First-Trimester Morphological Evaluation of Fetuses and Medical Law Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Inclusion and Exclusion Criteria
2.2. Statistical Analysis
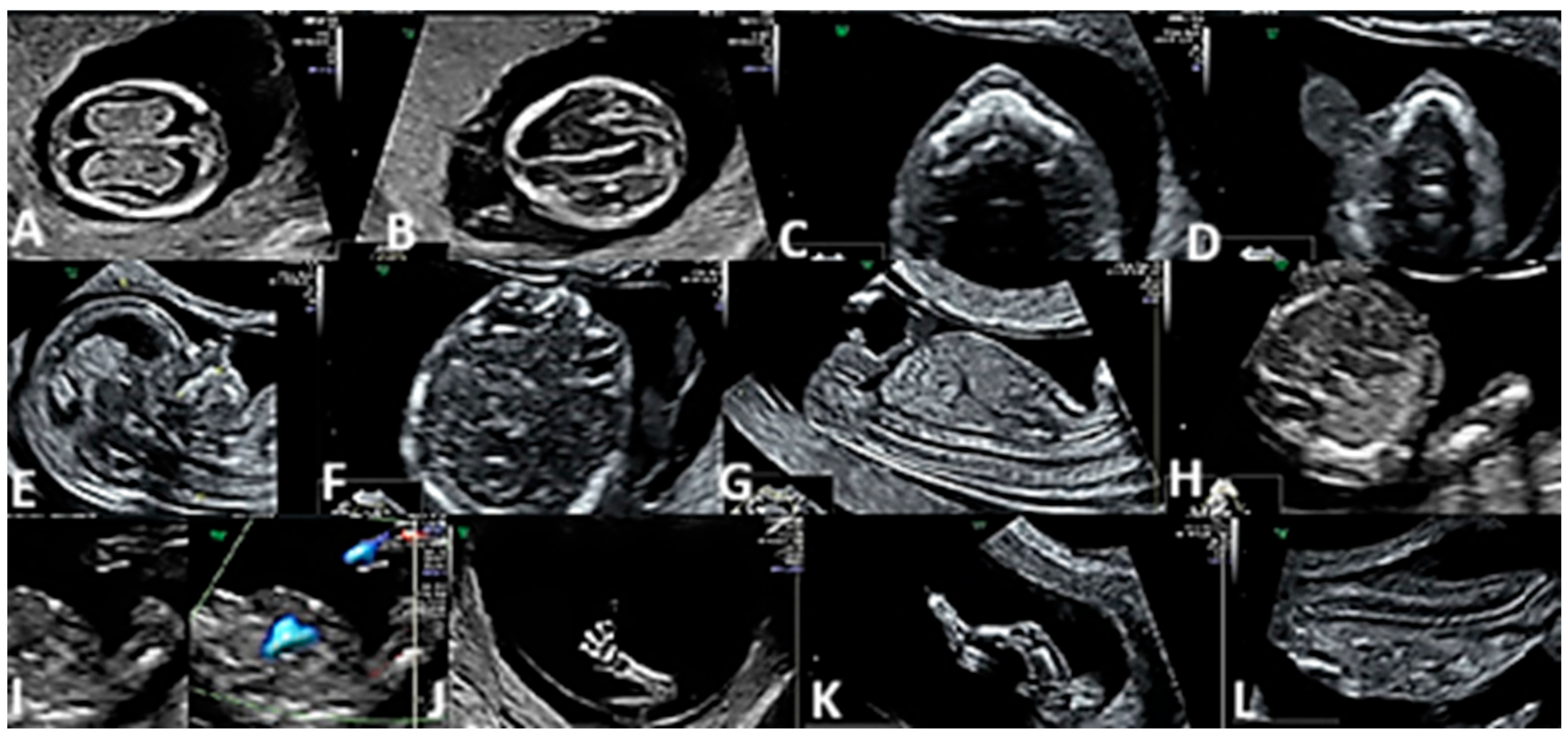
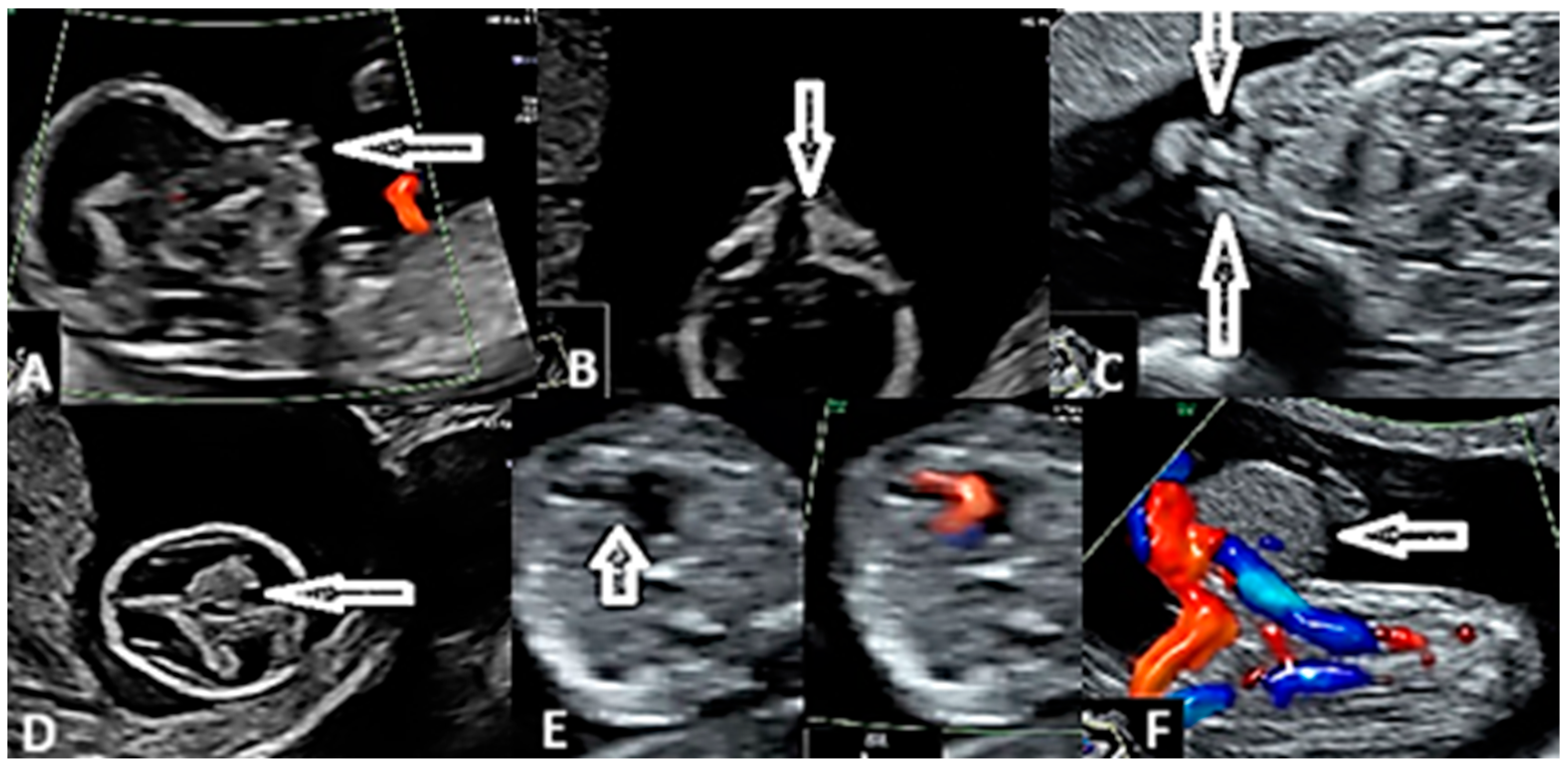
3. Results
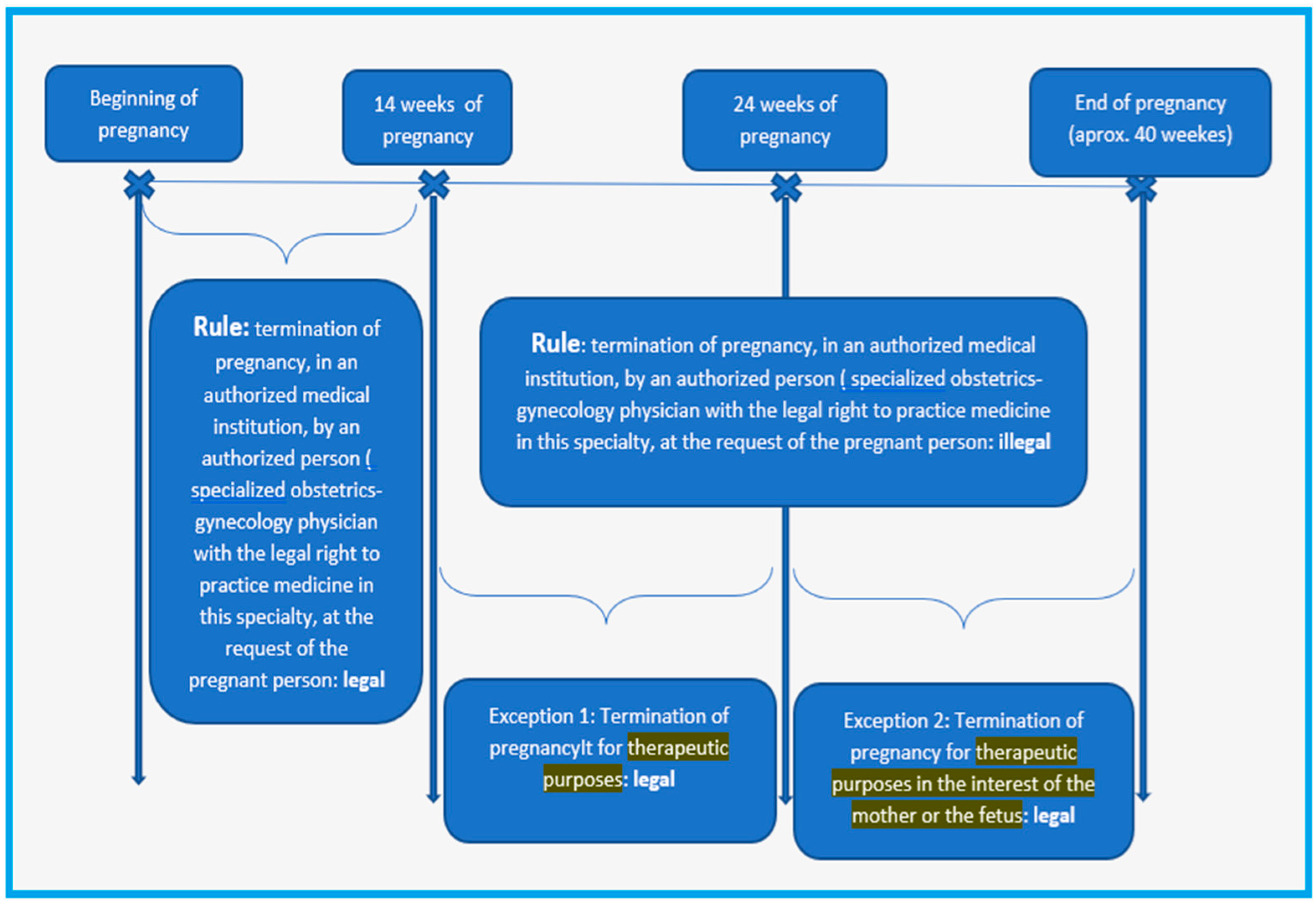
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicolades, K.H. Turning the pyramid of prenatal care. Fetal Diagn. Ther. 2011, 29, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, D.; Tudorache, S.; Comanescu, A.; Antsaklis, P.; Cotarcea, S.; Novac, L.; Cernea, N.; Antsaklis, A. Improved detection rate of structural abnormalities in the first trimester using an extended examination protocol. Ultrasound Obstet. Gynecol. 2013, 42, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Johnson, J.; Norton, M.E. ISPD 2022 debate—When offering a first trimester ultrasound at 11 + 0 to 13 + 6 weeks, a detailed review of fetal anatomy should be included. Prenat. Diagn. 2022, 43, 421–427. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Sebire, N.J.; Snijders, R.J. Down’s syndrome screening with nuchal translucency. Lancet 1997, 349, 438. [Google Scholar] [CrossRef] [PubMed]
- Crossley, J.A.; Aitken, D.A.; Cameron, A.D.; McBride, E.; Connor, J.M. Combined ultrasound and biochemical screening for Down’s syndrome in the first trimester: A Scottish multicentre study. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 667–676. [Google Scholar] [CrossRef]
- Park, S.Y.; Jang, I.A.; Lee, M.A.; Kim, Y.J.; Chun, S.H.; Park, M.H. Screening for chromosomal abnormalities using combined test in the first trimester of pregnancy. Obstet. Gynecol. Sci. 2016, 59, 357–366. [Google Scholar] [CrossRef]
- Brigatti, K.W.; Malone, F.D. First-trimester screening for aneuploidy. Obstet. Gynecol. Clin. N. Am. 2004, 31, 1–20. [Google Scholar] [CrossRef]
- Karim, J.N.; Roberts, N.W.; Salomon, L.J.; Papageorghiou, A.T. Systematic review of first trimester ultrasound screening for detection of fetal structural anomalies and factors that affect screening performance. Ultrasound Obstet. Gynecol. 2017, 50, 429–441. [Google Scholar] [CrossRef]
- Strauss, T.S.; Dutton, A.; Cary, C.; Boniferro, E.; Stoffels, G.; Feldman, K.; Hussain, F.; Ashmead, G.; Al-Ibraheemi, Z.; Brustman, L. The Role of the First Trimester Screen in the Face of Normal Cell Free DNA. J. Matern.-Fetal Neonatal Med. 2022, 35, 9907–9912. [Google Scholar] [CrossRef]
- Syngelaki, A.; Hammami, A.; Bower, S.; Zidere, V.; Akolekar, R.; Nicolaides, K.H. Diagnosis of fetal non-chromosomal abnormalities on routine ul trasound examination at 11–13weeks’ gestation. Ultrasound Obstet. Gynecol. 2019, 54, 468–476. [Google Scholar] [CrossRef]
- Syngelaki, A.; Chelemen, T.; Dagklis, T.; Allan, L.; Nicolaides, K.H. Challenges in the diagnosis of fetal non-chromosomal abnormalities at 11–13 weeks. Prenat. Diagn. 2011, 31, 90–102. [Google Scholar] [CrossRef]
- Rao, R.; Platt, L.D. Ultrasound screening: Status of markers and efficacy of screening for structural abnormalities. Semin. Perinatol. 2016, 40, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Alfirevic, Z.; Berghella, V.; Bilardo, C.; Hernandez-Andrade, E.; Johnsen, S.L.; Kalache, K.; Leung, K.Y.; Malinger, G.; Munoz, H.; et al. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet. Gynecol. 2011, 37, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.S.; Allan, L.D.; Chaoui, R.; Copel, J.A.; DeVore, G.R.; Hecher, K.; Lee, W.; Munoz, H.; Paladini, D.; Tutschek, B.; et al. ISUOG Practice Guidelines (updated): Sonographic screening examination of the fetal heart. Ultrasound Obstet. Gynecol. 2013, 41, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Colosi, E.; Musone, R.; Filardi, G.; Fabbo, A. First trimester fetal anatomy study and identification of major anomalies using 10 standardized scans. J. Prenat. Med. 2015, 9, 24–28. [Google Scholar] [CrossRef]
- Karim, J.N.; Bradburn, E.; Roberts, N.; Papageorghiou, A.T.; AC CEPTS Study. First-trimester ultrasound detection of fetal heart anomalies: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2022, 59, 11–25. [Google Scholar] [CrossRef]
- Quaresima, P.; Fesslova, V.; Farina, A.; Kagan, K.O.; Candiani, M.; Morelli, M.; Crispi, F.; Cavoretto, P.I. How to do a fetal cardiac scan. Arch. Gynecol. Obstet. 2023, 307, 1269–1276. [Google Scholar] [CrossRef]
- Turan, S.; Asoglu, M.R.; Ozdemir, H.; Seger, L.; Turan, O.M. Accuracy of the Standardized Early Fetal Heart Assessment in Excluding Major Congenital Heart Defects in High-Risk Population: A Single-Center Experience. J. Ultrasound Med. 2022, 41, 961–969. [Google Scholar] [CrossRef]
- Liao, Y.M.; Li, S.L.; Luo, G.Y.; Wen, H.X.; Ouyang, S.Y.; Chen, C.Y.; Yao, Y.; Bi, J.R.; Tian, X.X. Routine screening for fetal limb abnormalities in the first trimester. Prenat. Diagn. 2016, 36, 117–126. [Google Scholar] [CrossRef]
- Iliescu, D.G.; Pătru, C.L.; Drăgușin, R.; Florea, M.; Zorilă, G.L.; Căpitănescu, R.G.; Tudorache, Ș. Early Screening For Antenatal Congenital Fetal Limb Anomalies And Implications In The Pediatric Surgery. World J. Pharm. Med. Res. 2016, 2, 235–241. [Google Scholar]
- Kagan, K.O.; Avgidou, K.; Molina, F.S.; Gajewska, K.; Nicolaides, K.H. Relation between increased fetal nuchal translucency thickness and chromosomal defects. Obstet. Gynecol. 2006, 107, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.H. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am. J. Obstet. Gynecol. 2004, 191, 45–67. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Brizot, M.L.; Snijders, R.J. Fetal nuchal translucency: Ultrasound screening for fetal trisomy in the first trimester of pregnancy. Br. J. Obstet. Gynaecol. 1994, 101, 782–786. [Google Scholar] [CrossRef]
- Ghi, T.; Huggon, I.C.; Zosmer, N.; Nicolaides, K.H. Incidence of major structural cardiac defects associated with increased nuchal translucency but normal karyotype. Ultrasound Obstet. Gynecol. 2001, 18, 610–614. [Google Scholar] [CrossRef]
- Baer, R.J.; Norton, M.E.; Shaw, G.M.; Flessel, M.C.; Goldman, S.; Currier, R.J.; Jelliffe-Pawlowski, L.L. Risk of selected structural abnormalities in infants after increased nuchal translucency measurement. Am. J. Obstet. Gynecol. 2014, 211, 675.e1–675.e19. [Google Scholar] [CrossRef] [PubMed]
- Pătru, L.G.; Barbu, C.A.; Pătru, C.L. Digitalization of Medical Services-A New Ally for Malpractice Risk Management. Ovidius Univ. Ann. Econ. Sci. Ser. 2023, 23, 750–756. [Google Scholar]
- Franjić, S. European legal view on termination of pregnancy. J. Gynecol. Res. Obstet. 2017, 3, 051–055. [Google Scholar] [CrossRef]
- Abi Tayeh, G.; Jouannic, J.M.; Mansour, F.; Kesrouani, A.; Attieh, E. Complexity of consenting for medical termination of pregnancy: Prospective and longitudinal study in Paris. BMC Med. Ethics 2018, 19, 33. [Google Scholar] [CrossRef]
- Manian, M. The irrational woman: Informed consent and abortion decision-making. Duke J. Gend. L. Pol’y 2009, 16, 223. [Google Scholar]
- Woodcock, S. Abortion counselling and the informed consent dilemma. Bioethics 2011, 25, 495–504. [Google Scholar] [CrossRef]
- Morroni, C.; Buga, G.; Myer, L. More about....: Understanding aspects of the termination of pregnancy legislation. CME Your SA J. CPD 2006, 24, 37–38. [Google Scholar]
- Leitao, S.; O’Shaughnessy, E.; Campillo, I.S.L.; O’Donoghue, K. Healthcare professionals and students’ knowledge on termination of pregnancy legislation and clinical practice: A systematic review. Sex. Reprod. Healthc. 2022, 33, 100762. [Google Scholar] [CrossRef]
- World Health Organisation. Available online: https://www.who.int/publications/i/item/9789240039483 (accessed on 15 March 2024).
- Human Reproduction Program. 2020. Available online: https://hrp50.srhr.org/ (accessed on 15 March 2024).
- Octavian, O.G.; Ionescu, C.; Lesnic, A.; Filipescu, G.A.; Ples, L. Ethical and medico-legal aspects of the therapeutic abortion-our experience. Roman. J. Leg. Med. 2018, 26, 82–85. [Google Scholar]
- Berer, M. Making Abortions Safe: A Matter of Good Public Health Policy and Practice. Reprod. Health Matters 2002, 10, 31–44. [Google Scholar] [CrossRef]
- Safe Abortion: Technical and Policy Guidance for Health Systems, 2nd ed.; World Health Organization: Geneva, Switzerland, 2012; Available online: https://iris.who.int/bitstream/handle/10665/70914/9789241548434_eng.pdf?sequence=1&isAllowed=y (accessed on 20 April 2024).
- Law no. 286/2009 of the Romanian Criminal Code. Available online: https://www.just.ro/wp-content/uploads/2021/11/Noul-cod-penal-EN.doc (accessed on 20 April 2024).
- Kashyap, N.; Pradhan, M.; Singh, N.; Yadav, S. Early detection of fetal malformation, a long distance yet to cover! present status and potential of first trimester ultrasonography in detection of fetal congenital malformation in a developing country: Experience at a tertiary care centre in India. JPregnancy 2015, 2015, 623059. [Google Scholar] [CrossRef]
- Iliescu, D.G.; Dragusin, R.C.; Cernea, D.; Patru, C.L.; Florea, M.; Tudorache, S. Intrapartum ultrasound—An integrated approach for best prognosis. Med. Ultrason. 2017, 19, 932. [Google Scholar] [CrossRef]
- Ruican, D.; Petrescu, A.M.; Ungureanu, A.L.; Marinaş, M.C.; Pirici, D.; Istrate-Ofiţeru, A.M.; Roşu, G.C.; Badiu, A.M.; Simionescu, C.E.; Şerbănescu, M.-S.; et al. Virtual autopsy and confirmation of normal fetal heart anatomy in the first trimester using three-dimensional (3D) reconstruction of histological sections. Rom. J. Morphol. Embryol. 2021, 62, 101–108. [Google Scholar] [CrossRef]
| Anomaly | Number of Cases | Percent | p Value | |
|---|---|---|---|---|
| Nuchal translucency (NT) | NT < 3.5 mm—15 | NT > 3.5 mm—43 | 43/58 (74.1%) | <0.01 |
| Holoprosencephaly | 4 | 4 | 4/58 (6.9%) | <0.01 |
| Double outlet right ventricle | 0 | 2 | 2/58 (3.4%) | <0.01 |
| D-transposition of the great arteries | 0 | 2 | 2/58 (3.4%) | <0.01 |
| Ventricular septal defect | 0 | 1 | 1/58 (1.7%) | <0.01 |
| Omphalocele | 3 | 6 | 6/58 (10.3%) | <0.01 |
| Fetal Structural Anomalies First Trimester | Cases | Percentage | p Value |
|---|---|---|---|
| Nervous system | 23 | 23/58 (39.6%) | <0.01 |
| Holoprosencephaly | 11 | 11/58 (18.9%) | |
| Anencephaly | 7 | 7/58 (12%) | <0.01 |
| Exencephaly | 5 | 5/58 (8.62%) | <0.01 |
| Face | 7 | 7/58 (12%) | |
| Cleft lip only | 4 | 4/58 (6.89%) | <0.01 |
| Cleft palate only | 2 | 2/58 (3.4%) | |
| Cleft lip and palate | 1 | 1/58 (1.7%) | |
| Thorax and abdomen | 12 | 12/58 (20.6%) | |
| Omphalocele | 9 | 9/58 (15.5%) | |
| Gastroschisis | 3 | 3/58 (5.17%) | |
| Heart | 16 | 16/58 (27.5%) | |
| Double outlet right ventricle | 2 | 2/58 (3.4%) | <0.01 |
| D-transposition of the great arteries | 2 | 2/58 (3.4%) | <0.01 |
| Univentricular heart | 4 | 4/58 (6.8%) | <0.01 |
| Complex cardiac malformation | 5 | 5/58 (8.62%) | <0.01 |
| Tricuspid valve atresia with VSD | 1 | 1/58 (1.7%) | <0.01 |
| Major interventricular septal defect (VSD) | 1 | 1/58 (1.7%) | <0.01 |
| Tetralogy of Fallot | 1 | 1/58 (1.7%) | <0.01 |
| Limbs | 2 | 2/58 (3.4%) | |
| Club foot | 1 | 1/58 (1.7%) | |
| Polydactyly | 1 | 1/58 (1.7%) | |
| TOTAL | 58 | 100% | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Căpitănescu, R.G.; Marinaș, M.C.; Pătru, L.; Popa, D.G.; Andrei, E.C.; Popa, A.I.; Mogoș, G.F.R.; Mărgăritescu, N.D.; Pătru, C.L. First-Trimester Morphological Evaluation of Fetuses and Medical Law Implications. Diagnostics 2025, 15, 1277. https://doi.org/10.3390/diagnostics15101277
Căpitănescu RG, Marinaș MC, Pătru L, Popa DG, Andrei EC, Popa AI, Mogoș GFR, Mărgăritescu ND, Pătru CL. First-Trimester Morphological Evaluation of Fetuses and Medical Law Implications. Diagnostics. 2025; 15(10):1277. https://doi.org/10.3390/diagnostics15101277
Chicago/Turabian StyleCăpitănescu, Răzvan Grigoraș, Marius Cristian Marinaș, Larisa Pătru, Dragoș George Popa, Elena Cristina Andrei, Aura Iuliana Popa, Gabriel Florin Răzvan Mogoș, Nicolae Dragoș Mărgăritescu, and Ciprian Laurențiu Pătru. 2025. "First-Trimester Morphological Evaluation of Fetuses and Medical Law Implications" Diagnostics 15, no. 10: 1277. https://doi.org/10.3390/diagnostics15101277
APA StyleCăpitănescu, R. G., Marinaș, M. C., Pătru, L., Popa, D. G., Andrei, E. C., Popa, A. I., Mogoș, G. F. R., Mărgăritescu, N. D., & Pătru, C. L. (2025). First-Trimester Morphological Evaluation of Fetuses and Medical Law Implications. Diagnostics, 15(10), 1277. https://doi.org/10.3390/diagnostics15101277






