Involvement in Systemic Lupus Erythematosus: A Controlled Study. Arthritis Rheum. 2002, 46, 1228–1232. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lin, W.-M.; Hsieh, S.-T. Effects of Aging on Human Skin Innervation. Neuroreport 2004, 15, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Hoitsma, E.; Marziniak, M.; Faber, C.; Reulen, J.; Sommer, C.; De Baets, M.; Drent, M. Small Fibre Neuropathy in Sarcoidosis. Lancet 2002, 359, 2085–2086. [Google Scholar] [CrossRef]
- Polydefkis, M.; Yiannoutsos, C.T.; Cohen, B.A.; Hollander, H.; Schifitto, G.; Clifford, D.B.; Simpson, D.M.; Katzenstein, D.; Shriver, S.; Hauer, P.; et al. Reduced Intraepidermal Nerve Fiber Density in HIV-Associated Sensory Neuropathy. Neurology 2002, 58, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, D.N.; McDermott, M.P.; Henderson, D.; Chen, L.; Akowuah, K.; Schifitto, G. Epidermal Nerve Fiber Density, Axonal Swellings and QST as Predictors of HIV Distal Sensory Neuropathy. Muscle Nerve 2004, 29, 420–427. [Google Scholar] [CrossRef]
- Shun, C.; Chang, Y.; Wu, H.; Hsieh, S.; Lin, W.; Lin, Y.; Tai, T.; Hsieh, S. Skin Denervation in Type 2 Diabetes: Correlations with Diabetic Duration and Functional Impairments. Brain 2004, 127, 1593–1605. [Google Scholar] [CrossRef]
- Polydefkis, M.; Hauer, P.; Sheth, S.; Sirdofsky, M.; Griffin, J.W.; McArthur, J.C. The Time Course of Epidermal Nerve Fibre Regeneration: Studies in Normal Controls and in People with Diabetes, with and without Neuropathy. Brain 2004, 127, 1606–1615. [Google Scholar] [CrossRef]
- Umapathi, T.; Tan, W.L.; Loke, S.C.; Soon, P.C.; Tavintharan, S.; Chan, Y.H. Intraepidermal Nerve Fiber Density as a Marker of Early Diabetic Neuropathy. Muscle Nerve 2007, 35, 591–598. [Google Scholar] [CrossRef]
- Nolano, M.; Manganelli, F.; Provitera, V.; Pisciotta, C.; Stancanelli, A.; Caporaso, G.; Iodice, R.; Shy, M.E.; Santoro, L. Small Nerve Fiber Involvement in CMT1A. Neurology 2015, 84, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Løseth, S.; Lindal, S.; Stålberg, E.; Mellgren, S.I. Intraepidermal Nerve Fibre Density, Quantitative Sensory Testing and Nerve Conduction Studies in a Patient Material with Symptoms and Signs of Sensory Polyneuropathy. Eur. J. Neurol. 2006, 13, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, M.; Merkies, I.S.J.; Lauria, G.; Devigili, G.; Penza, P.; Lombardi, R.; Hermans, M.C.E.; van Nes, S.I.; De Baets, M.; Faber, C.G. Intraepidermal Nerve Fiber Density and Its Application in Sarcoidosis. Neurology 2009, 73, 1142–1148. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- McArthur, J.C.; Stocks, E.A.; Hauer, P.; Cornblath, D.R.; Griffin, J.W. Epidermal Nerve Fiber Density. Arch. Neurol. 1998, 55, 1513. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-C.; Lin, Y.-H.; Pan, C.-L.; Tseng, T.-J.; Lin, W.-M.; Hsieh, S.-T. Cutaneous Innervation in Chronic Inflammatory Demyelinating Polyneuropathy. Neurology 2002, 59, 1094–1098. [Google Scholar] [CrossRef]
- Nolano, M.; Provitera, V.; Crisci, C.; Stancanelli, A.; Wendelschafer-Crabb, G.; Kennedy, W.R.; Santoro, L. Quantification of Myelinated Endings and Mechanoreceptors in Human Digital Skin. Ann. Neurol. 2003, 54, 197–205. [Google Scholar] [CrossRef]
- Gøransson, L.G.; Mellgren, S.I.; Lindal, S.; Omdal, R. The Effect of Age and Gender on Epidermal Nerve Fiber Density. Neurology 2004, 62, 774–777. [Google Scholar] [CrossRef]
- Umapathi, T.; Tan, W.L.; Tan, N.C.K.; Chan, Y.H. Determinants of Epidermal Nerve Fiber Density in Normal Individuals. Muscle Nerve 2006, 33, 742–746. [Google Scholar] [CrossRef]
- Lauria, G.; Bakkers, M.; Schmitz, C.; Lombardi, R.; Penza, P.; Devigili, G.; Smith, A.G.; Hsieh, S.; Mellgren, S.I.; Umapathi, T.; et al. Intraepidermal Nerve Fiber Density at the Distal Leg: A Worldwide Normative Reference Study. J. Peripher. Nerv. Syst. 2010, 15, 202–207. [Google Scholar] [CrossRef]
- Provitera, V.; Gibbons, C.H.; Wendelschafer-Crabb, G.; Donadio, V.; Vitale, D.F.; Stancanelli, A.; Caporaso, G.; Liguori, R.; Wang, N.; Santoro, L.; et al. A Multi-center, Multinational Age- and Gender-adjusted Normative Dataset for Immunofluorescent Intraepidermal Nerve Fiber Density at the Distal Leg. Eur. J. Neurol. 2016, 23, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Hsieh, S.T.; Johansson, O.; Kennedy, W.R.; Leger, J.M.; Mellgren, S.I.; Nolano, M.; Merkies, I.S.J.; Polydefkis, M.; Smith, A.G.; et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the Use of Skin Biopsy in the Diagnosis of Small Fiber Neuropathy. Report of a Joint Task Force of the European Fe-deration of Neurological Societies and the Peripheral Nerve Society. Eur. J. Neurol. 2010, 17, 903. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, G.J.; McArthur, J.C.; Thomas, D.; Murinson, B.; Hauer, P.; Polydefkis, M.; Griffin, J.W. Denervation of Skin in Neuropathies: The Sequence of Axonal and Schwann Cell Changes in Skin Biopsies. Brain 2007, 130, 2703–2714. [Google Scholar] [CrossRef]
- Niemann, A.; Ruegg, M.; La Padula, V.; Schenone, A.; Suter, U. Ganglioside-Induced Differentiation Associated Protein 1 Is a Regulator of the Mitochondrial Network. J. Cell Biol. 2005, 170, 1067–1078. [Google Scholar] [CrossRef] [PubMed]


| Studies by Authors [Citation] | Year | Status | Sample | Type of Neuropathy | Mean Age of H/N | H/N by Gender | |
|---|---|---|---|---|---|---|---|
| H/N | N | Total | Females (%) | Males (%) | |||
| McArthur et al. [35] | 1998 | 98/0 | 98 | - | 47.5/- | 32.7/- | 67.3/- |
| Lauria et al. [15] | 1999 | 10/6 | 16 | Sensory Fiber Neuropathy | 49.0/56.5 | 50.0/66.7 | 50.0/33.3 |
| Chien et al. [22] | 2001 | 55/35 | 90 | Cutaneous Nerve Terminal Degeneration | 45.9/47.3 | 65.5/51.4 | 34.5/48.6 |
| Lauria et al. [21] | 2001 | 15/16 | 31 | Sensory Ganglionopathies | NA/NA | NA/NA | NA/NA |
| Nolano et al. [18] | 2001 | 51/14 | 65 | Friedreich’s Ataxia | 31.7/29.4 | 52.9/71.4 | 47.1/28.6 |
| Pan et al. [19] | 2001 | 55/35 | 90 | Peripheral Neuropathy | 45.9/47.3 | 65.5/51.4 | 34.5/48.6 |
| Smith et al. [20] | 2001 | 5/8 | 13 | Impaired Glucose Tolerance, Diabetes Mellitus | NA/NA | NA/NA | NA/NA |
| Chiang et al. [36] | 2002 | 18/18 | 36 | Chronic Inflammatory Demyelinating Polyneuropathy | 45.1/45.1 | 33.3/33.3 | 66.7/66.7 |
| Hoitsma et al. [25] | 2002 | 6/7 | 13 | Sarcoidosis | 35.0/36.0 | 50.0/14.3 | 50.0/85.7 |
| Omdal et al. [23] | 2002 | 15/15 | 30 | Systemic Lupus Erythematosus | 47.3/47.3 | 93.3/93.3 | 6.7/6.7 |
| Lauria et al. [3] | 2003 | 15/15 | 30 | Axonal Swellings in Painful Neuropathies | NA/49.5 | 60.0/46.7 | 40.0/53.3 |
| Nolano et al. [37] | 2003 | 14/0 | 14 | - | 33.7/- | 57.1/- | 42.9/- |
| Goransson et al. * [38] | 2004 | 40/0 M | 106 | - | 57.5/- | 0.0/- | 100.0/- |
| Goransson et al. * [38] | 2004 | 66/0 F | - | 43.8/- | 100.0/- | 0/- | |
| Hermann et al. [27] | 2004 | 19/40 | 59 | Axonal Swellings and QST in HIV Distal Sensory Neuropathy | 34.4/42.0 | NA/25.0 | NA/75.0 |
| Polydefkis et al. [29] | 2004 | 31/8 | 39 | Diabetes Mellitus | 37.5/54.4 | 58.1/50.0 | 41.9/50.0 |
| Shun et al. [28] | 2004 | 38/38 | 76 | Diabetes Mellitus | 55.5/56.2 | 34.2/34.2 | 65.8/65.8 |
| Umapathi et al. [30] | 2007 | 0/29 | 29 | Early Diabetic Neuropathy | -/48.4 | -/24.1 | -/75.9 |
| Umapathi * [30] | 2006 | 45/0 M | 84 | - | 43.2/- | 0.0/- | 100.0/- |
| Umapathi * [30] | 2006 | 39/0 F | - | 35.1/- | 100.0/- | 0/- | |
| Bakkers * [33] | 2009 | 91/0 M | 188 | - | 48.4/- | 0.0/- | 100.0/- |
| Bakkers * [33] | 2009 | 97/0 F | - | 51.6/- | 100.0/- | 0/- | |
| Nolano et al. [31] | 2014 | 40/20 | 60 | Charcot-Marie-Tooth type 1A | 44.2/42.9 | 75.0/75.0 | 25.0/25.0 |
| All studies | 863/304 | 1167 | |||||
| IENFD (Fibers/mm) in Different Areas Among Studies | Population | Observations by Anatomical Area | ||
|---|---|---|---|---|
| Healthy (n = 36) | Neuropathic (n = 26) | Total (n = 62) | ||
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Mean n (Healthy/Neuropathic among studies) | ||||
| Forearm (3/2) | 17.4 (17.1, 20.3) | 5.7 (5.6, 5.8) | Significant loss: stable median and SD suggest diagnostic clarity | |
| Fingers (1/0) | 11.3 (11.3, 11.3) | - | Limited data. Used occasionally | |
| Thigh (9/8) | 22.8 (21.6, 24.4) | 17.7 (13.7, 18.9) | The least affected site in neuropathy | |
| Distal leg (21/14) | 13.2 (10.5, 14.5) | 5.0 (2.7, 8.0) | Common biopsy site; large diagnostic gap | |
| Toes (2/2) | 10.5 (7.9, 13.1) | 2.5 (1.8, 3.3) | Distal-most loss; very high variation | |
| Overall N (36/26) | 14.4 (11.9, 20.4) | 6.5 (3.3, 13.7) | 13.1 (7.9, 17.4) | |
| Standard Deviation (SD) | ||||
| Forearm | 6.5 (6.2, 7.4) | 5.7 (5.0, 6.5) | Significant loss: stable median and SD suggest diagnostic clarity | |
| Fingers | 2.9 (2.9, 2.9) | - | Limited data. Used occasionally | |
| Thigh | 5.6 (5.4, 6.9) | 6.1 (5.0, 7.5) | The least affected site in neuropathy | |
| Distal leg | 3.9 (3.1, 5.2) | 2.6 (2.1, 4.0) | Common biopsy site; large diagnostic gap | |
| Toes | 2.0 (1.5, 2.4) | 2.0 (1.8, 2.1) | Distal-most loss; very high variation | |
| Overall | 4.7 (3.1, 5.7) | 3.5 (2.2, 5.6) | 4.4 (2.7, 5.6) | |
| Coefficient of variation (CV) (%) | ||||
| Forearm | 36.5 (35.7, 38.1) | 99.8 (88.0, 111.7) | Significant loss: stable median and SD suggest diagnostic clarity | |
| Fingers | 25.7 (25.7, 25.7) | - | Limited data. Used occasionally | |
| Thigh | 23.8 (21.2, 30.3) | 40.8 (27.3, 46.1) | The least affected site in neuropathy | |
| Distal leg | 33.8 (28.9, 37.1) | 60.5 (44.8, 78.0) | Common biopsy site; large diagnostic gap | |
| Toes | 20.9 (11.5, 30.4) | 81.8 (63.6, 100.0) | Distal-most loss; very high variation | |
| Overall | 31.5 (23.5, 36.7) | 54.5 (40.9, 78.0) | 36.1 (25.7, 50.1) | |
| Healthy | Differences | Values | |||
|---|---|---|---|---|---|
| Measurement Point | Estimate | 95% CI | p Value | Estimate | 95% CI |
| Distal leg | 12.89 | (11.75, 14.03) | |||
| Forearm vs. Distal leg | 4.80 | (2.57, 7.03) | <0.001 | 17.69 | (15.30, 20.09) |
| Fingers vs. Distal leg | −1.59 | (−6.93, 3.74) | 0.558 | 11.30 | (6.09, 16.51) |
| Thigh vs. Distal leg | 8.49 | (7.25, 9.73) | <0.001 | 21.38 | (19.87, 22.90) |
| Toes vs. Distal leg | −6.39 | (−8.34, −4.45) | <0.001 | 6.50 | (4.37, 8.64) |
| Neuropathic | Differences | Values | |||
| Measurement Point | Estimate | 95% CI | p Value | Estimate | 95% CI |
| Distal leg | 5.11 | (3.81, 6.41) | |||
| Forearm vs. Distal leg | 1.14 | (−2.93, 5.22) | 0.582 | 6.26 | (2.28, 10.23) |
| Thigh vs. Distal leg | 12.06 | (9.75, 14.36) | <0.001 | 17.17 | (15.15, 19.18) |
| Toes vs. Distal leg | −3.04 | (−6.84, 0.76) | 0.117 | 2.07 | (0.00, 5.69) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samolis, A.; Troupis, T.; Politis, C.; Pantazis, N.; Triantafyllou, G.; Tsakotos, G.; Tegos, T.; Lazaridis, N.; Natsis, K.; Piagkou, M. Intraepidermal Nerve Fiber Density as an Indicator of Neuropathy Predisposition: A Systematic Review with Meta-Analysis. Diagnostics 2025, 15, 1311. https://doi.org/10.3390/diagnostics15111311
Samolis A, Troupis T, Politis C, Pantazis N, Triantafyllou G, Tsakotos G, Tegos T, Lazaridis N, Natsis K, Piagkou M. Intraepidermal Nerve Fiber Density as an Indicator of Neuropathy Predisposition: A Systematic Review with Meta-Analysis. Diagnostics. 2025; 15(11):1311. https://doi.org/10.3390/diagnostics15111311
Chicago/Turabian StyleSamolis, Alexandros, Theodore Troupis, Constantinus Politis, Nikos Pantazis, George Triantafyllou, George Tsakotos, Thomas Tegos, Nikolaos Lazaridis, Konstantinos Natsis, and Maria Piagkou. 2025. "Intraepidermal Nerve Fiber Density as an Indicator of Neuropathy Predisposition: A Systematic Review with Meta-Analysis" Diagnostics 15, no. 11: 1311. https://doi.org/10.3390/diagnostics15111311
APA StyleSamolis, A., Troupis, T., Politis, C., Pantazis, N., Triantafyllou, G., Tsakotos, G., Tegos, T., Lazaridis, N., Natsis, K., & Piagkou, M. (2025). Intraepidermal Nerve Fiber Density as an Indicator of Neuropathy Predisposition: A Systematic Review with Meta-Analysis. Diagnostics, 15(11), 1311. https://doi.org/10.3390/diagnostics15111311







