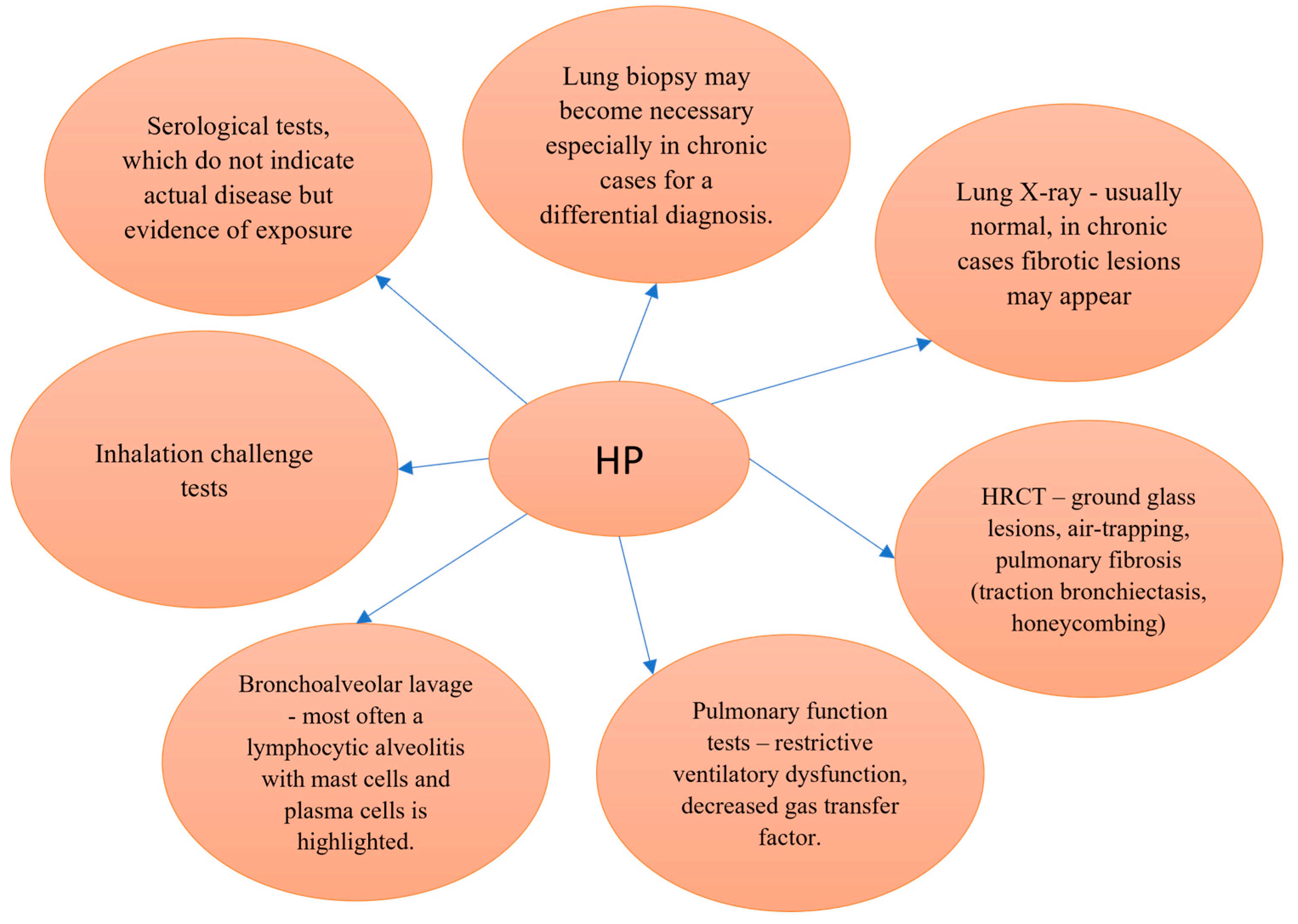
Fibrotic Hypersensitivity Pneumonitis: A Diagnostic Challenge Leading to Lung Transplantation
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HP | Hypersensitivity Pneumonitis |
| HRCT | High-Resolution Computed Tomography |
| ILD | Interstitial Lung Disease |
| IPF | Idiopathic Pulmonary Fibrosis |
| PFT | Pulmonary Function Test |
| FVC | Forced Vital Capacity |
| DLCO | Diffusing Capacity of the Lung for Carbon Monoxide |
| BAL | Bronchoalveolar Lavage |
| EKG | Electrocardiogram |
| ANA | Anti-Nuclear-Antigen antibody panel |
| IgG | Immunoglobulin G |
| FEV | Forced Expiratory Volume |
| TLC | Total Lung Capacity |
| RV | Residual Volume |
| IgE | Immunoglobulin E |
| COPD | Chronic Obstructive Pulmonary Disease |
| ERV | Expiratory Reserve Volume |
| RB-ILD | Respiratory Bronchiolitis-Associated Interstitial Lung Disease |
| PaO2 | Partial Pressure of Oxygen |
| HE | Hematoxylin and Eosin |
| PaCO2 | Partial Pressure of Carbon Dioxide |
| PPF | Progressive Pulmonary Fibrosis |
| ABG | Arterial Blood Gas |
| MDD | Multidisciplinary discussion |
| ATS | American Thoracic Society |
| JRS | Japanese Respiratory Society |
| ALAT | Latin American Thoracic Association |
| Anti-nRNP/Sm | Anti-nuclear Ribonucleoprotein/Smith antibodies |
| Anti-Sm | Anti-Smith antibodies |
| Anti-SS-A (Ro) | Anti-SS-A (Ro) antibodies |
| Anti-Ro-52 | Anti-Ro-52 antibodies |
| Anti-SS-B (La) | Anti-SS-B (La) antibodies |
| Anti-Scl-70 | Anti-topoisomerase I (Scl-70) antibodies |
| Anti-PM-Scl 100 | Anti-PM-Scl 100 antibodies |
| Anti-Jo-1 | Anti-histidyl-tRNA synthetase (Jo-1) antibodies |
| Anti-Centromere B | Anti-centromere B antibodies |
| Anti-PCNA | Anti-proliferating cell nuclear antigen (PCNA) antibodies |
| Anti-dsDNA | Anti-double-stranded DNA antibodies |
| Anti-Nucleosome | Anti-nucleosome antibodies |
| Anti-Histone | Anti-histone antibodies |
| Anti-Ribosomal P Protein | Anti-ribosomal P protein antibodies |
| Anti-AMA-M2 | Anti-mitochondrial antibodies type M2 |
| Anti-DFS70 | Anti-dense fine speckled 70 antibodies |
| DIP | Desquamative Interstitial Pneumonia |
| SaO2 | Arterial Oxygen Saturation |
| HCO3− | Bicarbonate |
| MEF25 | Maximal Expiratory Flow at 25% of Forced Vital Capacity |
References
- Dabiri, M.; Jehangir, M.; Khoshpouri, P.; Chalian, H. Hypersensitivity Pneumonitis: A Pictorial Review Based on the New ATS/JRS/ALAT Clinical Practice Guideline for Radiologists and Pulmonologists. Diagnostics 2022, 12, 2874. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Paul, I. Insights on immune profile, pathogenesis and differential diagnosis of hypersensitivity pneumonitis and pulmonary sarcoidosis–A holistic review and bibliometric analysis. Respir. Investig. 2025, 63, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Vasakova, M.; Morell, F.; Walsh, S.; Leslie, K.; Raghu, G. Hypersensitivity Pneumonitis: Perspectives in Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2017, 196, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Montero-Fernandez, M.A.; Kern, I.; Pezzuto, F.; Lunardi, F.; Hofman, P.; Berezowska, S.; Attanoos, R.; Burke, L.; Mason, P.; et al. The role of pathologists in the diagnosis of occupational lung diseases: An expert opinion of the European Society of Pathology Pulmonary Pathology Working Group. Virchows Arch. 2024, 485, 173–195. [Google Scholar] [CrossRef]
- Calaras, D.; David, A.; Vasarmidi, E.; Antoniou, K.; Corlateanu, A. Hypersensitivity Pneumonitis: Challenges of a Complex Disease. Can. Respir. J. 2024, 2024, 4919951. [Google Scholar] [CrossRef]
- Oliveira, M.; Oliveira, D.; Lisboa, C.; Boechat, J.L.; Delgado, L. Clinical Manifestations of Human Exposure to Fungi. J. Fungi 2023, 9, 381. [Google Scholar] [CrossRef]
- Salisbury, M.L.; Myers, J.L.; Belloli, E.A.; Kazerooni, E.A.; Martinez, F.J.; Flaherty, K.R. Diagnosis and Treatment of Fibrotic Hypersensitivity Pneumonia. Where We Stand and Where We Need to Go. Am. J. Respir. Crit. Care Med. 2017, 196, 690–699. [Google Scholar] [CrossRef]
- Petnak, T.; Thongprayoon, C.; Baqir, M.; Ryu, J.H.; Moua, T. Antigen identification and avoidance on outcomes in fibrotic hypersensitivity pneumonitis. Eur. Respir. J. 2022, 60, 2101336. [Google Scholar] [CrossRef]
- Sanduzzi Zamparelli, S.; Sanduzzi Zamparelli, A.; Bocchino, M. The Evolving Concept of the Multidisciplinary Approach in the Diagnosis and Management of Interstitial Lung Diseases. Diagnostics 2023, 13, 2437. [Google Scholar] [CrossRef]
- Bergantini, L.; Nardelli, G.; d’Alessandro, M.; Montuori, G.; Piccioli, C.; Rosi, E.; Gangi, S.; Cavallaro, D.; Cameli, P.; Bargagli, E. Combined Sarcoidosis and Idiopathic Pulmonary Fibrosis (CSIPF): A New Phenotype or a Fortuitous Overlap? Scoping Review and Case Series. J. Clin. Med. 2022, 11, 2065. [Google Scholar] [CrossRef]
- Pereira, J.O.; Fernandes, V.; Alfaro, T.M.; Freitas, S.; Cordeiro, C.R. Diagnosis of Fibrotic Hypersensitivity Pneumonitis: Is There a Role for Biomarkers? Life 2023, 13, 565. [Google Scholar] [CrossRef]
- Rafique, M.; Arslan, F.; Khan, J.; Zaki, S.; Hussain, A. Hypersensitivity Pneumonitis: An Interesting Case of Acute Shortness of Breath in a Young Patient. Cureus 2024, 16, e68683. [Google Scholar] [CrossRef]
- Rea, G.; Bocchino, M.; Lieto, R.; Ledda, R.E.; D’Alto, M.; Sperandeo, M.; Lucci, R.; Pasquinelli, P.; Sanduzzi Zamparelli, S.; Bocchini, G.; et al. The Unveiled Triad: Clinical, Radiological and Pathological Insights into Hypersensitivity Pneumonitis. J. Clin. Med. 2024, 13, 797. [Google Scholar] [CrossRef]
- Rittig, A.H.; Hilberg, O.; Ibsen, R.; Løkke, A. Incidence, Comorbidity and Survival Rate of Hypersensitivity Pneumonitis: A National Population-Based Study. ERJ Open Res. 2019, 5, 00259–02018. [Google Scholar] [CrossRef]
- Alberti, M.L.; Rincon-Alvarez, E.; Buendia-Roldan, I.; Selman, M. Hypersensitivity Pneumonitis: Diagnostic and Therapeutic Challenges. Front. Med. 2021, 8, 718299. [Google Scholar] [CrossRef]
- Cîrjaliu, R.E.; Gurrala, S.V.; Nallapati, B.; Krishna, V.; Oancea, C.; Tudorache, E.; Marc, M.; Bratosin, F.; Bogdan, I.; Rosca, O.; et al. Prognostic Implications of Initial Radiological Findings of Pulmonary Fibrosis in Patients with Acute SARS-CoV-2 Infection: A Prospective Multicentric Study. Diseases 2024, 12, 285. [Google Scholar] [CrossRef]
- Guler, S.A.; Wohlfarth, E.; Berezowska, S.; Geiser, T.K.; Ebner, L.; Funke-Chambour, M. Performance of a diagnostic algorithm for fibrotic hypersensitivity pneumonitis. A case-control study. Respir. Res. 2021, 22, 120. [Google Scholar] [CrossRef]
- Fernández Pérez, E.R.; Travis, W.D.; Lynch, D.A.; Brown, K.K.; Johannson, K.A.; Selman, M.; Ryu, J.H.; Wells, A.U.; Tony Huang, Y.C.; Pereira, C.A.C.; et al. Diagnosis and Evaluation of Hypersensitivity Pneumonitis: CHEST Guideline and Expert Panel Report. Chest 2021, 160, e97–e156. [Google Scholar] [CrossRef]
- Reichardt, S.D.; Amouret, A.; Muzzi, C.; Vettorazzi, S.; Tuckermann, J.P.; Lühder, F.; Reichardt, H.M. The Role of Glucocorticoids in Inflammatory Diseases. Cells 2021, 10, 2921. [Google Scholar] [CrossRef]
- Hamblin, M.; Prosch, H.; Vašáková, M. Diagnosis, course and management of hypersensitivity pneumonitis. Eur. Respir. Rev. 2022, 31, 210169. [Google Scholar] [CrossRef]
- Akkale, T.; Sarı, G.; Şimşek, C. Occupational hypersensitivity pneumonia. Tuberk. Toraks 2023, 71, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Trushenko, N.V.; Suvorova, O.A.; Pershina, E.S.; Nekludova, G.V.; Chikina, S.Y.; Levina, I.A.; Chernyaev, A.L.; Samsonova, M.V.; Tyurin, I.E.; Mustafina, M.K.; et al. Predictors of Progression and Mortality in Patients with Chronic Hypersensitivity Pneumonitis: Retrospective Analysis of Registry of Fibrosing Interstitial Lung Diseases. Life 2023, 13, 467. [Google Scholar] [CrossRef]
- Amati, F.; Stainer, A.; Polelli, V.; Mantero, M.; Gramegna, A.; Blasi, F.; Aliberti, S. Efficacy of Pirfenidone and Nintedanib in Interstitial Lung Diseases Other than Idiopathic Pulmonary Fibrosis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7849. [Google Scholar] [CrossRef] [PubMed]
- Ntiamoah, P.; Mehta, A.C. Beyond the Graft: Recurrence of Interstitial Lung Diseases Post Transplant. J. Clin. Med. 2025, 14, 1093. [Google Scholar] [CrossRef] [PubMed]
- Nosotti, M.; Leiva-Juarez, M.; D’Ovidio, F.; Van Raemdonck, D.; Ceulemans, L.; Keshavjee, S.; Rackauskas, M.; Paladini, P.; Luzzi, L.; Casado, P.M.; et al. Survival after lung transplantation for chronic hypersensitivity pneumonitis: Results from a large international cohort study. Transpl. Int. 2022, 35, 10450. [Google Scholar] [CrossRef]
- Cano-Jiménez, E.; Villar Gómez, A.; Velez Segovia, E.; Aburto Barrenechea, M.; Sellarés Torres, J.; Francesqui, J.; Portillo Carroz, K.; Solis Solis, A.J.; Acosta Fernández, O.; Llanos González, A.B.; et al. Prognostic factors of progressive fibrotic hypersensitivity pneumonitis: A large, retrospective, multicentre, observational cohort study. ERJ Open Res. 2024, 10, 00405–02023. [Google Scholar] [CrossRef]
- Ahmad, Y.; Mooney, J.; Allen, I.E.; Seaman, J.; Kalra, A.; Muelly, M.; Reicher, J. A Machine Learning System to Indicate Diagnosis of Idiopathic Pulmonary Fibrosis Non-Invasively in Challenging Cases. Diagnostics 2024, 14, 830. [Google Scholar] [CrossRef]
- Ruaro, B.; Pozzan, R.; Confalonieri, P.; Tavano, S.; Hughes, M.; Matucci Cerinic, M.; Baratella, E.; Zanatta, E.; Lerda, S.; Geri, P.; et al. Gastroesophageal Reflux Disease in Idiopathic Pulmonary Fibrosis: Viewer or Actor? To Treat or Not to Treat? Pharmaceuticals 2022, 15, 1033. [Google Scholar] [CrossRef]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Meiser, B.; Potena, L.; Robinson, A.; Rossano, J.W.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report—2019; Focus theme: Donor and recipient size match. J. Heart Lung Transpl. 2019, 38, 1056–1066. [Google Scholar] [CrossRef]
- Churg, A.; Tazelaar, H.; Matej, R.; Vasakova, M.K.; Stewart, B.; Patel, D.; Duarte, E.; Gomez Manjarres, D.C.; Mehta, H.J.; Wright, J.L. Pathologic criteria for the diagnosis of usual interstitial pneumonia vs fibrotic hypersensitivity pneumonitis in transbronchial cryobiopsies. Mod. Pathol. 2023, 36, 100221. [Google Scholar] [CrossRef]
- Tinoco, E.M.; Bermudo, G.; Vicens-Zygmunt, V.; Luburich, P.; Llatjós, R.; Molina-Molina, M. Hypersensitivity Pneumonitis, a Differential Diagnosis of Cystic Lung Diseases. Pulmonology 2023, 29, 347–349. [Google Scholar] [CrossRef]
- Vindis, K.; Nemeth, N.; Marge, C.; Pantis, C.; Pop, M.G.; Pop, M.S.; Bondar, L.I.; Jurcau, M.C.; Babeș, K. Effects of Physical Exercise on Walking Distance and Functional Limitations in Patients with Chronic Dyspnea. Medicina 2025, 61, 636. [Google Scholar] [CrossRef]
- Leone, P.M.; Richeldi, L. Current Diagnosis and Management of Hypersensitivity Pneumonitis. Tuberc. Respir. Dis. 2020, 83, 122–131. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef]
- Ageely, G.; Souza, C.; De Boer, K.; Zahra, S.; Gomes, M.; Voduc, N. The Impact of Multidisciplinary Discussion (MDD) in the Diagnosis and Management of Fibrotic Interstitial Lung Diseases. Can. Respir. J. 2020, 2020, 9026171. [Google Scholar] [CrossRef]
- Gredic, M.; Karnati, S.; Ruppert, C.; Guenther, A.; Avdeev, S.N.; Kosanovic, D. Combined Pulmonary Fibrosis and Emphysema: When Scylla and Charybdis Ally. Cells 2023, 12, 1278. [Google Scholar] [CrossRef]
- Intra, J.; Biffi, A.; Basta, F.; Delfini, C.; Novati, N.; Zucchetti, E.; Luppi, F.; Casati, M. The Role of Serum IgG Precipitins against Six Typical Organic Antigens Involved in Hypersensitivity Pneumonitis: A 10-Year Retrospective Study of a Referral Interstitial Lung Disease Centre. Int. J. Transl. Med. 2024, 4, 381–386. [Google Scholar] [CrossRef]
- Shalmon, T.; Freund, O.; Wand, O.; Schneer, S.; Hershko, T.; Hadad, Y.; Aviram, G.; Bar-Shai, A.; Adir, Y.; Shitrit, D.; et al. Hypersensitivity pneumonitis radiologic features in interstitial lung diseases. Respir. Med. 2025, 236, 107901. [Google Scholar] [CrossRef]
- Moua, T.; Petnak, T.; Charokopos, A.; Baqir, M.; Ryu, J.H. Challenges in the Diagnosis and Management of Fibrotic Hypersensitivity Pneumonitis: A Practical Review of Current Approaches. J. Clin. Med. 2022, 11, 1473. [Google Scholar] [CrossRef]
- Sobiecka, M.; Szturmowicz, M.; Lewandowska, K.B.; Barańska, I.; Zimna, K.; Łyżwa, E.; Dybowska, M.; Langfort, R.; Radwan-Röhrenschef, P.; Roży, A.; et al. Bronchoalveolar Lavage Cell Count and Lymphocytosis Are the Important Discriminators between Fibrotic Hypersensitivity Pneumonitis and Idiopathic Pulmonary Fibrosis. Diagnostics 2023, 13, 935. [Google Scholar] [CrossRef]
- Barkas, G.I.; Daniil, Z.; Kotsiou, O.S. The Role of Small Airway Disease in Pulmonary Fibrotic Diseases. J. Pers. Med. 2023, 13, 1600. [Google Scholar] [CrossRef] [PubMed]
- Vongvivitpatana, T.S.; Nambiar, A.M. A low forced vital capacity (FVC)/diffusing capacity of the lung for carbon monoxide (DLCO) ratio increases clinical suspicion for fibrotic hypersensitivity pneumonitis (FHP) over idiopathic pulmonary fibrosis (IPF). Cureus 2024, 16, e73008. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, K.; Lewandowska, K.B.; Kowalik, A.; Bartoszuk, I.; Radwan-Röhrenschef, P.; Sobiecka, M.; Dybowska, M.; Tomkowski, W.Z.; Szturmowicz, M. Does a Type of Inciting Antigen Correlate with the Presence of Lung Fibrosis in Patients with Hypersensitivity Pneumonitis? J. Clin. Med. 2024, 13, 5074. [Google Scholar] [CrossRef]
- Lassandro, G.; Picchi, S.G.; Corvino, A.; Massimo, C.; Tamburrini, S.; Vanore, L.; Urraro, G.; Russo, G.; Lassandro, F. Noninfectious Granulomatous Lung Disease: Radiological Findings and Differential Diagnosis. J. Pers. Med. 2024, 14, 134. [Google Scholar] [CrossRef]
- Feng, N.; Yasukawa, L.L.; Sen, A.; Greenberg, H.B. Permissive replication of homologous murine rotavirus in the mouse intestine is primarily regulated by VP4 and NSP1. J. Virol. 2013, 87, 8307–8316. [Google Scholar] [CrossRef]
- Bhatta, R.; Abou-Ghaida, J.; Bhattarai, S.; Blavo, C. A Case of Immunomodulator-Responsive Hypersensitivity Pneumonitis Secondary to Chronic Passive Smoke Inhalation. Cureus 2024, 16, e58723. [Google Scholar] [CrossRef]
- Sumi, T.; Takahashi, T.; Michimata, H.; Nagayama, D.; Koshino, Y.; Watanabe, H.; Yamada, Y.; Kodama, K.; Nishikiori, H.; Chiba, H. Exacerbation of Hypersensitivity Pneumonitis Induced by COVID-19. QJM 2023, 116, 235–236. [Google Scholar] [CrossRef]
- Fukihara, J.; Kondoh, Y. COVID-19 and interstitial lung diseases: A multifaceted look at the relationship between the two diseases. Respir. Investig. 2023, 61, 601–617. [Google Scholar] [CrossRef]
- Leong, S.W.; Bos, S.; Lordan, J.L.; Nair, A.; Fisher, A.J.; Meachery, G. Lung Transplantation for Interstitial Lung Disease: Evolution over Three Decades. BMJ Open Respir. Res. 2023, 10, e001387. [Google Scholar] [CrossRef]
- Irina, B.P.; Steluta, M.M.; Emanuela, T.; Diana, M.; Cristina, O.D.; Mirela, F.; Cristian, O. Respiratory Muscle Training Program Supplemented by a Cell-Phone Application in COPD Patients with Severe Airflow Limitation. Respir. Med. 2021, 190, 106679. [Google Scholar] [CrossRef]
- Cameli, P.; Alonzi, V.; d’Alessandro, M.; Bergantini, L.; Pordon, E.; Guerrieri, M.; Refini, R.M.; Sestini, P.; Bargagli, E. The Effectiveness of Nintedanib in Patients with Idiopathic Pulmonary Fibrosis, Familial Pulmonary Fibrosis and Progressive Fibrosing Interstitial Lung Diseases: A Real-World Study. Biomedicines 2022, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Kypreos, M.; Barbera, T.; Newton, C.A.; Glazer, C.S.; Adams, T.N. Addition of antifibrotic therapy to immunosuppression in hypersensitivity pneumonitis: A case series. Respir. Med. Case Rep. 2021, 34, 101562. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mumtaz, S.; Baig, H.Z.; Mira-Avendano, I.; Wang, B.; Rojas, C.A.; Stowell, J.T.; Lesser, E.R.; Borkar, S.R.; Majithia, V.; et al. Longitudinal Study of Patients with Connective Tissue Disease–Interstitial Lung Disease and Response to Mycophenolate Mofetil and Rituximab. Diagnostics 2024, 14, 2702. [Google Scholar] [CrossRef] [PubMed]
- Tzilas, V.; Tzouvelekis, A.; Bouros, E.; Karampitsakos, T.; Ntassiou, M.; Avdoula, E.; Trachalaki, A.; Antoniou, K.; Raghu, G.; Bouros, D. Clinical experience with antifibrotics in fibrotic hypersensitivity pneumonitis: A 3-year real-life observational study. ERJ Open Res. 2020, 6, 00152–02020. [Google Scholar] [CrossRef]
- Lewandowska, K.B.; Barańska, I.; Sobiecka, M.; Radwan-Rohrenschef, P.; Dybowska, M.; Franczuk, M.; Roży, A.; Skoczylas, A.; Bestry, I.; Kuś, J.; et al. Factors Predictive for Immunomodulatory Therapy Response and Survival in Patients with Hypersensitivity Pneumonitis—Retrospective Cohort Analysis. Diagnostics 2022, 12, 2767. [Google Scholar] [CrossRef]
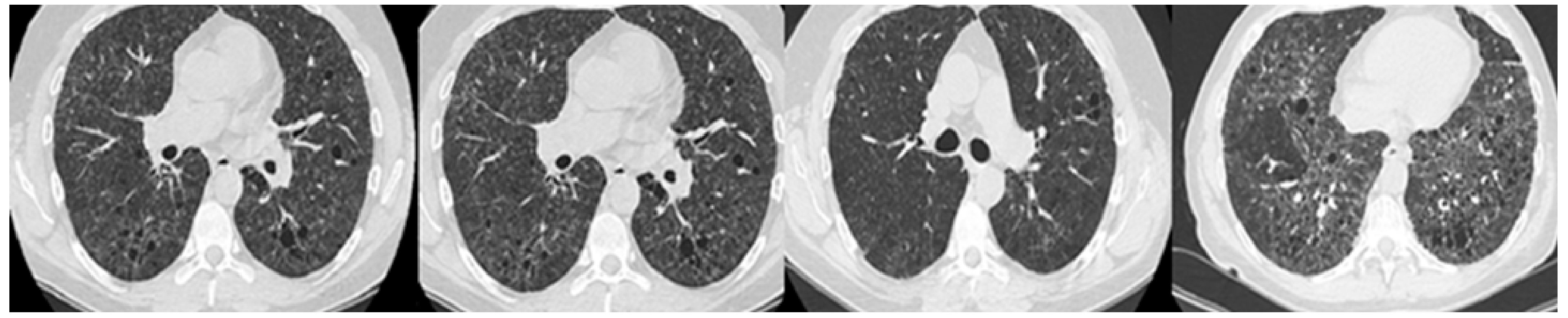
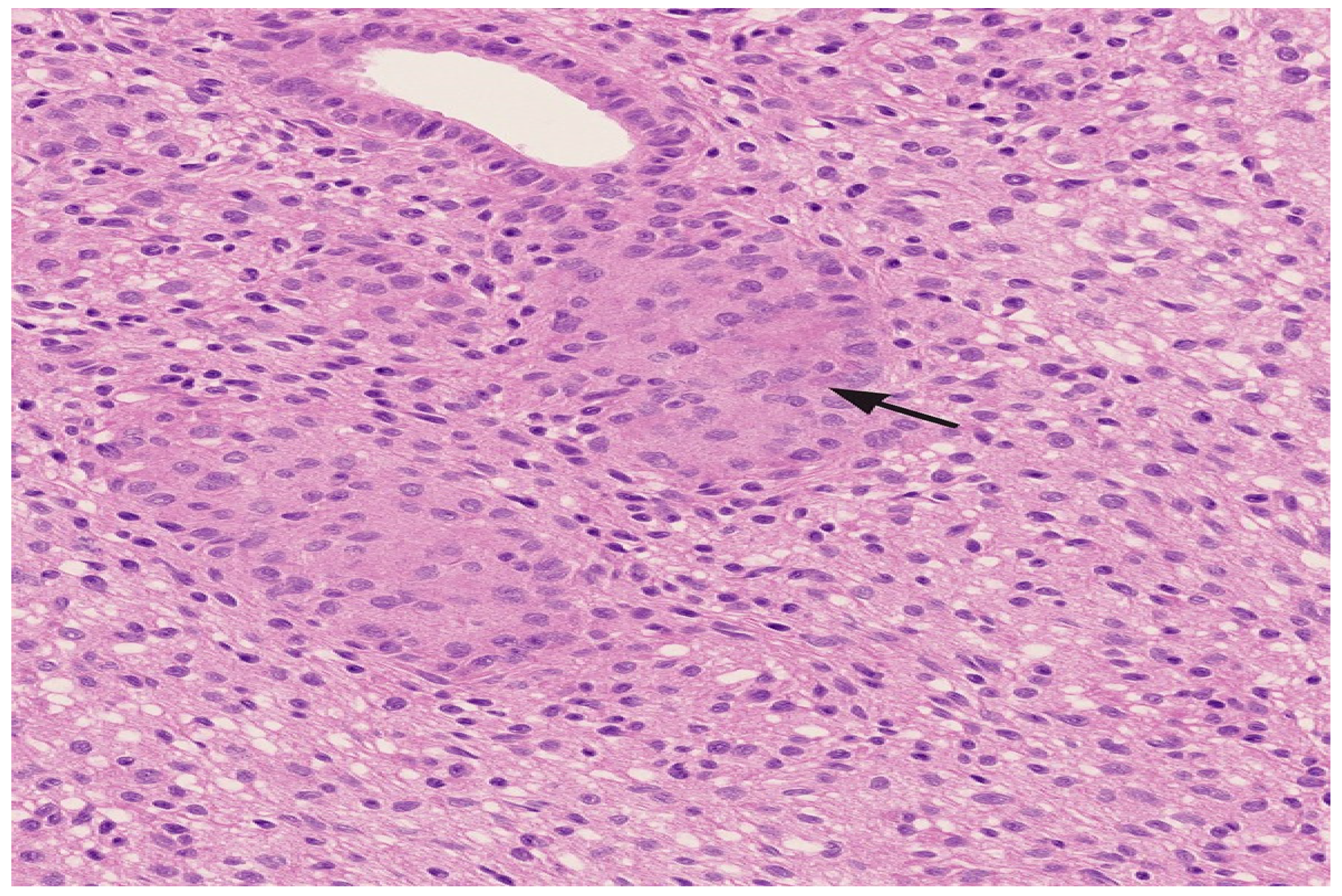
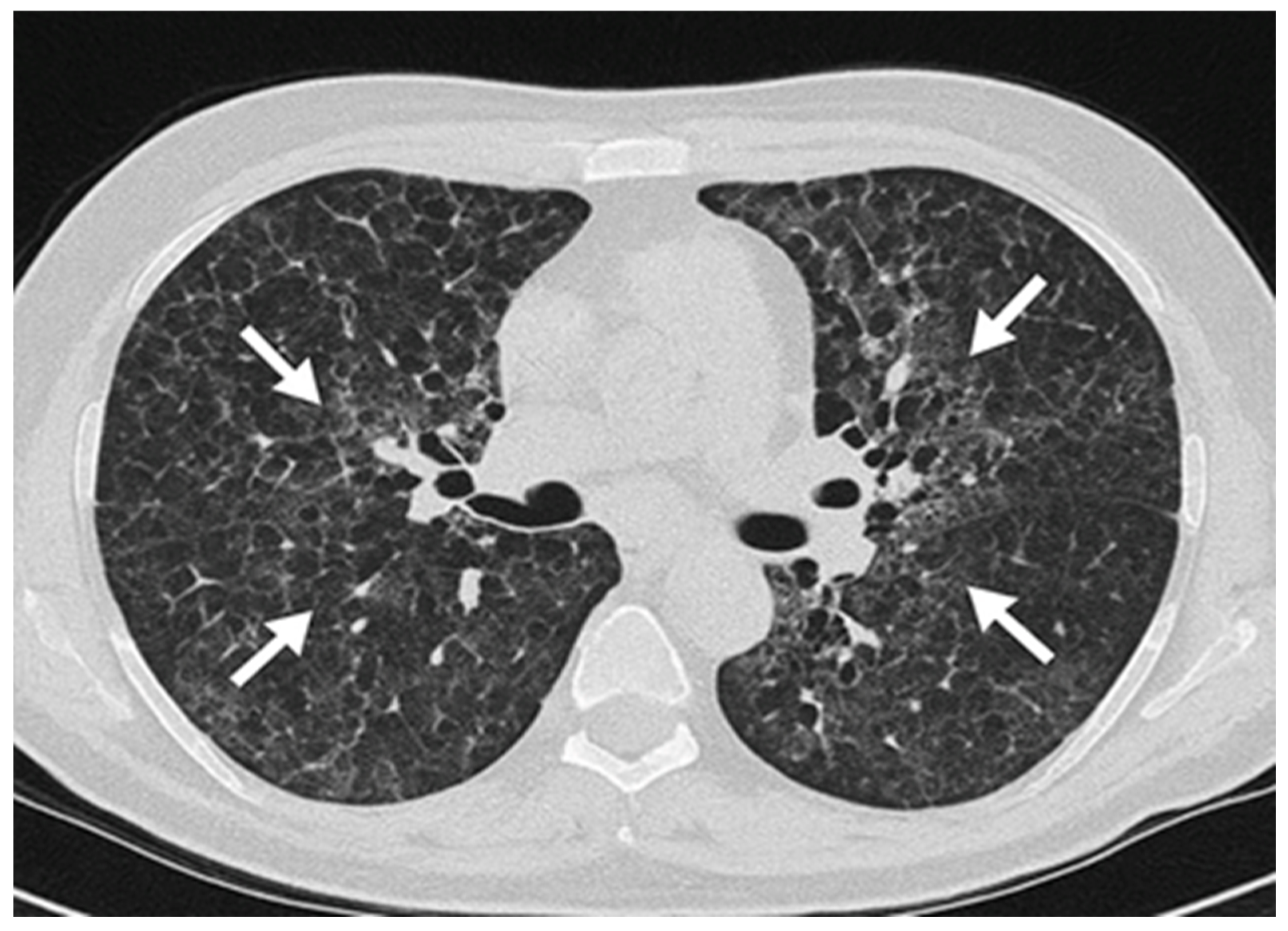
| Diffusion Capacity and Lung Volumes/Pulmonary Function Test | ||||||
|---|---|---|---|---|---|---|
| Data | FVC % | DLCO mmol/(min*kPa) | TLC L | RV/TLC % | ERV % | RV % |
| 2018 | 80 | 42 | 85 | 70 | 185 | 58 |
| 2020 | 78 | 38 | 75 | 50 | 179 | 55 |
| 2022 | 109 | 36 | 74 | 38 | 191 | 50 |
| 2024 | 93 | 32 | 75 | 62 | 177 | 49 |
| Antibody | Result | Reference Value |
|---|---|---|
| Anti-nRNP/Sm | Negative | (Negative) |
| Anti-Sm | Negative | (Negative) |
| Anti-SS-A (Ro) | Negative | (Negative) |
| Anti-Ro-52 | Negative | (Negative) |
| Anti-SS-B (La) | Negative | (Negative) |
| Anti-Scl-70 | Negative | (Negative) |
| Anti-PM-Scl 100 | Negative | (Negative) |
| Anti-Jo-1 | Negative | (Negative) |
| Anti-Centromere B | Negative | (Negative) |
| Anti-PCNA | Negative | (Negative) |
| Anti-dsDNA | Negative | (Negative) |
| Anti-Nucleosome | Negative | (Negative) |
| Anti-Histone | Negative | (Negative) |
| Anti-Ribosomal P Protein | Negative | (Negative) |
| Anti-AMA-M2 | Negative | (Negative) |
| Anti-DFS70 | Negative | (Negative) |
| CAP-Class Reference Ranges | ||
|---|---|---|
| CAP-Class | AU/mL Range | Interpretation |
| Class 0 | 0.10–0.35 | Negative |
| Class 1 | 0.35–0.70 | Threshold value |
| Class 2 | 0.70–3.50 | Weakly positive |
| Class 3 | 3.50–17.5 | Positive |
| Class 4 | 17.5–50.0 | Highly positive |
| Class 5 | 50.0–100 | Intensely positive |
| Class 6 | >100 | Exceptionally high value |
| Allergen-Specific IgE Results with CAP-Class Interpretation | ||
| Allergen | Result (AU/mL) | CAP-Class |
| Chicken feathers | <0.10 (Negative) | Class 0 |
| Chicken droppings | <0.10 (Negative) | Class 0 |
| Parameter | Value (On Room Air) |
|---|---|
| pH | 7.43 |
| PaO2 | 61 mmHg |
| PaCO2 | 36 mmHg |
| HCO3− | 23 mEq/L |
| SaO2 | 90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mot, M.-D.; Olar, D.C.; Vulciu, P.A.; Barata, P.-I.; Bouros-Tataru, A.-L.; Butari, D.B.; Șandor, F.M.; Bondar, L.I. Fibrotic Hypersensitivity Pneumonitis: A Diagnostic Challenge Leading to Lung Transplantation. Diagnostics 2025, 15, 1267. https://doi.org/10.3390/diagnostics15101267
Mot M-D, Olar DC, Vulciu PA, Barata P-I, Bouros-Tataru A-L, Butari DB, Șandor FM, Bondar LI. Fibrotic Hypersensitivity Pneumonitis: A Diagnostic Challenge Leading to Lung Transplantation. Diagnostics. 2025; 15(10):1267. https://doi.org/10.3390/diagnostics15101267
Chicago/Turabian StyleMot, Maria-Daniela, Dana Cristina Olar, Paula Alexandra Vulciu, Paula-Irina Barata, Ana-Liana Bouros-Tataru, Denis Bogdan Butari, Florin Mihai Șandor, and Laura Ioana Bondar. 2025. "Fibrotic Hypersensitivity Pneumonitis: A Diagnostic Challenge Leading to Lung Transplantation" Diagnostics 15, no. 10: 1267. https://doi.org/10.3390/diagnostics15101267
APA StyleMot, M.-D., Olar, D. C., Vulciu, P. A., Barata, P.-I., Bouros-Tataru, A.-L., Butari, D. B., Șandor, F. M., & Bondar, L. I. (2025). Fibrotic Hypersensitivity Pneumonitis: A Diagnostic Challenge Leading to Lung Transplantation. Diagnostics, 15(10), 1267. https://doi.org/10.3390/diagnostics15101267