Spectralis Optical Coherence Tomography for Evaluating Ocular Hypertensive and Glaucoma Suspect Eyes: Real-World Data from Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Spectralis OCT Imaging
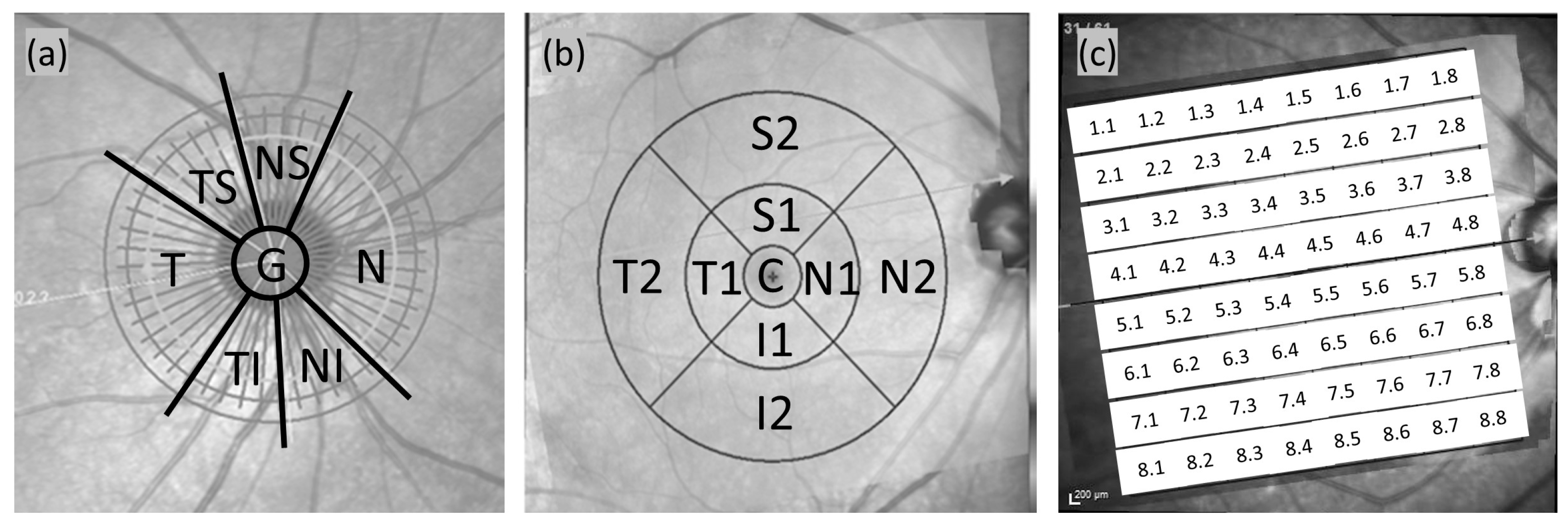
2.3. Statistical Analysis
3. Results
Demographic and Clinical Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults-Screening, Diagnosis, and Management: A Review. JAMA 2021, 325, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A.; Zangwill, L.M.; Bowd, C.; Mansouri, K.; Weinreb, R.N. The structure and function relationship in glaucoma: Implications for detection of progression and measurement of rates of change. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6939–6946. [Google Scholar] [CrossRef] [PubMed]
- Oddone, F.; Lucenteforte, E.; Michelessi, M.; Rizzo, S.; Donati, S.; Parravano, M.; Virgili, G. Macular versus Retinal Nerve Fiber Layer Parameters for Diagnosing Manifest Glaucoma: A Systematic Review of Diagnostic Accuracy Studies. Ophthalmology 2016, 123, 939–949. [Google Scholar] [CrossRef]
- Gardiner, S.K.; Ren, R.; Yang, H.; Fortune, B.; Burgoyne, C.F.; Demirel, S. A method to estimate the amount of neuroretinal rim tissue in glaucoma: Comparison with current methods for measuring rim area. Am. J. Ophthalmol. 2014, 157, 540–549.e542. [Google Scholar] [CrossRef]
- Chauhan, B.C.; Burgoyne, C.F. From clinical examination of the optic disc to clinical assessment of the optic nerve head: A paradigm change. Am. J. Ophthalmol. 2013, 156, 218–227.e2. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.C.; Danthurebandara, V.M.; Sharpe, G.P.; Demirel, S.; Girkin, C.A.; Mardin, C.Y.; Scheuerle, A.F.; Burgoyne, C.F. Bruch’s membrane opening minimum rim width and retinal nerve fiber layer thickness in a normal white population: A multicenter study. Ophthalmology 2015, 122, 1786–1794. [Google Scholar] [CrossRef]
- Asrani, S.; Rosdahl, J.A.; Allingham, R.R. Novel software strategy for glaucoma diagnosis: Asymmetry analysis of retinal thickness. Arch. Ophthalmol. 2011, 129, 1205–1211. [Google Scholar] [CrossRef]
- Gordon, M.O.; Beiser, J.A.; Brandt, J.D.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 714–720. [Google Scholar] [CrossRef]
- Lalezary, M.; Medeiros, F.A.; Weinreb, R.N.; Bowd, C.; Sample, P.A.; Tavares, I.M.; Tafreshi, A.; Zangwill, L.M. Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am. J. Ophthalmol. 2006, 142, 576–582.e1. [Google Scholar] [CrossRef]
- Keltner, J.L.; Johnson, C.A.; Anderson, D.R.; Levine, R.A.; Fan, J.; Cello, K.E.; Quigley, H.A.; Budenz, D.L.; Parrish, R.K.; Kass, M.A. The association between glaucomatous visual fields and optic nerve head features in the Ocular Hypertension Treatment Study. Ophthalmology 2006, 113, 1603–1612. [Google Scholar] [CrossRef]
- Garway-Heath, D.F.; Ruben, S.T.; Viswanathan, A.; Hitchings, R.A. Vertical cup/disc ratio in relation to optic disc size: Its value in the assessment of the glaucoma suspect. Br. J. Ophthalmol. 1998, 82, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.T.; Singh, K. Glaucoma Suspect: Diagnosis and Management. Asia-Pac. J. Ophthalmol. 2016, 5, 32–37. [Google Scholar] [CrossRef]
- Li, A.; Thompson, A.C.; Asrani, S. Impact of Artifacts From Optical Coherence Tomography Retinal Nerve Fiber Layer and Macula Scans on Detection of Glaucoma Progression. Am. J. Ophthalmol. 2021, 221, 235–245. [Google Scholar] [CrossRef]
- Wu, C.W.; Chen, H.Y.; Chen, J.Y.; Lee, C.H. Glaucoma Detection Using Support Vector Machine Method Based on Spectralis OCT. Diagnostics 2022, 12, 391. [Google Scholar] [CrossRef]
- Jeoung, J.W.; Kim, T.-W.; Weinreb, R.N.; Kim, S.H.; Park, K.H.; Kim, D.M. Diagnostic ability of spectral-domain versus time-domain optical coherence tomography in preperimetric glaucoma. J. Glaucoma 2014, 23, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical coherence tomography (OCT): Principle and technical realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Springer: Cham, Switzerland, 2019; pp. 59–85. [Google Scholar]
- Banister, K.; Boachie, C.; Bourne, R.; Cook, J.; Burr, J.M.; Ramsay, C.; Garway-Heath, D.; Gray, J.; McMeekin, P.; Hernández, R. Can automated imaging for optic disc and retinal nerve fiber layer analysis aid glaucoma detection? Ophthalmology 2016, 123, 930–938. [Google Scholar] [CrossRef]
- Tian, J.; Varga, B.; Tatrai, E.; Fanni, P.; Somfai, G.M.; Smiddy, W.E.; Debuc, D.C. Performance evaluation of automated segmentation software on optical coherence tomography volume data. J. Biophotonics 2016, 9, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Dyrda, A.A.; Biarnés, M.; Gómez, A.; Martín, C.; Mora, C.; Fatti, G.; Antón, A. Diagnostic accuracy of spectralis SD OCT automated macular layers segmentation to discriminate normal from early glaucomatous eyes. Ophthalmology 2017, 124, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Schuman, J.S.; Pedut-Kloizman, T.; Hertzmark, E.; Hee, M.R.; Wilkins, J.R.; Coker, J.G.; Puliafito, C.A.; Fujimoto, J.G.; Swanson, E.A. Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology 1996, 103, 1889–1898. [Google Scholar] [CrossRef]
- Nakano, N.; Hangai, M.; Nakanishi, H.; Mori, S.; Nukada, M.; Kotera, Y.; Ikeda, H.O.; Nakamura, H.; Nonaka, A.; Yoshimura, N. Macular ganglion cell layer imaging in preperimetric glaucoma with speckle noise–reduced spectral domain optical coherence tomography. Ophthalmology 2011, 118, 2414–2426. [Google Scholar] [CrossRef]
- Kim, E.K.; Park, H.-Y.L.; Park, C.K. Segmented inner plexiform layer thickness as a potential biomarker to evaluate open-angle glaucoma: Dendritic degeneration of retinal ganglion cell. PLoS ONE 2017, 12, e0182404. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, P.; Hohberger, B.; Lämmer, R.; Mardin, C. Extended Ganglion Cell Layer Thickness Deviation Maps With OCT in Glaucoma Diagnosis. Front. Med. 2021, 8, 684676. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Canorea, P.; Ruiz-Medrano, J.; Gutierrez-Bonet, R.; Peña-Garcia, P.; Saenz-Frances, F.; Garcia-Feijoo, J.; Martinez-de-la-Casa, J.M. Analysis of inner and outer retinal layers using spectral domain optical coherence tomography automated segmentation software in ocular hypertensive and glaucoma patients. PLoS ONE 2018, 13, e0196112. [Google Scholar] [CrossRef]
- Edlinger, F.S.; Schrems-Hoesl, L.M.; Mardin, C.Y.; Laemmer, R.; Kruse, F.E.; Schrems, W.A. Structural changes of macular inner retinal layers in early normal-tension and high-tension glaucoma by spectral-domain optical coherence tomography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1245–1256. [Google Scholar] [CrossRef]
- Zeimer, R.; Asrani, S.; Zou, S.; Quigley, H.; Jampel, H. Quantitative detection of glaucomatous damage at the posterior pole by retinal thickness mapping: A pilot study. Ophthalmology 1998, 105, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.; Challa, P.; Herndon, L.; Lee, P.; Stinnett, S.; Allingham, R.R. Correlation among retinal thickness, optic disc, and visual field in glaucoma patients and suspects: A pilot study. J. Glaucoma 2003, 12, 119–128. [Google Scholar] [CrossRef]
- Salgarello, T.; Colotto, A.; Valente, P.; Petrocelli, G.; Galan, M.E.; Scullica, L.; Falsini, B. Posterior pole retinal thickness in ocular hypertension and glaucoma: Early changes detected by hemispheric asymmetries. J. Glaucoma 2005, 14, 375–383. [Google Scholar] [CrossRef]
- Silverman, A.L.; Hammel, N.; Khachatryan, N.; Sharpsten, L.; Medeiros, F.A.; Girkin, C.A.; Liebmann, J.M.; Weinreb, R.N.; Zangwill, L.M. Diagnostic accuracy of the spectralis and cirrus reference databases in differentiating between healthy and early glaucoma eyes. Ophthalmology 2016, 123, 408–414. [Google Scholar] [CrossRef]
- Diekmann, T.; Schrems-Hoesl, L.M.; Mardin, C.Y.; Laemmer, R.; Horn, F.K.; Kruse, F.E.; Schrems, W.A. Predictive factors for visual field conversion: Comparison of scanning laser polarimetry and optical coherence tomography. J. Glaucoma 2018, 27, 157–163. [Google Scholar] [CrossRef]
- Poon, L.Y.-C.; Antar, H.; Tsikata, E.; Guo, R.; Papadogeorgou, G.; Freeman, M.; Khoueir, Z.; Lee, R.; Shieh, E.; Simavli, H. Effects of age, race, and ethnicity on the optic nerve and peripapillary region using spectral-domain OCT 3D volume scans. Transl. Vis. Sci. Technol. 2018, 7, 12. [Google Scholar] [CrossRef]
- Khanal, S.; Thapa, M.; Racette, L.; Johnson, R.; Davey, P.G.; Joshi, M.R.; Shrestha, G.S. Retinal nerve fiber layer thickness in glaucomatous Nepalese eyes and its relation with visual field sensitivity. J. Optom. 2014, 7, 217–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, C.W.; Chang, Y.C.; Chen, H.Y. Early Detection of Primary Open Angle, Angle Closure, and Normal Tension Glaucoma in an Asian Population Using Optical Coherence Tomography. J. Glaucoma 2023, 32, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Yusof, A.M.Z.; Othman, O.; Tang, S.F.; Hassan, M.R.; Din, N.M. Diagnostic evaluation of optical coherence tomography parameters in normal, preperimetric and perimetric glaucoma patients. Int. J. Ophthalmol. 2022, 15, 1782–1790. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, K.H.; Jeoung, J.W. False-positive classification and associated factors in segmented macular layers and retinal nerve fiber layer analysis: Spectralis OCT deviation map study. Sci. Rep. 2023, 13, 6782. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.K.-S.; Mohamed, S.; Leung, K.S.; Cheung, C.Y.-L.; Chan, S.L.; Cheng, D.K.; Lee, A.K.; Leung, G.Y.; Rao, S.K.; Lam, D.S.C. Retinal nerve fiber layer measurements in myopia: An optical coherence tomography study. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5171–5176. [Google Scholar] [CrossRef]
- Alasil, T.; Wang, K.; Keane, P.A.; Lee, H.; Baniasadi, N.; de Boer, J.F.; Chen, T.C. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J. Glaucoma 2013, 22, 532–541. [Google Scholar] [CrossRef]
- Perez, C.I.; Chansangpetch, S.; Mora, M.; Nguyen, A.; Zhao, J.; Han, Y.; Lin, S.C. Ethnicity-specific database improves the diagnostic ability of peripapillary retinal nerve fiber layer thickness to detect glaucoma. Am. J. Ophthalmol. 2021, 221, 311–322. [Google Scholar] [CrossRef]

| Features | Healthy Controls (n = 393; 742 Eyes) | OH (n = 139; 258 Eyes) | GS (n = 208; 380 Eyes) | ||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | p | Mean ± SD | p | |
| Age (years) a | 51.77 ± 16.22 | 41.48 ± 16.06 | <0.001 | 45.21 ± 15.29 | <0.001 |
| Sex (male/female) b | 149:244 | 52:87 | 1.000 | 99:109 | 0.024 |
| Refraction (D) a | −2.31 ± 3.22 | −4.79 ± 3.66 | <0.001 | −3.87 ± 3.50 | <0.001 |
| MD (dB) a | −1.47 ± 3.29 | −1.10 ± 1.75 | 0.099 | −1.24 ± 2.63 | 0.384 |
| PSD (dB) a | 2.60 ± 2.25 | 2.02 ± 1.26 | <0.001 | 2.11 ± 1.38 | 0.001 |
| Scan | Best Parameter | Thickness (µM) (Mean ± SD) | p * | AUC (95% CI) | Sensitivity at 95% Specificity (%) | Sensitivity at 80% Specificity (%) | |
|---|---|---|---|---|---|---|---|
| OH | Control | ||||||
| RNFL | Temporal (T) | 85.14 ± 18.68 | 84.51 ± 32.73 | 0.790 | 0.538 (0.497, 0.580) | 4.2 | 24.7 |
| BMO-MRW | Temporal (T) | 222.60 ± 46.31 | 220.39 ± 53.23 | 0.681 | 0.535 (0.495, 0.575) | 2.7 | 20.5 |
| ETDRS | |||||||
| RETINA | Outer superior (S2) | 299.00 ± 16.03 | 295.30 ± 15.62 | 0.020 | 0.566 (0.525, 0.606) | 10.4 | 28.2 |
| NFL | Outer temporal (T2) | 19.45 ± 4.74 | 19.59 ± 2.72 | 0.657 | 0.611 (0.570, 0.653) | 3.9 | 21.6 |
| GCL | Outer inferior (I2) | 32.42 ± 3.69 | 32.06 ± 3.93 | 0.431 | 0.540 (0.499, 0.582) | 8.9 | 27.0 |
| IPL | Outer inferior (I2) | 27.07 ± 3.08 | 26.58 ± 3.20 | 0.109 | 0.566 (0.524, 0.607) | 9.3 | 32.0 |
| PPAA | RAT_18 | 0.285 ± 0.02 | 0.292 ± 0.02 | 0.008 | 0.578 (0.538, 0.617) | 7.4 | 24.5 |
| RAT_74 | 0.295 ± 0.02 | 0.291 ± 0.02 | 0.025 | 0.568 (0.527, 0.609) | 9.3 | 26.3 | |
| RAT_82 | 0.240 ± 0.01 | 0.237 ± 0.01 | 0.013 | 0.568 (0.526, 0.609) | 10.1 | 28.3 | |
| Scan | Best Parameter | Thickness (µM) (Mean ± SD) | p * | AUC (95% CI) | Sensitivity at 95% Specificity (%) | Sensitivity at 80% Specificity (%) | |
|---|---|---|---|---|---|---|---|
| GS | Control | ||||||
| RNFL | Temporal inferior (TI) | 152.27 ± 25.05 | 160.52 ± 30.59 | <0.001 | 0.591 (0.556, 0.626) | 8.4 | 30.7 |
| BMO-MRW | Mean global (G) | 249.84 ± 43.88 | 294.63 ± 58.35 | <0.001 | 0.737 (0.707, 0.767) | 17.3 | 49.6 |
| ETDRS | |||||||
| RETINA | Inner inferior (I1) | 330.78 ± 16.60 | 332.57 ± 17.31 | 0.184 | 0.520 (0.485, 0.555) | 7.3 | 23.6 |
| NFL | Outer temporal (T2) | 19.35 ± 3.37 | 19.59 ± 2.72 | 0.287 | 0.558 (0.523, 0.594) | 3.4 | 14.2 |
| GCL | Outer superior (S2) | 34.45 ± 3.45 | 35.16 ± 3.92 | 0.015 | 0.552 (0.517, 0.587) | 8.9 | 25.5 |
| IPL | Outer temporal (T2) | 31.81 ± 2.74 | 32.24 ± 2.95 | 0.054 | 0.544 (0.509, 0.579) | 5.0 | 29.4 |
| PPAA | RAT_28 | 0.312 ± 0.02 | 0.316 ± 0.02 | 0.074 | 0.543 (0.507, 0.578) | 8.2 | 24.0 |
| Subtypes | Parameters Included | AUC (95% CI) | p-Value |
|---|---|---|---|
| OH | Model 1I: age, refraction, MRW (temporal), RETINA (outer superior) | 0.694 (0.658, 0.730) | |
| Model 1: age, refraction, MRW (temporal) | 0.694 (0.658, 0.730) | ||
| Model 0: age, refraction | 0.694 (0.658, 0.730) | ||
| GS | Model II: age, refraction, MRW (mean global), RNFL (temporal inferior) | 0.643 (0.609, 0.676) | <0.001 b |
| Model I: age, refraction, MRW (mean global) | 0.646 (0.613, 0.679) | <0.001 a | |
| Model 0: age, refraction | 0.630 (0.596, 0.664) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, M.-S.; Wu, C.-W.; Chang, Y.-C.; Chen, H.-Y. Spectralis Optical Coherence Tomography for Evaluating Ocular Hypertensive and Glaucoma Suspect Eyes: Real-World Data from Taiwan. Diagnostics 2025, 15, 1256. https://doi.org/10.3390/diagnostics15101256
Wong M-S, Wu C-W, Chang Y-C, Chen H-Y. Spectralis Optical Coherence Tomography for Evaluating Ocular Hypertensive and Glaucoma Suspect Eyes: Real-World Data from Taiwan. Diagnostics. 2025; 15(10):1256. https://doi.org/10.3390/diagnostics15101256
Chicago/Turabian StyleWong, Man-Sze, Chao-Wei Wu, Yue-Cune Chang, and Hsin-Yi Chen. 2025. "Spectralis Optical Coherence Tomography for Evaluating Ocular Hypertensive and Glaucoma Suspect Eyes: Real-World Data from Taiwan" Diagnostics 15, no. 10: 1256. https://doi.org/10.3390/diagnostics15101256
APA StyleWong, M.-S., Wu, C.-W., Chang, Y.-C., & Chen, H.-Y. (2025). Spectralis Optical Coherence Tomography for Evaluating Ocular Hypertensive and Glaucoma Suspect Eyes: Real-World Data from Taiwan. Diagnostics, 15(10), 1256. https://doi.org/10.3390/diagnostics15101256





