Screening Blood and Vitreous for Biomarkers Associated with Proliferative Diabetic Retinopathy
Abstract
1. Introduction
2. Materials and Methods
3. Results
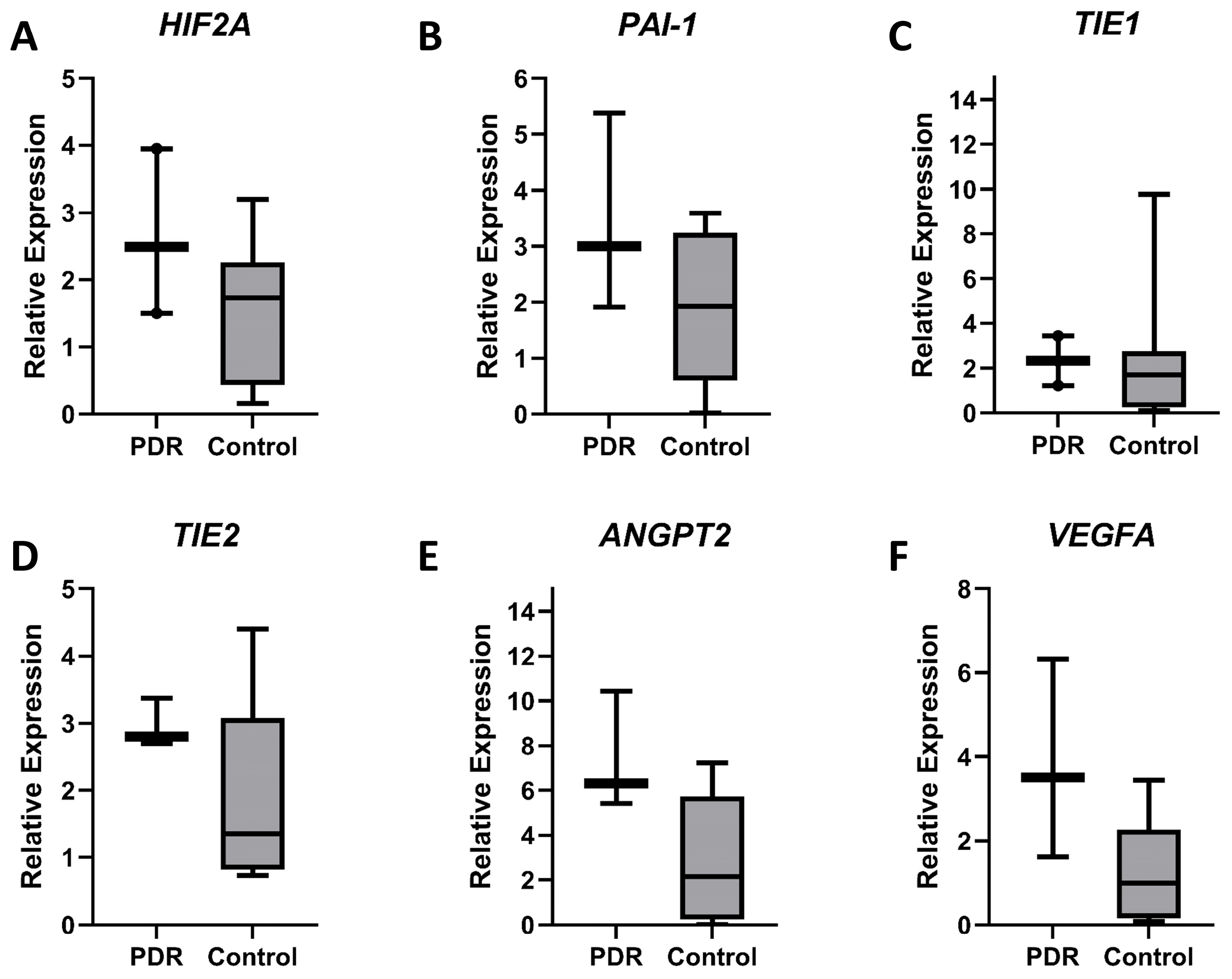
3.1. Vitreous Gene Expression Analysis
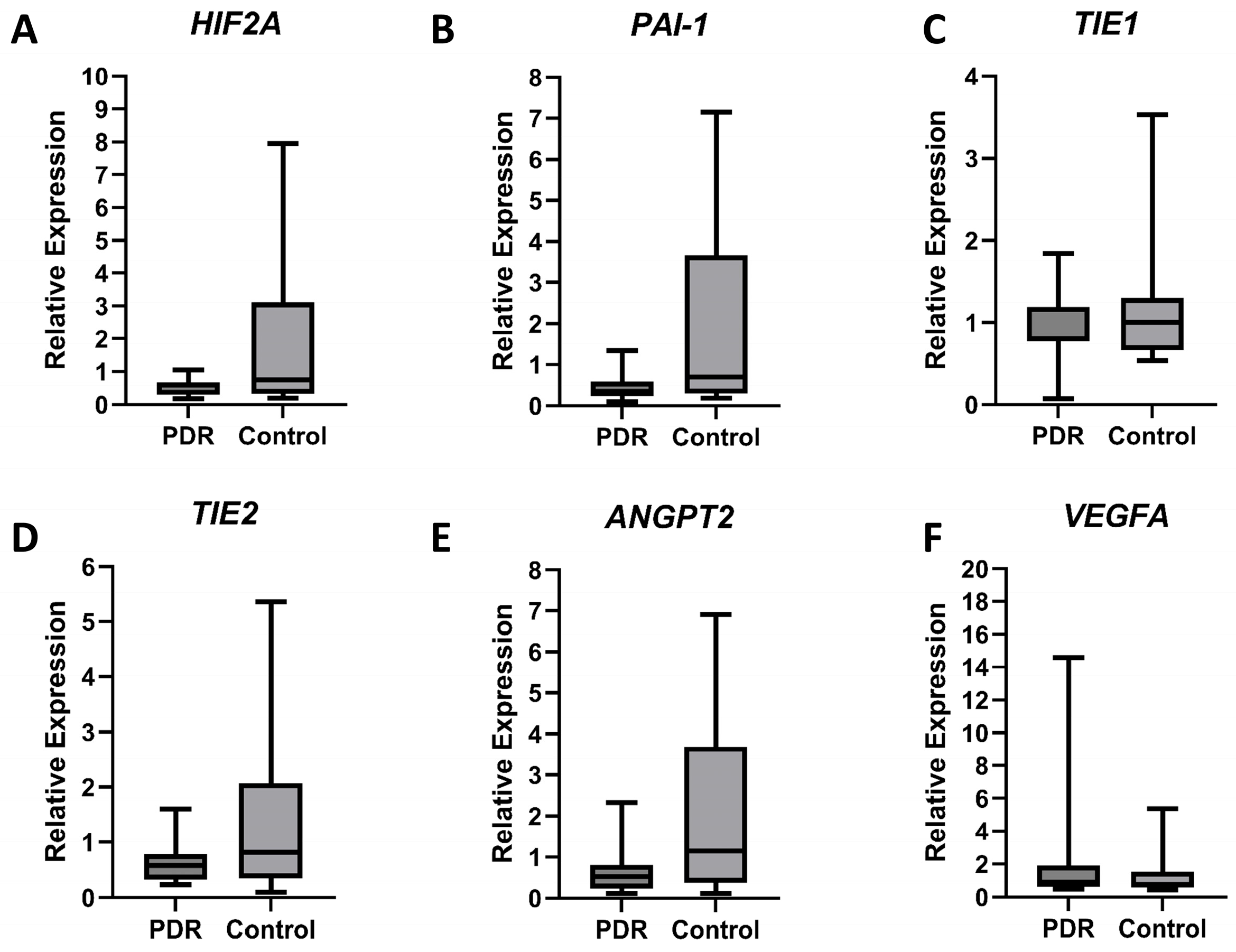
3.2. Blood Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDR | Proliferative diabetic retinopathy |
| NS | Not significant |
| mRNA | Messenger ribonucleic acid |
References
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ogura, A.; Ishikawa, K.; Yoshida, A.; Kohno, R.; Yamaji, Y.; Ikeo, K.; Gojobori, T.; Kono, T.; Ishibashi, T. Gene expression profile of fibrovascular membranes from patients with proliferative diabetic retinopathy. Br. J. Ophthalmol. 2010, 94, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; He, S.; Ye, Z.; Zhu, X.; Sun, W.; Fu, W.; Ma, T.; Li, Z. Identification of potential molecular targets associated with proliferative diabetic retinopathy. BMC Ophthalmol. 2020, 20, 143. [Google Scholar] [CrossRef]
- Durham, J.T.; Herman, I.M. Microvascular modifications in diabetic retinopathy. Curr. Diab. Rep. 2011, 11, 253–264. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Bressler, N.M.; Beck, R.W.; Ferris, F.L., 3rd. Panretinal photocoagulation for proliferative diabetic retinopathy. N. Engl. J. Med. 2011, 365, 1520–1526. [Google Scholar] [CrossRef]
- Wu, S.L.; Zhan, D.M.; Xi, S.H.; He, X.L. Roles of tissue plasminogen activator and its inhibitor in proliferative diabetic retinopathy. Int. J. Ophthalmol. 2014, 7, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, J.; Babapoor-Farrokhran, S.; Applewhite, B.; Deshpande, M.; Megarity, H.; Flores-Bellver, M.; Aparicio-Domingo, S.; Ma, T.; Rui, Y.; et al. PAI-1 is a vascular cell-specific HIF-2-dependent angiogenic factor that promotes retinal neovascularization in diabetic patients. Sci. Adv. 2022, 8, eabm1896. [Google Scholar] [CrossRef]
- Basu, A.; Menicucci, G.; Maestas, J.; Das, A.; McGuire, P. Plasminogen activator inhibitor-1 (PAI-1) facilitates retinal angiogenesis in a model of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4974–4981. [Google Scholar] [CrossRef]
- Nawaz, I.M.; Rezzola, S.; Cancarini, A.; Russo, A.; Costagliola, C.; Semeraro, F.; Presta, M. Human vitreous in proliferative diabetic retinopathy: Characterization and translational implications. Prog. Retin. Eye Res. 2019, 72, 100756. [Google Scholar] [CrossRef]
- Ponsioen, T.L.; Hooymans, J.M.; Los, L.I. Remodelling of the human vitreous and vitreoretinal interface—A dynamic process. Prog. Retin. Eye Res. 2010, 29, 580–595. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A.; Toth, L.Z.; Eckl, E.A.; Mitchell, A.P. Studies on the structure of the vitreous body. xii. cytological and histochemical studies on the cortical tissue layer. Exp. Eye Res. 1964, 3, 57–71. [Google Scholar] [CrossRef] [PubMed]
- El-Ghrably, I.A.; Dua, H.S.; Orr, G.M.; Fischer, D.; Tighe, P.J. Detection of cytokine mRNA production in infiltrating cells in proliferative vitreoretinopathy using reverse transcription polymerase chain reaction. Br. J. Ophthalmol. 1999, 83, 1296–1299. [Google Scholar] [CrossRef]
- Maranian, M.; Snead, M. A novel transcriptome approach to the investigation of the molecular pathology of vitreous and retinal detachment. Genes 2022, 13, 1885. [Google Scholar] [CrossRef]
- Gärtner, J. The fine structure of the vitreous base of the human eye and pathogenesis of pars planitis. Am. J. Ophthalmol. 1971, 71, 1317–1327. [Google Scholar] [CrossRef]
- Gärtner, J. Electron-microscopic study on the fibrillar network and fibrocyte--collagen interactions in the vitreous cortex at the ora serrata of human eyes with special regard to the role of disintegrating cells. Exp. Eye Res. 1986, 42, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, G.; Wang, Y. Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am. J. Ophthalmol. 2009, 148, 883–889. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Heier, J.S.; Do, D.V.; Mirando, A.C.; Pandey, N.B.; Sheng, H.; Heah, T. The Tie2 signaling pathway in retinal vascular diseases: A novel therapeutic target in the eye. Int. J. Retin. Vitr. 2020, 6, 48. [Google Scholar] [CrossRef]
- Cai, J.; Kehoe, O.; Smith, G.M.; Hykin, P.; Boulton, M.E. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2163–2171. [Google Scholar] [CrossRef]
- Joussen, A.M.; Ricci, F.; Paris, L.P.; Korn, C.; Quezada-Ruiz, C.; Zarbin, M. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: A review of preclinical data. Eye 2021, 35, 1305–1316. [Google Scholar] [CrossRef]
- Dan-Brezis, I.; Zahavi, A.; Axer-Siegel, R.; Nisgav, Y.; Dahbash, M.; Weinberger, D.; Ehrlich, R.; Livnat, T. Inflammation, angiogenesis and coagulation interplay in a variety of retinal diseases. Acta Ophthalmol. 2020, 98, e559–e562. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Zhang, J.; Sharma, D.; Dinabandhu, A.; Sanchez, J.; Applewhite, B.; Jee, K.; Deshpande, M.; Flores-Bellver, M.; Hu, M.W.; Guo, C.; et al. Targeting hypoxia-inducible factors with 32-134D safely and effectively treats diabetic eye disease in mice. J. Clin. Investig. 2023, 133, e163290. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Mansoor, S.; Sharma, A.; Sapkal, A.; Sheth, J.; Falatoonzadeh, P.; Kuppermann, B.; Kenney, M. Diabetic retinopathy and VEGF. Open Ophthalmol. J. 2013, 7, 4–10. [Google Scholar] [CrossRef]
- Mowat, F.M.; Luhmann, U.F.; Smith, A.J.; Lange, C.; Duran, Y.; Harten, S.; Shukla, D.; Maxwell, P.H.; Ali, R.R.; Bainbridge, J.W. HIF-1alpha and HIF-2alpha are differentially activated in distinct cell populations in retinal ischaemia. PLoS ONE 2010, 5, e11103. [Google Scholar] [CrossRef] [PubMed]
- McMullin, M.F. HIF pathway mutations and erythrocytosis. Expert Rev. Hematol. 2010, 3, 93–101. [Google Scholar] [CrossRef]
- Zhou, A.; Huntington, J.A.; Pannu, N.S.; Carrell, R.W.; Read, R.J. How vitronectin binds PAI-1 to modulate fibrinolysis and cell migration. Nat. Struct. Biol. 2003, 10, 541–544. [Google Scholar] [CrossRef]
- Ambati, B.K.; Ambati, J.; Singh, N. Modulation of Angiogenesis. U.S. Patent 9,198,981, 1 December 2015. Available online: https://uknowledge.uky.edu/ophthalmology_patents/11 (accessed on 26 February 2025).
- Arrigo, A.; Aragona, E.; Bandello, F. VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann. Med. 2022, 54, 1089–1111. [Google Scholar] [CrossRef]
- Matsunaga, N.; Chikaraishi, Y.; Izuta, H.; Ogata, N.; Shimazawa, M.; Matsumura, M.; Hara, H. Role of soluble vascular endothelial growth factor receptor-1 in the vitreous in proliferative diabetic retinopathy. Ophthalmology 2008, 115, 1916–1922. [Google Scholar] [CrossRef]
- Zhao, Y.; Singh, R.P. The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. Drugs Context 2018, 7, 212532. [Google Scholar] [CrossRef]
- Gong, Q.Y.; Hu, G.Y.; Yu, S.Q.; Qian, T.W.; Xu, X. Comprehensive assessment of growth factors, inflammatory mediators, and cytokines in vitreous from patients with proliferative diabetic retinopathy. Int. J. Ophthalmol. 2022, 15, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, J.; Castro-de-Sousa, J.P.; Vaz-Pereira, S.; Neves, A.; Passarinha, L.A.; Tomaz, C.T. Evaluation of the growth factors VEGF-a and VEGF-B in the vitreous and serum of patients with macular and retinal vascular diseases. Growth Factors 2018, 36, 48–57. [Google Scholar] [CrossRef] [PubMed]
| HIF2α_F | 5′ GATGACAGAATCACAGAACTGATTGG 3′ |
| HIF2α_R | 5′ GCACAAGTTCTGGTGACTCTTG 3′ |
| PAI-1_F | 5′ AGATTGATGACAAGGGCATGGC 3′ |
| PAI-1_R | 5′ GAAGATCGCGTCTGTGGTGC 3′ |
| TIE1_F | 5′ GGAAGAGCAACGGATCCTACT 3′ |
| TIE1_R | 5′ TAGATGCCGCTCGATGGTGG 3′ |
| TIE2_F | 5′ ATAGTCCGGAGATGTGAAGCC 3′ |
| TIE2_R | 5′ GCATTCTCCAGTATCTTCATGGCA 3′ |
| ANGPT2_F | 5′ TGGAAGCTGGAGGAGGCG 3′ |
| ANGPT2_R | 5′ TGAAGGGTTACCAAATCCCACT 3′ |
| VEGFA_F | 5′ ACCTCCACCATGCCAAGTGG 3′ |
| VEGFA_R | 5′ GTAGCTGCGCTGATAGACATCC 3′ |
| ACTB_F | 5′ CTCTTCCAGCCTTCCTTCCT 3′ |
| ACTB_R | 5′ AGCACTGTGTTGGCGTACAG 3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richard, S.; Kharouba, R.; Abu-dbai, J.; Gagarin, O.; Kratz, A.; Obied, B.; Zahavi, A.; Goldenberg-Cohen, N. Screening Blood and Vitreous for Biomarkers Associated with Proliferative Diabetic Retinopathy. Diagnostics 2025, 15, 1344. https://doi.org/10.3390/diagnostics15111344
Richard S, Kharouba R, Abu-dbai J, Gagarin O, Kratz A, Obied B, Zahavi A, Goldenberg-Cohen N. Screening Blood and Vitreous for Biomarkers Associated with Proliferative Diabetic Retinopathy. Diagnostics. 2025; 15(11):1344. https://doi.org/10.3390/diagnostics15111344
Chicago/Turabian StyleRichard, Stephen, Rawan Kharouba, Jawad Abu-dbai, Oksana Gagarin, Assaf Kratz, Basel Obied, Alon Zahavi, and Nitza Goldenberg-Cohen. 2025. "Screening Blood and Vitreous for Biomarkers Associated with Proliferative Diabetic Retinopathy" Diagnostics 15, no. 11: 1344. https://doi.org/10.3390/diagnostics15111344
APA StyleRichard, S., Kharouba, R., Abu-dbai, J., Gagarin, O., Kratz, A., Obied, B., Zahavi, A., & Goldenberg-Cohen, N. (2025). Screening Blood and Vitreous for Biomarkers Associated with Proliferative Diabetic Retinopathy. Diagnostics, 15(11), 1344. https://doi.org/10.3390/diagnostics15111344






