Risk Factors Influencing Right and Left Ventricular Variables Assessed with Gated Cadmium–Zinc–Telluride Equilibrium Radionuclide Angiocardiography in Oncology Patients
Abstract
1. Introduction
2. Materials and Methods
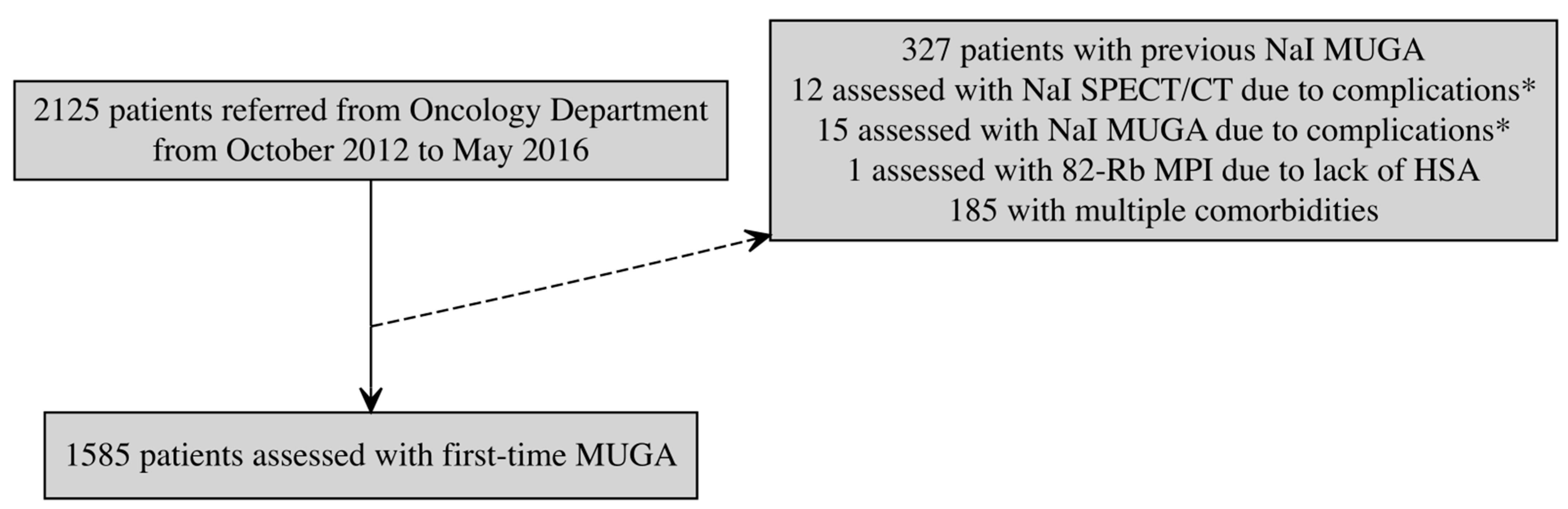
2.1. Study Design, Population, and Data Collection
2.2. Ethical Considerations
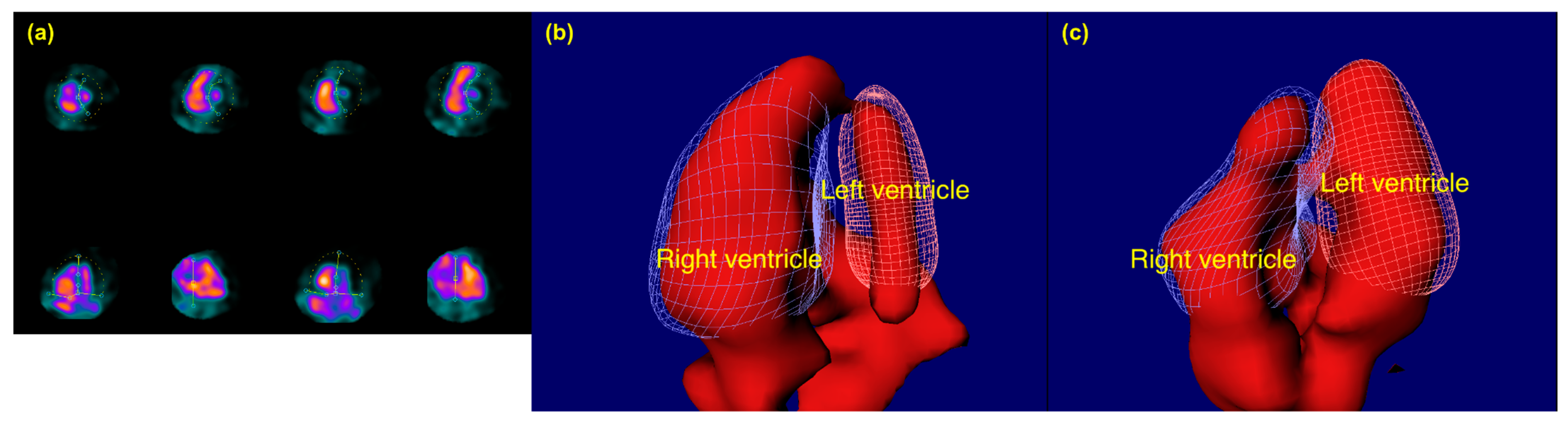
2.3. Gated CZT-SPECT-ERNA Procedure
2.4. General Considerations
2.5. Statistical Analysis
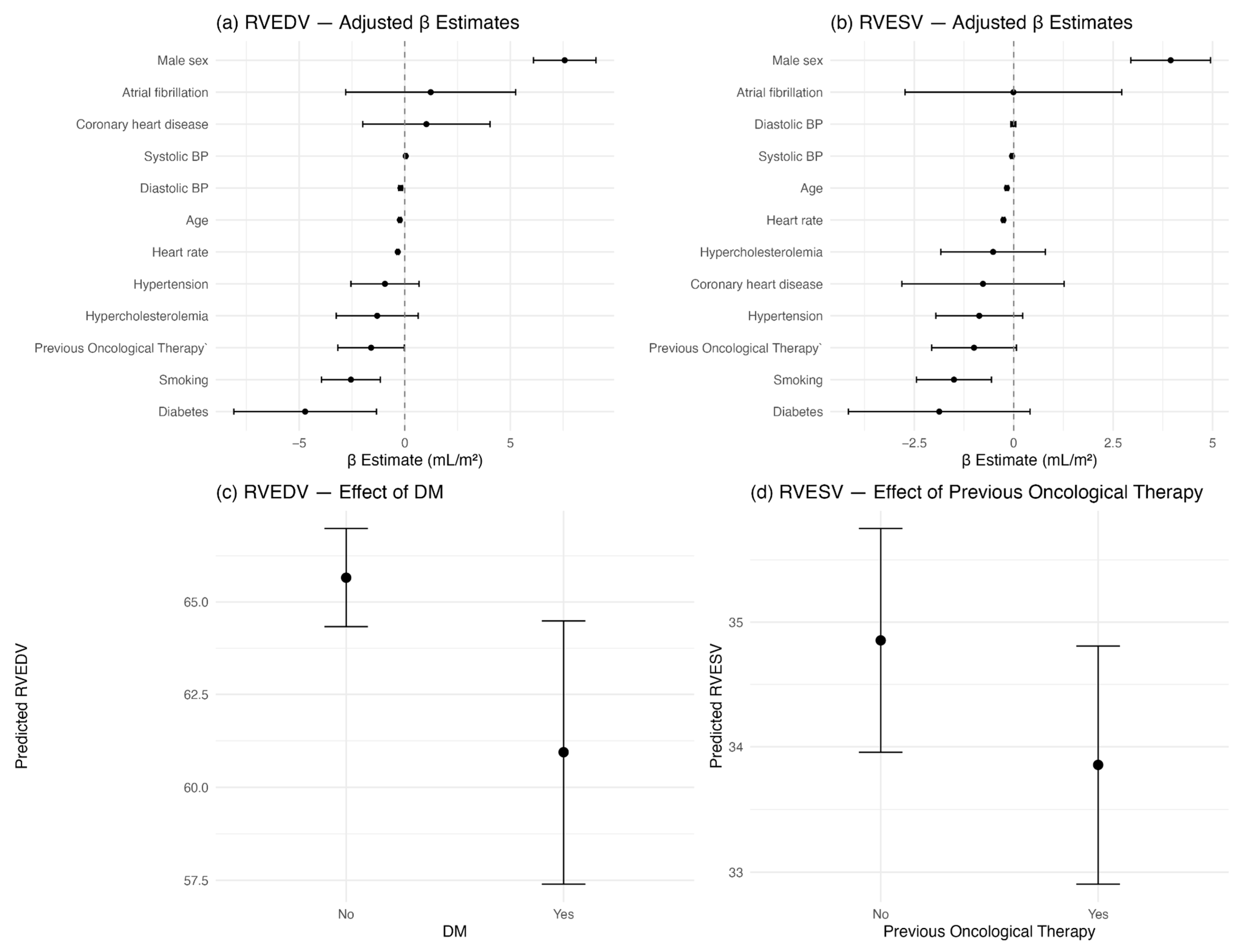
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D-TTE | Two-Dimensional Transthoracic Echocardiography |
| 3D-TTE | Three-Dimensional Transthoracic Echocardiography |
| AF | Atrial Fibrillation |
| CHD | Coronary Heart Disease |
| COPD | Chronic Obstructive Pulmonary Disease |
| CZT | Cadmium–Zinc–Telluride |
| CZT-SPECT-ERNA | Cadmium–Zinc–Telluride Single-Photon Emission Computed Tomography Equilibrium Radionuclide Angiocardiography |
| DM | Diabetes Mellitus |
| EPJ | Elektronisk Patientjournal (Electronic Patient Record) |
| ERNA | Equilibrium Radionuclide Angiocardiography |
| FMK | Fælles Medicinkort (Shared Medication Record) |
| HbA1c | Hemoglobin A1c |
| HSA | Human Serum Albumin |
| LVEDV | Left Ventricular End-Diastolic Volume |
| LVEF | Left Ventricular Ejection Fraction |
| LVESV | Left Ventricular End-Systolic Volume |
| LVSV | Left Ventricular Stroke Volume |
| PE | Pulmonary Embolism |
| PH | Pulmonary Hypertension |
| RIS/PACS | Radiology Information System/Picture Archiving and Communication System |
| RVEDV | Right Ventricular End-Diastolic Volume |
| RVEF | Right Ventricular Ejection Fraction |
| RVESV | Right Ventricular End-Systolic Volume |
| RVSV | Right Ventricular Stroke Volume |
| SV | Stroke Volume |
References
- Jones, N.R.; Hobbs, F.R.; Taylor, C.J. Prognosis following a diagnosis of heart failure and the role of primary care: A review of the literature. BJGP Open 2017, 1, bjgpopen17X101013. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Kossaify, A. Echocardiographic Assessment of the Right Ventricle, from the Conventional Approach to Speckle Tracking and Three-Dimensional Imaging, and Insights into the “Right Way” to Explore the Forgotten Chamber. Clin. Med. Insights Cardiol. 2015, 9, 65–75. [Google Scholar] [CrossRef]
- Meyer, P.; Filippatos, G.S.; Ahmed, M.I.; Iskandrian, A.E.; Bittner, V.; Perry, G.J.; White, M.; Aban, I.B.; Mujib, M.; Dell’Italia, L.J.; et al. Effects of Right Ventricular Ejection Fraction on Outcomes in Chronic Systolic Heart Failure. Circulation 2010, 121, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Ishizu, T.; Ieda, M.; Ohte, N. Right ventricular three-dimensional echocardiography: The current status and future perspectives. J. Echocardiogr. 2020, 18, 149–159. [Google Scholar] [CrossRef]
- Kartas, A.; Samaras, A.; Akrivos, E.; Vrana, E.; Papazoglou, A.S.; Moysidis, D.V.; Papanastasiou, A.; Baroutidou, A.; Botis, M.; Liampas, E.; et al. Τhe association of heart failure across left ventricular ejection fraction with mortality in atrial fibrillation. ESC Heart Fail. 2021, 8, 3189–3197. [Google Scholar] [CrossRef]
- Yoon, G.J.; Telli, M.L.; Kao, D.P.; Matsuda, K.Y.; Carlson, R.W.; Witteles, R.M. Left Ventricular Dysfunction in Patients Receiving Cardiotoxic Cancer Therapies. J. Am. Coll. Cardiol. 2010, 56, 1644–1650. [Google Scholar] [CrossRef]
- Hendriks, T.; Van Dijk, R.; Alsabaan, N.A.; Van Der Harst, P. Active Tobacco Smoking Impairs Cardiac Systolic Function. Sci. Rep. 2020, 10, 6608. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Holzmann, M.J. Association between reduced left ventricular ejection fraction following non-ST-segment elevation myocardial infarction and long-term mortality in patients of advanced age. Int. J. Cardiol. 2019, 296, 15–20. [Google Scholar] [CrossRef]
- Ehl, N.F.; Kühne, M.; Brinkert, M.; Müller-Brand, J.; Zellweger, M.J. Diabetes reduces left ventricular ejection fraction-irrespective of presence and extent of coronary artery disease. Eur. J. Endocrinol. 2011, 165, 945–951. [Google Scholar] [CrossRef]
- Hesse, B.; Lindhardt, T.B.; Acampa, W.; Anagnostopoulos, C.; Ballinger, J.; Bax, J.J.; Edenbrandt, L.; Flotats, A.; Germano, G.; Stopar, T.G.; et al. EANM/ESC guidelines for radionuclide imaging of cardiac function. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 851–885. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.M.; Schmidt, U.; Huang, C.; Zerahn, B. Gated tomographic radionuclide angiography using cadmium-zinc-telluride detector gamma camera; comparison to traditional gamma cameras. J. Nucl. Cardiol. 2014, 21, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Duvall, W.L.; Guma-Demers, K.A.; George, T.; Henzlova, M.J. Radiation reduction and faster acquisition times with SPECT gated blood pool scans using a high-efficiency cardiac SPECT camera. J. Nucl. Cardiol. 2016, 23, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.M.; Haase, C.; Zerahn, B. Interstudy repeatability of left and right ventricular volume estimations by serial-gated tomographic radionuclide angiographies using a cadmium-zinc-telluride detector gamma camera. Clin. Physiol. Funct. Imaging 2015, 35, 418–424. [Google Scholar] [CrossRef]
- Hansen, M.N.; Haarmark, C.; Kristensen, B.; Zerahn, B. An Algorithm for Individual Dosage in Cadmium–Zinc–Telluride SPECT-Gated Radionuclide Angiography. Diagnostics 2021, 11, 2268. [Google Scholar] [CrossRef]
- Haarmark, C.; Haase, C.; Jensen, M.M.; Zerahn, B. Pre-chemotherapy values for left and right ventricular volumes and ejection fraction by gated tomographic radionuclide angiography using a cadmium-zinc-telluride detector gamma camera. J. Nucl. Cardiol. 2016, 23, 87–97. [Google Scholar] [CrossRef]
- Hansen, N.L.; Haarmark, C.; Zerahn, B. Ventricular peak emptying and filling rates measured by gated tomographic radionuclide angiography using a cadmium–zinc–telluride SPECT camera in chemotherapy-naïve cancer patients. J. Nucl. Cardiol. 2020, 27, 1193–1201. [Google Scholar] [CrossRef]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 87. [Google Scholar] [CrossRef]
- Theetha Kariyanna, P.; Kumar, A.; Jayarangaiah, A.; Shetty, M.; Chowdhury, Y.; Das, S.; Jayarangaiah, A. Chemotherapy induced right ventricular cardiomyopathy; a systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1103941. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Leong, D.P.; Chakrabarty, A.; Joerg, L.; Kotasek, D.; Cheong, K.; Joshi, R.; Joseph, M.X.; DePasquale, C.; Koczwara, B.; et al. Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: A prospective study using novel cardiac imaging and biochemical markers. Int. J. Cardiol. 2013, 168, 5465–5467. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Baudisch, A.; Haßfeld, S.; Heinzel, F.; Cuspidi, C.; Burkhardt, F.; Escher, F.; Attanasio, P.; Pieske, B.; Genger, M. Right ventricular function and mechanics in chemotherapy- and radiotherapy-naïve cancer patients. Int. J. Cardiovasc. Imaging 2018, 34, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Tanindi, A.; Demirci, U.; Tacoy, G.; Buyukberber, S.; Alsancak, Y.; Coskun, U.; Yalcin, R.; Benekli, M. Assessment of right ventricular functions during cancer chemotherapy. Eur. J. Echocardiogr. 2011, 12, 834–840. [Google Scholar] [CrossRef]
- Piper, S.E.; McDonagh, T.A. Chemotherapy-related Cardiomyopathy. Eur. Cardiol. Rev. 2015, 10, 19–24. [Google Scholar] [CrossRef]
- Parmentier, S.; Melin, J.A.; Piret, L.; Beckers, C. Assessment of left ventricular diastolic function in patients receiving anthracycline therapy. Eur. J. Nucl. Med. 1988, 13, 563–567. [Google Scholar] [CrossRef]
- Kawut, S.M.; Lima, J.A.C.; Barr, R.G.; Chahal, H.; Jain, A.; Tandri, H.; Praestgaard, A.; Bagiella, E.; Kizer, J.R.; Johnson, W.C.; et al. Sex and Race Differences in Right Ventricular Structure and Function: The Multi-Ethnic Study of Atherosclerosis–Right Ventricle Study. Circulation 2011, 123, 2542–2551. [Google Scholar] [CrossRef]
- Walker, L.A.; Buttrick, P.M. The Right Ventricle: Biologic Insights and Response to Disease: Updated. Curr. Cardiol. Rev. 2013, 9, 73–81. [Google Scholar]
- Maggioni, A.P.; Dahlström, U.; Filippatos, G.; Chioncel, O.; Leiro, M.C.; Drozdz, J.; Fruhwald, F.; Gullestad, L.; Logeart, D.; Fabbri, G.; et al. EURObservational Research Programme: Regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur. J. Heart Fail. 2013, 15, 808–817. [Google Scholar] [CrossRef]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L. Heart Failure With Reduced Ejection Fraction: A Review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef]
- Goldstein, J.A. Right heart ischemia: Pathophysiology, natural history, and clinical management. Prog. Cardiovasc. Dis. 1998, 40, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Lahm, T.; Douglas, I.S.; Archer, S.L.; Bogaard, H.J.; Chesler, N.C.; Haddad, F.; Hemnes, A.R.; Kawut, S.M.; Kline, J.A.; Kolb, T.M.; et al. Assessment of Right Ventricular Function in the Research Setting: Knowledge Gaps and Pathways Forward. An Official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2018, 198, e15–e43. [Google Scholar] [CrossRef] [PubMed]
- Van Wolferen, S.A.; Marcus, J.T.; Boonstra, A.; Marques, K.M.J.; Bronzwaer, J.G.F.; Spreeuwenberg, M.D.; Postmus, P.E.; Vonk-Noordegraaf, A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur. Heart J. 2007, 28, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Widya, R.L.; Van Der Meer, R.W.; Smit, J.W.A.; Rijzewijk, L.J.; Diamant, M.; Bax, J.J.; de Roos, A.; Lamb, H.J. Right Ventricular Involvement in Diabetic Cardiomyopathy. Diabetes Care 2013, 36, 457–462. [Google Scholar] [CrossRef]
- Apert, A.; Canu, M.; Jankowski, A.; Riou, L.; Broisat, A.; Charlon, C.; Augier, C.; Boignard, A.; Leenhardt, J.; Salvat, M.; et al. Comparison of Cadmium Zinc Telluride ECG-gated SPECT equilibrium radionuclide angiocardiography to magnetic resonance imaging to measure right ventricular volumes and ejection fraction in patients with cardiomyopathy. J. Nucl. Cardiol. 2022, 29, 1647–1656. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Bargiota, A.; Kitai, T.; Katsiki, N.; Farmakis, D.; Skoularigis, J.; Starling, R.C.; Iliodromitis, E. Diabetes Mellitus and Heart Failure. J. Clin. Med. 2021, 10, 3682. [Google Scholar] [CrossRef]
- Mgbemena, O.; Zhang, Y.; Velarde, G. Role of Diabetes Mellitus in Heart Failure With Preserved Ejection Fraction: A Review Article. Cureus 2021, 13, e19398. Available online: https://www.cureus.com/articles/75988-role-of-diabetes-mellitus-in-heart-failure-with-preserved-ejection-fraction-a-review-article (accessed on 5 June 2024). [CrossRef]
- Abudureyimu, M.; Luo, X.; Wang, X.; Sowers, J.R.; Wang, W.; Ge, J.; Ren, J.; Zhang, Y. Heart failure with preserved ejection fraction (HFpEF) in type 2 diabetes mellitus: From pathophysiology to therapeutics. J. Mol. Cell Biol. 2022, 14, mjac028. [Google Scholar] [CrossRef]
- Alpert, J.S.; Petersen, P.; Godtfredsen, J. Atrial Fibrillation: Natural History, Complications, and Management. Annu. Rev. Med. 1988, 39, 41–52. [Google Scholar] [CrossRef]
- Marcusohn, E.; Kobo, O.; Postnikov, M.; Epstein, D.; Agmon, Y.; Gepstein, L.; Hellman, Y.; Zukermann, R. Left Ventricular Systolic Dysfunction Due to Atrial Fibrillation: Clinical and Echocardiographic Predictors. Card. Fail. Rev. 2021, 7, e16. [Google Scholar] [CrossRef]
- Kerr, A.J.; Williams, M.J.A.; Stewart, R.A.H. Ventricular rate and beat-to-beat variation of stroke volume in atrial fibrillation. Am. J. Cardiol. 2001, 87, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Grau, M.; Barr, R.G.; Lima, J.A.; Hoffman, E.A.; Bluemke, D.A.; Carr, J.J.; Chahal, H.; Enright, P.L.; Jain, A.; Prince, M.R.; et al. Percent Emphysema and Right Ventricular Structure and Function. Chest 2013, 144, 136–144. [Google Scholar] [CrossRef] [PubMed]

| Variable | Control (n = 811) | AF (n = 54) | CHD (n = 104) | DM (n = 81) | Previous Oncological Therapy (n = 535) |
|---|---|---|---|---|---|
| Gender (men) | 367 (45%) | 30 (56%) | 66 (63%) | 58 (72%) | 103 (19%) |
| Age | 61 (±15) | 73 (±7) * | 69 (±11) * | 67 (±8) * | 62 (±11) * |
| Height (women) [cm] | 166 (±7) | 167 (±5) | 164 (±6) | 164 (±8) | 166 (±6) |
| Height (men) [cm] | 178 (±7) | 179 (±5) | 176 (±6) * | 177 (±7) | 180 (±7) |
| Weight (women) [kg] | 70 (±14) | 72 (±12) | 68 (±12) | 77 (±25) | 70 (±14) |
| Weight (men) [kg] | 82 (±14) | 81 (±9) | 80 (±12) | 87 (±15) * | 83 (±15) |
| Systolic BP [mmHg] | 129 (±19) | 128 (±20) | 129 (±20) | 127 (±16) | 125 (±18) * |
| Diastolic BP [mmHg] | 77 (±10) | 76 (±11) | 75 (±10) | 73 (±10) * | 76 (±10) |
| Heart rate [bpm] | 75 (±13) | 76 (±17) | 73 (±15) | 77 (±15) | 77 (±14) * |
| Arterial hypertension | 229 (28%) | 32 (59%) | 64 (62%) | 55 (68%) | 138 (26%) |
| Hypercholesterolemia | 95 (12%) | 21 (39%) | 56 (54%) | 38 (47%) | 56 (10%) |
| Smoking (prior or current) | 350 (43%) | 27 (50%) | 65 (63%) | 47 (58%) | 208 (39%) |
| Variable | Left Ventricle | Right Ventricle |
|---|---|---|
| AF | ||
| EDV | 2.4 (−8.2–13, p = 0.65) | −1.3 (−5.9–3.3, p = 0.56) |
| ESV | 5.5 (−1.7–13, p = 0.13) | −1.1 (−5.3–3, p = 0.58) |
| EF | −3.6 (−7.9–0.64, p = 0.094) | 1.5 (−1.8–4.8, p = 0.37) |
| SV | −3.1 (−9.4–3.2, p = 0.33) | −0.2 (−2.9–2.5, p = 0.88) |
| CHD | ||
| EDV | 22 (13–32, p < 0.001) | 0.6 (−3.7–4.9, p = 0.78) |
| ESV | 21 (13–29, p < 0.001) | −1.1 (−4.2–2, p = 0.48) |
| EF | −9.2 (−13–(−6), p < 0.001) | 3 (0.79–5.2, p = 0.008) |
| SV | 0.9 (−3.2–5, p = 0.66) | 1.7 (−0.35–3.8, p = 0.1) |
| DM | ||
| EDV | −6.8 (−13–(−0.8), p = 0.027) | −5.1 (−9.2–(−1), p = 0.015) |
| ESV | −4.5 (−8–(−1), p = 0.012) | −3 (−5.5–(−0.4), p = 0.024) |
| EF | 3.5 (0.7–6.2, p = 0.015) | 0.2 (−2–2.4, p = 0.83) |
| SV | −2.3 (−6.2–1.5, p = 0.23) | −2.2 (−4.6–0.23, p = 0.076) |
| Previous oncological therapy | ||
| EDV | −3.8 (−6.5–(−1.2), p = 0.005) | −4.4 (−6.1–(−2.7), p < 0.001) |
| ESV | 0.9 (−0.68–2.5, p = 0.26) | −2.3 (−3.4–(−1.2), p < 0.001) |
| EF | −2.2 (−3.4–(−1), p < 0.001) | −0.01 (−0.92–0.90, p = 0.98) |
| SV | −4.8 (−6.4–(−3.1), p < 0.001) | −2.1 (−3–(−1.2), p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monsson, O.; Nielsen, M.; Kümler, T.; Haarmark, C.; Zerahn, B. Risk Factors Influencing Right and Left Ventricular Variables Assessed with Gated Cadmium–Zinc–Telluride Equilibrium Radionuclide Angiocardiography in Oncology Patients. Diagnostics 2025, 15, 1274. https://doi.org/10.3390/diagnostics15101274
Monsson O, Nielsen M, Kümler T, Haarmark C, Zerahn B. Risk Factors Influencing Right and Left Ventricular Variables Assessed with Gated Cadmium–Zinc–Telluride Equilibrium Radionuclide Angiocardiography in Oncology Patients. Diagnostics. 2025; 15(10):1274. https://doi.org/10.3390/diagnostics15101274
Chicago/Turabian StyleMonsson, Olav, Marc Nielsen, Thomas Kümler, Christian Haarmark, and Bo Zerahn. 2025. "Risk Factors Influencing Right and Left Ventricular Variables Assessed with Gated Cadmium–Zinc–Telluride Equilibrium Radionuclide Angiocardiography in Oncology Patients" Diagnostics 15, no. 10: 1274. https://doi.org/10.3390/diagnostics15101274
APA StyleMonsson, O., Nielsen, M., Kümler, T., Haarmark, C., & Zerahn, B. (2025). Risk Factors Influencing Right and Left Ventricular Variables Assessed with Gated Cadmium–Zinc–Telluride Equilibrium Radionuclide Angiocardiography in Oncology Patients. Diagnostics, 15(10), 1274. https://doi.org/10.3390/diagnostics15101274






