Carotid Stump Syndrome: A Case That Highlights the Necessity of Digital Subtraction Angiography for the Prompt Management of the Syndrome
Abstract
1. Introduction
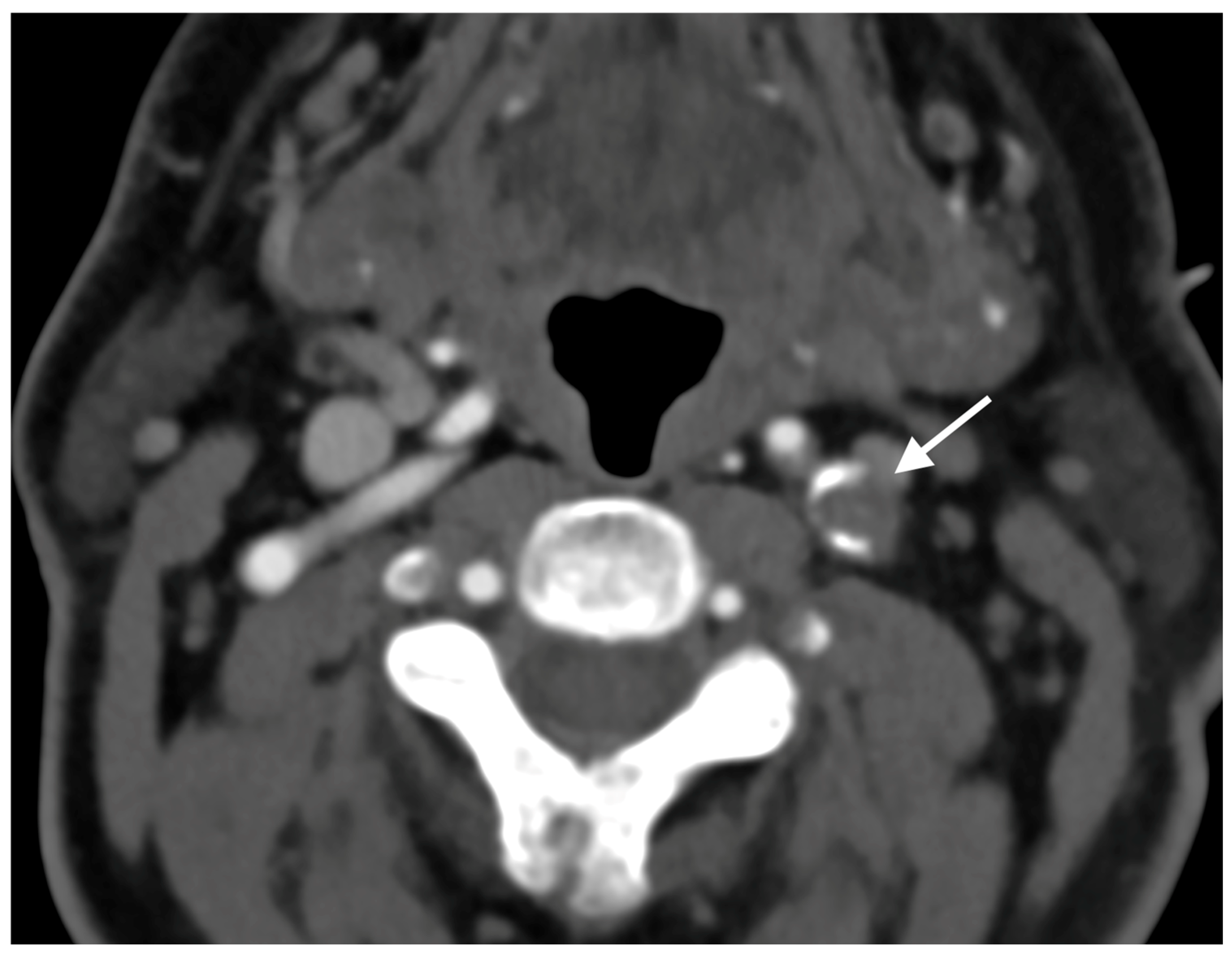
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSS | carotid stump syndrome |
| ICA | internal carotid artery |
| ECA | external carotid artery |
| CT | computed tomography |
| NIHSS | National Institutes of Health Stroke Scale |
| CTA | computed tomography angiography |
| DSA | digital subtraction angiography |
| CEA | carotid endarterectomy |
| AIS | acute ischemic strokes |
| TIA | transient ischemic attacks |
| MRI | magnetic resonance imaging |
| MRA | magnetic resonance angiography |
| CAS | carotid artery stenting |
References
- Barrett, K.M.; Brott, T.G. Stroke Caused by Extracranial Disease. Circ. Res. 2017, 120, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. Editor’s Choice—2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef] [PubMed]
- European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998, 351, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Radu, R.A.; Cagnazzo, F.; Derraz, I.; Dargazanli, C.; Rapido, F.; Lefevre, P.H.; Gascou, G.; Costalat, V. Modern endovascular management of chronic total carotid artery occlusion: Technical results and procedural challenges. J. Neurointerv. Surg. 2023, 15, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Sherrod, B.; Gamboa, N.T.; Taussky, P.; Grandhi, R. Carotid Stump Syndrome with Stent-Assisted Coil Embolization. Cureus 2022, 14, e22746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kargiotis, O.; Psychogios, K.; Safouris, A.; Spiliopoulos, S.; Karapanayiotides, T.; Bakola, E.; Mantatzis, M.; Dardiotis, E.; Ellul, J.; Giannopoulos, S.; et al. Diagnosis and treatment of acute isolated proximal internal carotid artery occlusions: A narrative review. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221136335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alenezi, S.; Saleem, A.; Alenezi, A.; Alhajri, O.; Khuraibet, S.; Ameer, A. Sudden unilateral eye pain with vision loss related to carotid stump syndrome; A case report and literature review. Int. J. Surg. Case Rep. 2023, 106, 108208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Croke, L. Dual Antiplatelet Therapy for High-Risk TIA and Minor Stroke: BMJ Rapid Recommendation. Am. Fam. Physician 2019, 100, 378–379. [Google Scholar] [PubMed]
- Liu, Y.; Zhao, J.; Gao, Y.; Chen, W.; Johnston, S.C.; Bath, P.M.; Amarenco, P.; Yan, H.; Wang, X.; Yang, Y.; et al. Clopidogrel and Aspirin Initiated Between 24 to 72 Hours for Mild Ischemic Stroke: A Subgroup Analysis of the INSPIRES Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2431938, Erratum in JAMA Netw. Open 2025, 8, e259310. https://doi.org/10.1001/jamanetworkopen.2025.9310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kniemeyer, H.W.; Aulich, A.; Schlachetzki, F.; Steinmetz, H.; Sandmann, W. Pseudo- and segmental occlusion of the internal carotid artery: A new classification, surgical treatment and results. Eur. J. Vasc. Endovasc. Surg. 1996, 12, 310–320. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.U.; Shao, S.; Zheng, X.; Gao, X.; Zhang, Y. Carotid stump syndrome: A case report. Exp. Ther. Med. 2015, 10, 1161–1164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eikelboom, B.C.; Ackerstaff, R.G. Preoperative prediction of cerebral ischaemia due to carotid occlusion. Eur. J. Vasc. Surg. 1993, 7 (Suppl. A), 21–24. [Google Scholar] [CrossRef] [PubMed]
- Liebeskind, D.S. Collateral circulation. Stroke 2003, 34, 2279–2284. [Google Scholar] [CrossRef]
- Akpinar, S.; Gelener, P.; Yilmaz, G. Aetiologies of internal carotid artery pseudo-occlusions in acute stroke patients: What neurointerventionalists can expect. Br. J. Radiol. 2017, 90, 20160352. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.R.; Bell, P.R.; Bolia, A. Endovascular treatment of carotid stump syndrome. J. Vasc. Surg. 2003, 38, 593–595. [Google Scholar] [CrossRef]
- Lakshminarayan, R.; Scott, P.M.; Robinson, G.J.; Ettles, D.F. Carotid stump syndrome: Pathophysiology and endovascular treatment options. Cardiovasc. Interv. Radiol. 2011, 34, 48–52. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Luo, B. Interventional recanalization as a treatment of carotid stump syndrome caused by right internal carotid artery occlusion: A case report. Medicine 2019, 98, e17152. [Google Scholar] [CrossRef]
- Kumar, S.M.; Wang, J.C.; Barry, M.C.; Farrell, L.; Kelly, C.J.; Fitzgerald, P.H.; Leahy, A.; Hayes, D.B. Carotid stump syndrome: Outcome from surgical management. Eur. J. Vasc. Endovasc. Surg. 2001, 21, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Chinchure, S.D.; Kataria, V. Carotid Stump Syndrome: Rare Cause of Recurrent Stroke Post-Ipsilateral Carotid Occlusion—A Case Report. Ann. Indian. Acad. Neurol. 2024, 27, 426–429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meza, H.T.; Roy, J.A.; Ponz, M.S.; Morte, S.G.; Moreno, J.M. Carotid pseudo-occlusion: A concept to consider in acute stroke. Neurologia (Engl. Ed.) 2020, 35, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Kappelhof, M.; Marquering, H.A.; Berkhemer, O.A.; Borst, J.; van der Lugt, A.; van Zwam, W.H.; Vos, J.A.; Lycklama, À.; Nijeholt, G.; Majoie, C.B.L.M.; et al. Accuracy of CT Angiography for Differentiating Pseudo-Occlusion from True Occlusion or High-Grade Stenosis of the Extracranial ICA in Acute Ischemic Stroke: A Retrospective MR CLEAN Substudy. AJNR Am. J. Neuroradiol. 2018, 39, 892–898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toruno, M.A.; Meier, T.; Elfil, M.; Varshika, K.; Cortese, J.; Ghozy, S.; Kadirvel, R.; Kallmes, D.F. Endovascular Thrombectomy for Carotid Pseudo-Occlusion in the Setting of Acute Ischemic Stroke: A Comparative Systematic Review and Meta-analysis. AJNR Am. J. Neuroradiol. 2024, 45, 1241–1245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lucero, L.; Dhawan, D.S.; O’Banion, L.A. Surgical management of carotid stump syndrome. J. Vasc. Surg. Cases Innov. Tech. 2023, 9, 101342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hrbáč, T.; Beneš, V.; Širůček, P.; Jonszta, T.; Herzig, R.; Procházka, V.; Skoloudík, D. Safety and efficacy of surgical treatment of carotid stump syndrome: Pilot study. Ann. Vasc. Surg. 2012, 26, 797–801. [Google Scholar] [CrossRef] [PubMed]
- AbuRahma, A.F.; Avgerinos, E.D.; Chang, R.W.; Darling, R.C., 3rd; Duncan, A.A.; Forbes, T.L.; Malas, M.B.; Murad, M.H.; Perler, B.A.; Powell, R.J.; et al. Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J. Vasc. Surg. 2022, 75, 4S–22S. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Aviv, R.I.; Fox, A.J.; Johansson, E. Symptomatic carotid near-occlusion causes a high risk of recurrent ipsilateral ischemic stroke. J. Neurol. 2020, 267, 522–530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grossberg, J.A.; Haussen, D.C.; Cardoso, F.B.; Rebello, L.C.; Bouslama, M.; Anderson, A.M.; Frankel, M.R.; Nogueira, R.G. Cervical Carotid Pseudo-Occlusions and False Dissections: Intracranial Occlusions Masquerading as Extracranial Occlusions. Stroke 2017, 48, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Fox, A.J. Near-Occlusion is a Common Variant of Carotid Stenosis: Study and Systematic Review. Can. J. Neurol. Sci. 2022, 49, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Fox, A.J. Carotid Near-Occlusion: A Comprehensive Review, Part 1—Definition, Terminology, and Diagnosis. AJNR Am. J. Neuroradiol. 2016, 37, 2–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dulai, M.; Elsherif, M.; Tawfick, W.; Kavanagh, E.P.; Hynes, N.; Sultan, S. Outcome following open and endovascular intervention for carotid stump syndrome. SAGE Open Med. Case Rep. 2018, 6, 2050313. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Das, B.; Goel, G.; Garg, A.; Sapra, H. Carotid stump syndrome treated with endovascular coiling: A rare cause of stroke in young patients. Neurol. India 2018, 66, 228–229. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenos, C.; Anastasiou, A.; Nikolopoulou, G.; Papanagiotou, P.; Papagiannis, G.; Koutroumpi, A.; Drakopoulou, D.; Anastasiou, P.; Yiannopoulou, K. Carotid Stump Syndrome: A Case That Highlights the Necessity of Digital Subtraction Angiography for the Prompt Management of the Syndrome. Diagnostics 2025, 15, 1273. https://doi.org/10.3390/diagnostics15101273
Stenos C, Anastasiou A, Nikolopoulou G, Papanagiotou P, Papagiannis G, Koutroumpi A, Drakopoulou D, Anastasiou P, Yiannopoulou K. Carotid Stump Syndrome: A Case That Highlights the Necessity of Digital Subtraction Angiography for the Prompt Management of the Syndrome. Diagnostics. 2025; 15(10):1273. https://doi.org/10.3390/diagnostics15101273
Chicago/Turabian StyleStenos, Christos, Aikaterini Anastasiou, Georgia Nikolopoulou, Panagiotis Papanagiotou, Georgios Papagiannis, Aikaterini Koutroumpi, Danai Drakopoulou, Periklis Anastasiou, and Konstantina Yiannopoulou. 2025. "Carotid Stump Syndrome: A Case That Highlights the Necessity of Digital Subtraction Angiography for the Prompt Management of the Syndrome" Diagnostics 15, no. 10: 1273. https://doi.org/10.3390/diagnostics15101273
APA StyleStenos, C., Anastasiou, A., Nikolopoulou, G., Papanagiotou, P., Papagiannis, G., Koutroumpi, A., Drakopoulou, D., Anastasiou, P., & Yiannopoulou, K. (2025). Carotid Stump Syndrome: A Case That Highlights the Necessity of Digital Subtraction Angiography for the Prompt Management of the Syndrome. Diagnostics, 15(10), 1273. https://doi.org/10.3390/diagnostics15101273





