Integrating Dynamic Red Blood Cell Distribution Width Monitoring and β-Blocker Therapy for Mortality Prediction in Intensive Care Unit Cardiomyopathy Patients: A Bayesian Multivariate Joint Model and Machine Learning Study
Abstract
1. Introduction
2. Methods
2.1. Data Source
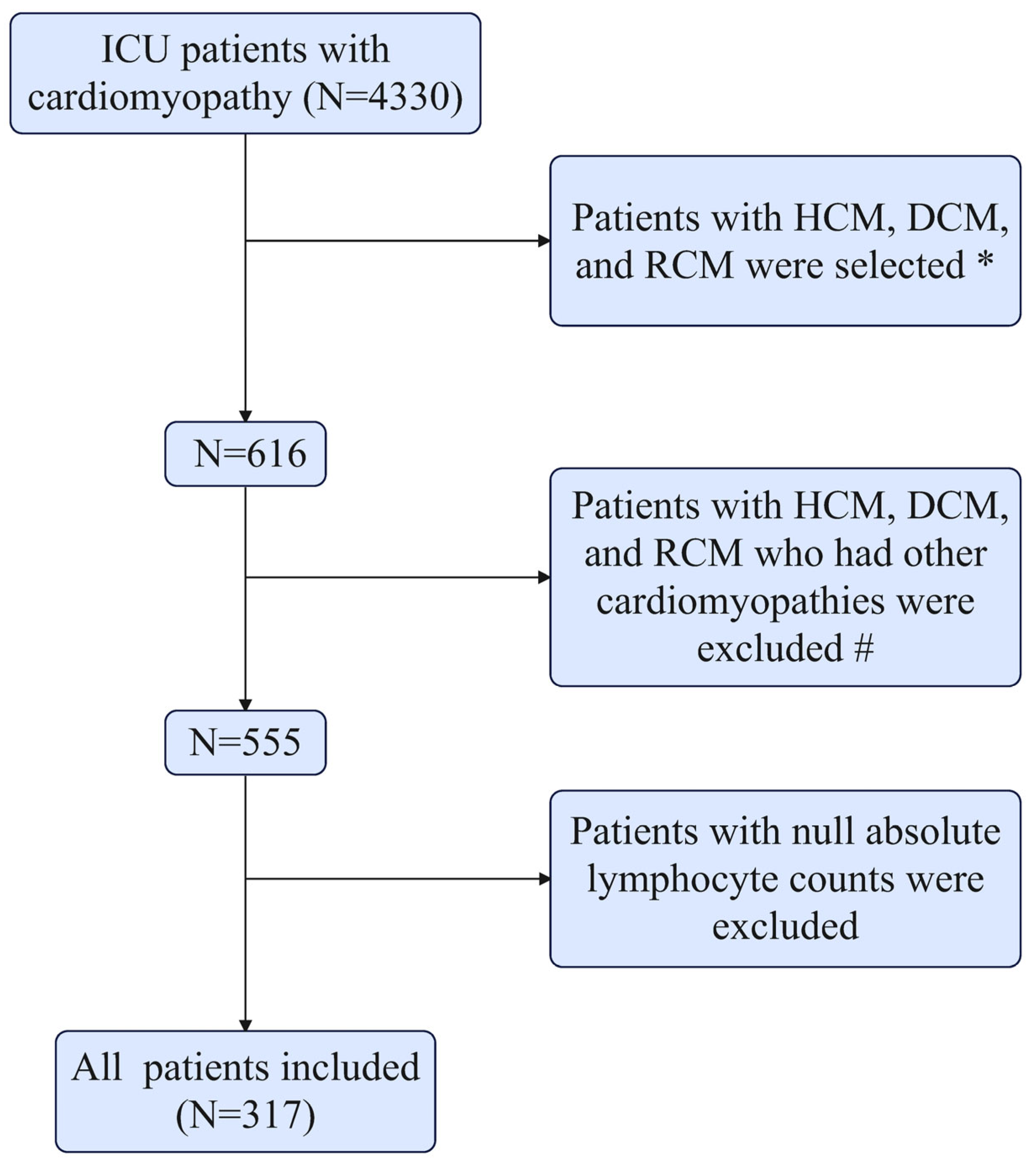
2.2. Study Population
2.3. Data Extraction and Definitions
2.4. Statistical Analysis
2.5. Machine Learning
2.6. Analysis of Repeated-Measures Data
2.7. BMJM Development
3. Results
3.1. Baseline Characteristics
3.2. Primary Outcomes of Cox Regression Analyses
3.3. Primary Outcomes of Logistic Regression Analyses
3.4. KM Survival Analyses
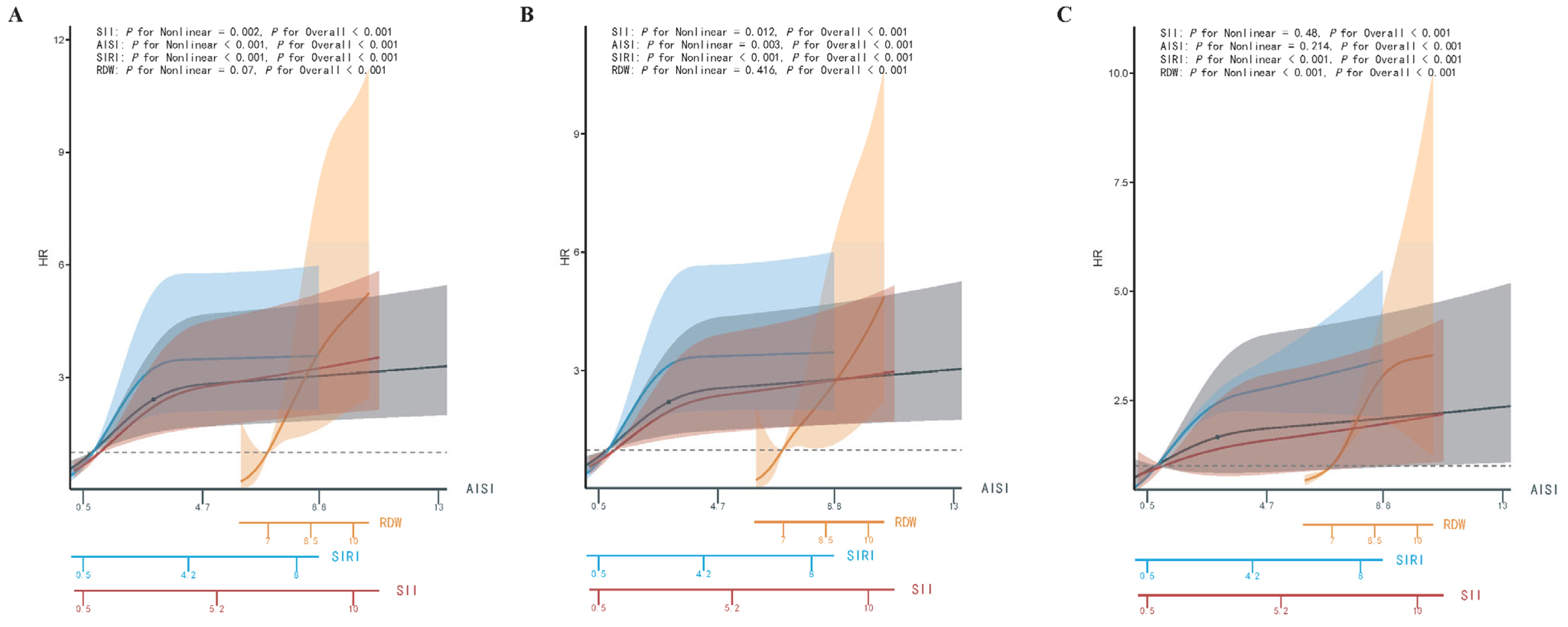
3.5. Nonlinear Analyses
3.6. Subgroup Interaction Regression Analysis
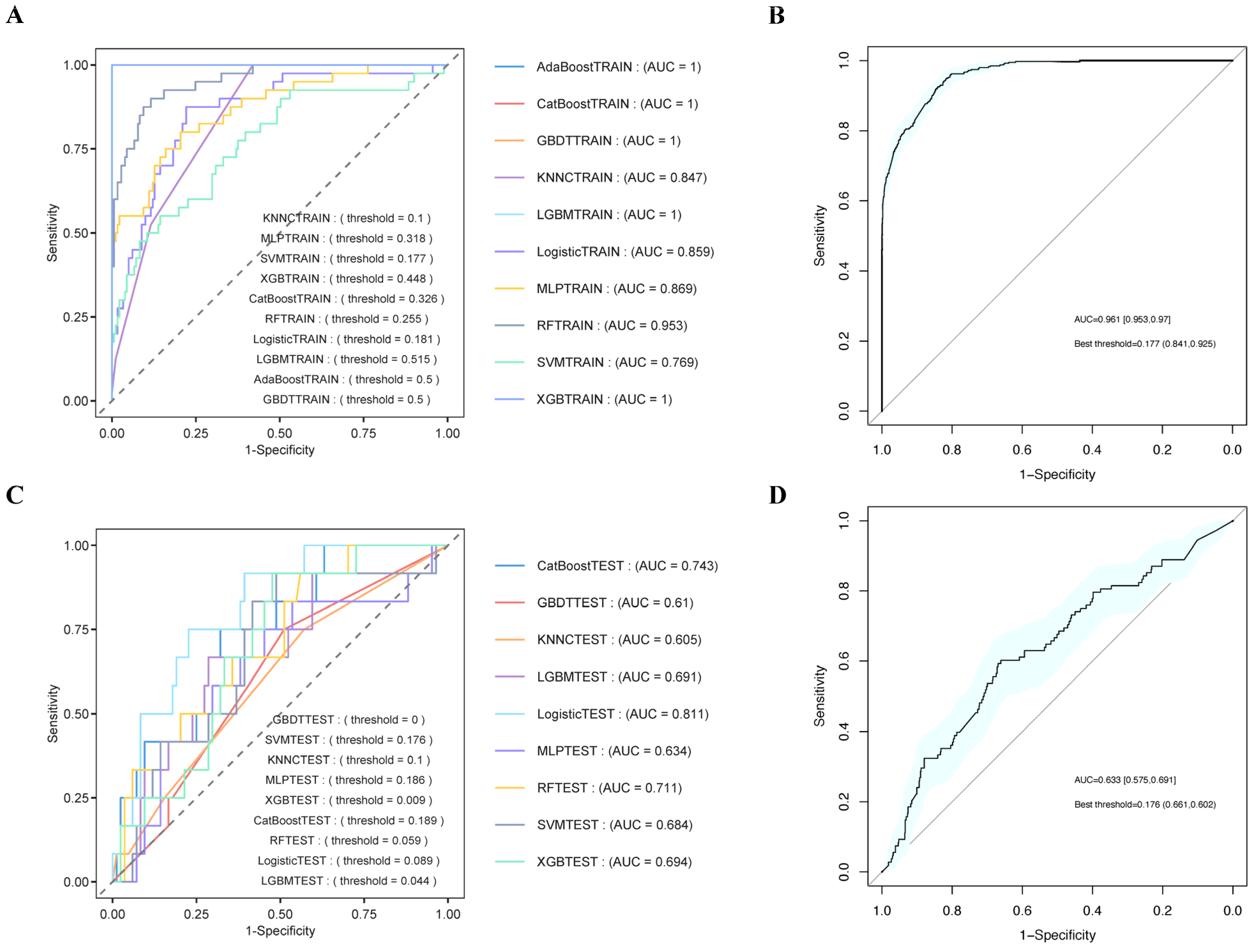
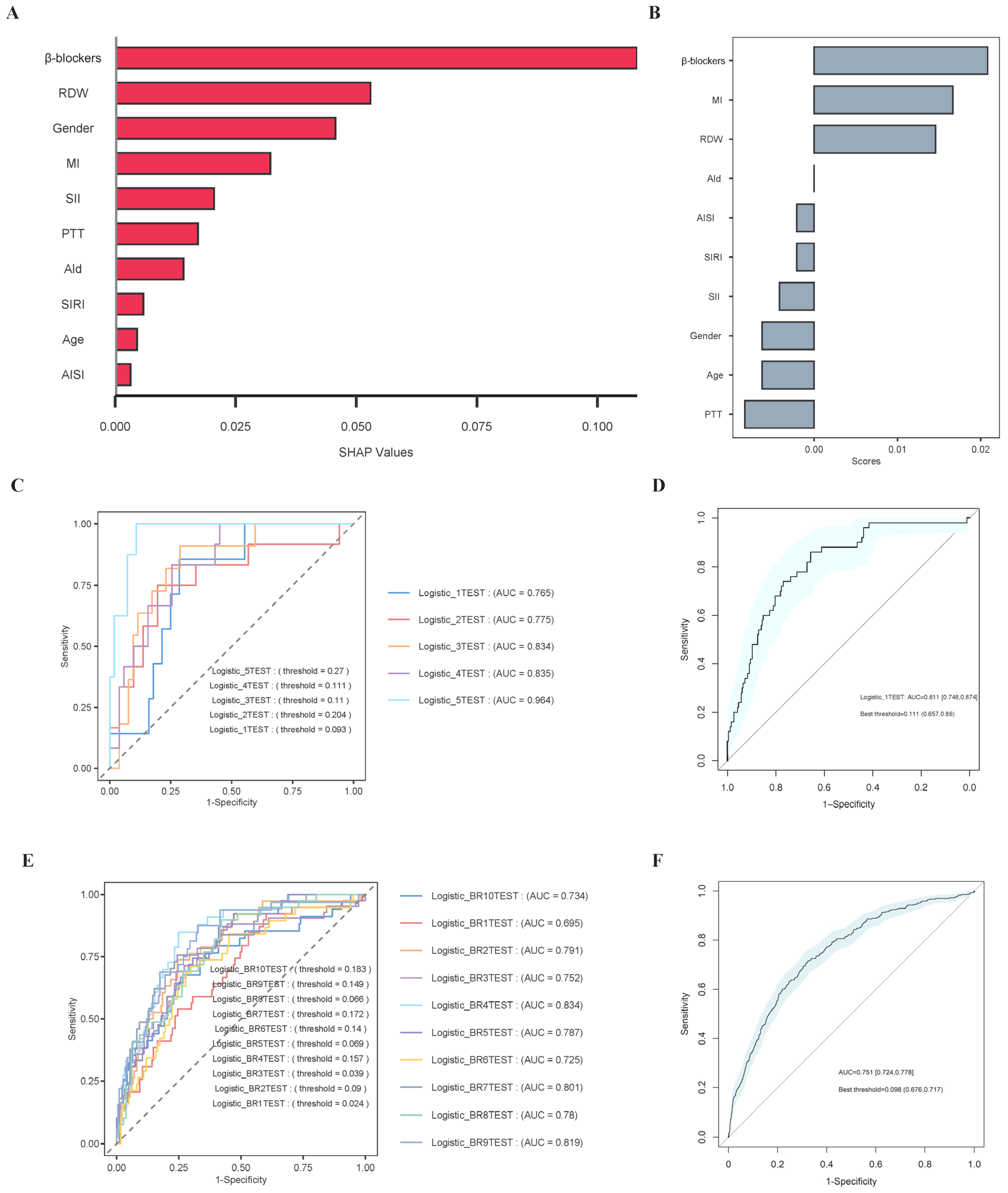
3.7. Boruta Algorithm
3.8. Establishment and Validation of the Prediction Models
3.9. The Results of Analysis of Repeated-Measures Data
3.10. The Results of BMJM Development
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; De Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [PubMed]
- Zhang, Y.; Liu, M.; Li, P.; Ma, L.; Yin, L.; Cui, Y.; Huang, K.; Li, W.; Xin, H.; Xiang, M.; et al. The burden and management competency of cardiomyopathies in China: A nationwide survey study. Lancet Reg. Health West. Pac. 2024, 46, 101062. [Google Scholar] [CrossRef]
- Codd, M.B.; Sugrue, D.D.; Gersh, B.J.; Melton, L.J., 3rd. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975–1984. Circulation 1989, 80, 564–572. [Google Scholar] [CrossRef]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2020, 76, e159–e240. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J. Clinical Course and Management of Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2018, 379, 655–668. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, C.; Chen, Z.; Lin, J.; Yang, L.; Zhang, Y.; Wu, C.; Zhao, D.; Jardin, B.; Pu, W.T.; et al. Non-Cell-Autonomous Cardiomyocyte Regulation Complicates Gene Supplementation Therapy for Lmna-Associated Cardiac Defects in Mice. JACC Basic Transl. Sci. 2024, 9, 1308–1325. [Google Scholar] [CrossRef]
- Yang, P.; Lou, Y.; Geng, Z.; Guo, Z.; Wu, S.; Li, Y.; Song, K.; Shi, T.; Zhang, S.; Xiong, J.; et al. Allele-Specific Suppression of Variant MHC with High-Precision RNA Nuclease CRISPR-Cas13d Prevents Hypertrophic Cardiomyopathy. Circulation 2024, 150, 283–298. [Google Scholar] [CrossRef]
- Chai, A.C.; Cui, M.; Chemello, F.; Li, H.; Chen, K.; Tan, W.; Atmanli, A.; McAnally, J.R.; Zhang, Y.; Xu, L.; et al. Base editing correction of hypertrophic cardiomyopathy in human cardiomyocytes and humanized mice. Nat. Med. 2023, 29, 401–411. [Google Scholar] [CrossRef]
- Reichart, D.; Newby, G.A.; Wakimoto, H.; Lun, M.; Gorham, J.M.; Curran, J.J.; Raguram, A.; DeLaughter, D.M.; Conner, D.A.; Marsiglia, J.D.C.; et al. Efficient in vivo genome editing prevents hypertrophic cardiomyopathy in mice. Nat. Med. 2023, 29, 412–421. [Google Scholar] [CrossRef]
- Sobrino-Márquez, J.; Grande-Trillo, A.; Cantero-Pérez, E.; Rangel-Sousa, D.; Lage-Galle, E.; Adsuar-Gómez, A. Prognostic Value of Blood Panel Parameters in Patients With Dilated Cardiomyopathy and Advanced Heart Failure. Transplant. Proc. 2018, 50, 650–652. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Kang, J.S.; Yu, J.X.; Yin, S.J.; Cong, X.F.; Chen, X. Differences in the predictive value of red cell distribution width for the mortality of patients with heart failure due to various heart diseases. J. Geriatr. Cardiol. 2015, 12, 647–654. [Google Scholar] [PubMed]
- Wang, Z.; Chen, X.; He, S. Prognostic value of red blood cell distribution width for mortality in patients with hypertrophic cardiomyopathy. Clin. Biochem. 2021, 96, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shen, R.; Yu, H.; Jin, N.; Hong, J.; Luo, Y.; Chen, X.; Rong, J. The levels of systemic inflammatory markers exhibit a positive correlation with the occurrence of heart failure: A cross-sectional study from NHANES. Front. Cardiovasc. Med. 2024, 11, 1457534. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, C.; Zhou, Y.; Che, H.; Wang, R.; Cheng, L.; Rao, C.; Zhong, Q.; Li, Z.; Duan, Y.; et al. The Associations of Two Novel Inflammation Biomarkers, SIRI and SII, with Mortality Risk in Patients with Chronic Heart Failure. J. Inflamm. Res. 2024, 17, 1255–1264. [Google Scholar] [CrossRef]
- Wang, Z.; Ruan, H.; Li, L.; Wei, X.; Zhu, Y.; Wei, J.; Chen, X.; He, S. Assessing the relationship between systemic immune-inflammation index and mortality in patients with hypertrophic cardiomyopathy. Upsala J. Med Sci. 2021, 126, 10-48101. [Google Scholar] [CrossRef]
- Johnson, A.E.; Bulgarelli, L.; Shen, L.; Gayles, A.; Shammout, A.; Horng, S.; Pollard, T.J.; Hao, S.; Moody, B.; Gow, B.; et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 2023, 10, 1. [Google Scholar] [CrossRef]
- Johnson, A.; Bulgarelli, L.; Pollard, T.; Gow, B.; Moody, B.; Horng, S.; Celi, L.A.; Mark, R. MIMIC-IV (version 3.0). PhysioNet. 2024. Available online: https://physionet.org/content/mimiciv/3.0/ (accessed on 18 March 2025).
- Team DC. DecisionLinnc. 1.0. 2023. Available online: https://www.statsape.com/ (accessed on 18 March 2025).
- Sylvester, S.; Sagehorn, M.; Gruber, T.; Atzmueller, M.; Schöne, B. SHAP value-based ERP analysis (SHERPA): Increasing the sensitivity of EEG signals with explainable AI methods. Behav. Res. Methods 2024, 56, 6067–6081. [Google Scholar] [CrossRef]
- Alvares, D.; Rubio, F.J. A tractable Bayesian joint model for longitudinal and survival data. Stat. Med. 2021, 40, 4213–4229. [Google Scholar] [CrossRef]
- Rizopoulos, D. The R Package JMbayes for fitting joint models for longitudinal and time-to-event data using MCMC. J. Stat. Softw. 2016, 72, 1–46. [Google Scholar] [CrossRef]
- Alsefri, M.; Sudell, M.; Garcia-Finana, M.; Kolamunnage-Dona, R. Bayesian joint modelling of longitudinal and time to event data: A methodological review. BMC Med. Res. Methodol. 2020, 20, 94. [Google Scholar] [CrossRef] [PubMed]
- Rizopoulos, D.; Ghosh, P. A Bayesian semiparametric multivariate joint model for multiple longitudinal outcomes and a time-to-event. Stat. Med. 2011, 30, 1366–1380. [Google Scholar] [CrossRef]
- Rizopoulos, D. Joint Models for Longitudinal and Time-to-Event Data: With Applications in R; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Kursa, M.B.; Rudnicki, W.R. Feature selection with the Boruta package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Yi, J.-E.; Lee, H.-J.; Kim, Y.J.; Kim, Y.; Joung, B.; Park, J. Additive prognostic value of red cell distribution width over late gadolinium enhancement on CMR in patients with non-ischemic dilated cardiomyopathy. Sci. Rep. 2020, 10, 9212. [Google Scholar] [CrossRef] [PubMed]
- Ozulku, M.; Caliskan, M.; Gullu, H.; Erdogan, D.; Caliskan, Z.; Muderrisoglu, H. Interrelation of RDW and coronary flow reserve in patient with idiopathic dilated cardiomyopathy. Anadolu Kardiyol. Dergisi/The Anatol. J. Cardiol. 2014, 14, 342–348. [Google Scholar] [CrossRef]
- Feng, J.; Huang, Y.; Huang, L.; Zhao, X.; Li, X.; Xin, A.; Wang, C.; Zhang, Y.; Zhang, J. Association between RDW-SD and prognosis across glycemic status in patients with dilated cardiomyopathy. BMJ Open Diabetes Res. Care 2024, 12, e004478. [Google Scholar] [CrossRef]
- Yang, H.-J.; Liu, X.; Qu, C.; Shi, S.-B.; Liang, J.-J.; Yang, B. Usefulness of Red Blood Cell Distribution Width to Predict Heart Failure Hospitalization in Patients with Hypertrophic Cardiomyopathy. Int. Heart J. 2018, 59, 779–785. [Google Scholar] [CrossRef]
- Li, J.; Gao, W.; Liu, J.; Zhang, X.; Tao, J.; Zhang, G. Red blood cell distribution width and maximum left ventricular wall thickness predict poor outcomes in patients with hypertrophic cardiomyopathy. Echocardiography 2022, 39, 278–285. [Google Scholar] [CrossRef]
- Wang, S.; Song, C.; Cui, H.; Zhu, C.; Wu, R.; Huang, X.; Lai, Y.; Wang, S. Red blood cell distribution width is associated with adverse cardiovascular events after septal myectomy. J. Thorac. Dis. 2021, 13, 582–591. [Google Scholar] [CrossRef]
- McCranor, B.J.; Kim, M.J.; Cruz, N.M.; Xue, Q.-L.; Berger, A.E.; Walston, J.D.; Civin, C.I.; Roy, C.N. Interleukin-6 directly impairs the erythroid development of human TF-1 erythroleukemic cells. Blood Cells Mol. Dis. 2014, 52, 126–133. [Google Scholar] [CrossRef]
- Ribeil, J.-A.; Arlet, J.-B.; Dussiot, M.; Cruz Moura, I.; Courtois, G.; Hermine, O. Ineffective erythropoiesis in beta -thalassemia. Sci. World J. 2013, 2013, 394295. [Google Scholar] [CrossRef]
- Pagani, A.; Nai, A.; Silvestri, L.; Camaschella, C. Hepcidin and Anemia: A Tight Relationship. Front. Physiol. 2019, 10, 1294. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Allen, L.A.; Pocock, S.J.; Shaw, L.K.; McMurray, J.J.; Pfeffer, M.A.; Swedberg, K.; Wang, D.; Yusuf, S.; Michelson, E.L.; et al. Red cell distribution width as a novel prognostic marker in heart failure: Data from the CHARM Program and the Duke Databank. J. Am. Coll. Cardiol. 2007, 50, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Arkew, M.; Gemechu, K.; Haile, K.; Asmerom, H. Red Blood Cell Distribution Width as Novel Biomarker in Cardiovascular Diseases: A Literature Review. J. Blood Med. 2022, 13, 413–424. [Google Scholar] [CrossRef]
- Xiu, J.; Lin, X.; Chen, Q.; Yu, P.; Lu, J.; Yang, Y.; Chen, W.; Bao, K.; Wang, J.; Zhu, J.; et al. The aggregate index of systemic inflammation (AISI): A novel predictor for hypertension. Front. Cardiovasc. Med. 2023, 10, 1163900. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, B.; Lu, W.; Chen, Y.; Peng, Y.; Chen, L.; Lin, Y. Association Between the Aggregate Index of Systemic Inflammation and Clinical Outcomes in Patients with Acute Myocardial Infarction: A Retrospective Study. J. Inflamm. Res. 2024, 17, 7057–7067. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Shen, Y. Higher systemic inflammation response index is associated with increased risk of heart failure in adults: An observational study. Medicine 2024, 103, e38625. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, C.; Wu, L.; Li, Z.; Li, H.; Zhang, J. Systemic Immune Inflammation Index (SII), System Inflammation Response Index (SIRI) and Risk of All-Cause Mortality and Cardiovascular Mortality: A 20-Year Follow-Up Cohort Study of 42,875 US Adults. J. Clin. Med. 2023, 12, 1128. [Google Scholar] [CrossRef]
- Özilhan, M.O.; Gökalp, G. The predictive role of systemic immune-inflammation index in non-ischemic cardiomyopathy. J. Med. Palliat. Care 2024, 5, 36–41. [Google Scholar] [CrossRef]
- Waagstein, F.; Hjalmarson, A.; Varnauskas, E.; Wallentin, I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Heart 1975, 37, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Furberg, C.D. Beta-adrenergic receptor blocking drugs in hypertrophic cardiomyopathy, autonomically mediated cardiovascular functional disorders and Fallot’s tetralogy. Drugs 1974, 7, 106–117. [Google Scholar] [CrossRef]
- Hjalmarson, A.; Waagstein, F. The role of beta-blockers in the treatment of cardiomyopathy and ischaemic heart failure. Drugs 1994, 47 (Suppl. S4), 31–39; discussion 39–40. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, R.G.; Semsarian, C.; Macdonald, P. Dilated cardiomyopathy. Lancet 2017, 390, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaghouli, M.; Ventura-Cots, M.; Wong, Y.J.; Genesca, J.; Bosques, F.; Brown, R.S.J.; Mathurin, P.; Louvet, A.; Shawcross, D.; Vargas, V.; et al. Relationship between updated MELD and prognosis in alcohol-associated hepatitis: Opportunities for more efficient trial design. Hepatol. Commun. 2024, 8, e0495. [Google Scholar] [CrossRef]
- Raynaud, M.; Aubert, O.; Divard, G.; Reese, P.P.; Kamar, N.; Yoo, D.; Chin, C.S.; Bailly, É.; Buchler, M.; Ladrière, M.; et al. Dynamic prediction of renal survival among deeply phenotyped kidney transplant recipients using artificial intelligence: An observational, international, multicohort study. Lancet Digit. Health 2021, 3, e795–e805. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, H.; Chang, W.; Sun, Q.; Yuan, X.; Wu, Z.; Xie, J.; Liu, L.; Yang, Y. Time-varying intensity of ventilatory inefficiency and mortality in patients with acute respiratory distress syndrome. Ann. Intensiv. Care 2025, 15, 6. [Google Scholar] [CrossRef]
- Wozniak, H.; Gaïa, N.; Lazarevic, V.; Le Terrier, C.; Beckmann, T.S.; Balzani, E.; Urner, M.; Pugin, J.; Schrenzel, J.; Heidegger, C.-P.; et al. Early reduction in gut microbiota diversity in critically ill patients is associated with mortality. Ann. Intensiv. Care 2024, 14, 174. [Google Scholar] [CrossRef]
- Haines, R.W.; Prowle, J.R.; Day, A.; Bear, D.E.; Heyland, D.K.; Puthucheary, Z. Association between urea trajectory and protein dose in critically ill adults: A secondary exploratory analysis of the effort protein trial (RE-EFFORT). Crit. Care 2024, 28, 24. [Google Scholar] [CrossRef]
- Urner, M.; Jüni, P.; Hansen, B.; Wettstein, M.S.; Ferguson, N.D.; Fan, E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: A registry-based, prospective cohort study. Lancet Respir. Med. 2020, 8, 905–913. [Google Scholar] [CrossRef]
- Petrosino, M.; Bogossian, E.G.; Rebora, P.; Galimberti, S.; Chesnut, R.; Bouzat, P.; Oddo, M.; Taccone, F.S.; Citerio, G.; Participants, O.R. Neurological Pupil Index and Intracranial Hypertension in Patients With Acute Brain Injury: A Secondary Analysis of the ORANGE Study. JAMA Neurol. 2025, 82, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Haines, R.W.M.; Fowler, A.J.M.; Wan, Y.I.; Flower, L.M.; Heyland, D.K.; Day, A.; Pearse, R.M.; Prowle, J.R.; Puthucheary, Z. Catabolism in Critical Illness: A Reanalysis of the REducing Deaths due to OXidative Stress (REDOXS) Trial. Crit. Care Med. 2022, 50, 1072–1082. [Google Scholar] [CrossRef] [PubMed]





| Variable | Total (n = 317) | Survivor (n = 265) | Non-Survivor (n = 52) | p-Value |
|---|---|---|---|---|
| Age, years | 65.00 (56.00–75.00) | 65.00 (54.00–75.00) | 68.50 (61.00–77.00) | 0.069 |
| Weight, kg | 85.10 (71.40–100.20) | 85.80 (71.55–101.40) | 80.65 (69.55–94.38) | 0.197 |
| Gender | 0.02 | |||
| Female | 97.00 (30.60%) | 74.00 (27.92%) | 23.00 (44.23%) | |
| Male | 220.00 (69.40%) | 191.00 (72.08%) | 29.00 (55.77%) | |
| Disease classification, n (%) | 0.601 | |||
| DCM | 190.00 (59.94%) | 157.00 (59.25%) | 33.00 (63.46%) | |
| HCM | 114.00 (35.96%) | 98.00 (36.98%) | 16.00 (30.77%) | |
| RCM | 13.00 (4.10%) | 10.00 (3.77%) | 3.00 (5.77%) | |
| Hypertension, n (%) | 0.758 | |||
| No | 251.00 (79.18%) | 209.00 (78.87%) | 42.00 (80.77%) | |
| Yes | 66.00 (20.82%) | 56.00 (21.13%) | 10.00 (19.23%) | |
| Stroke, n (%) | 0.166 | |||
| No | 289.00 (91.17%) | 239.00 (90.19%) | 50.00 (96.15%) | |
| Yes | 28.00 (8.83%) | 26.00 (9.81%) | 2.00 (3.85%) | |
| Chronic kidney disease, n (%) | 0.172 | |||
| No | 226.00 (71.29%) | 193.00 (72.83%) | 33.00 (63.46%) | |
| Yes | 91.00 (28.71%) | 72.00 (27.17%) | 19.00 (36.54%) | |
| Cancer, n (%) | 0.431 | |||
| No | 284.00 (89.59%) | 239.00 (90.19%) | 45.00 (86.54%) | |
| Yes | 33.00 (10.41%) | 26.00 (9.81%) | 7.00 (13.46%) | |
| Type 2 diabetes, n (%) | 0.524 | |||
| No | 225.00 (70.98%) | 190.00 (71.70%) | 35.00 (67.31%) | |
| Yes | 92.00 (29.02%) | 75.00 (28.30%) | 17.00 (32.69%) | |
| Hyperlipidemia, n (%) | 0.232 | |||
| No | 152.00 (47.95%) | 131.00 (49.43%) | 21.00 (40.38%) | |
| Yes | 165.00 (52.05%) | 134.00 (50.57%) | 31.00 (59.62%) | |
| Heart failure, n (%) | 0.823 | |||
| No | 77.00 (24.29%) | 65.00 (24.53%) | 12.00 (23.08%) | |
| Yes | 240.00 (75.71%) | 200.00 (75.47%) | 40.00 (76.92%) | |
| Myocardial infarction, n (%) | 0.001 | |||
| No | 276.00 (87.07%) | 238.00 (89.81%) | 38.00 (73.08%) | |
| Yes | 41.00 (12.93%) | 27.00 (10.19%) | 14.00 (26.92%) | |
| Chronic obstructive pulmonary disease, n (%) | 0.482 | |||
| No | 272.00 (85.80%) | 229.00 (86.42%) | 43.00 (82.69%) | |
| Yes | 45.00 (14.20%) | 36.00 (13.58%) | 9.00 (17.31%) | |
| Ventilation, n (%) | 0.291 | |||
| No | 66.00 (20.82%) | 58.00 (21.89%) | 8.00 (15.38%) | |
| Yes | 251.00 (79.18%) | 207.00 (78.11%) | 44.00 (84.62%) | |
| Sepsis, n (%) | <0.001 | |||
| No | 180.00 (56.78%) | 168.00 (63.40%) | 12.00 (23.08%) | |
| Yes | 137.00 (43.22%) | 97.00 (36.60%) | 40.00 (76.92%) | |
| Hospital mortality, n (%) | <0.001 | |||
| No | 310.00 (97.79%) | 265.00 (100.00%) | 45.00 (86.54%) | |
| Yes | 7.00 (2.21%) | 0.00 (0.00%) | 7.00 (13.46%) | |
| ICU mortality, n (%) | <0.001 | |||
| No | 271.00 (85.49%) | 262.00 (98.87%) | 9.00 (17.31%) | |
| Yes | 46.00 (14.51%) | 3.00 (1.13%) | 43.00 (82.69%) | |
| LOS of hospital, day | 10.17 (6.10–16.72) | 9.99 (6.05–15.94) | 12.11 (7.00–18.74) | 0.369 |
| LOS of ICU, day | 2.68 (1.30–5.29) | 2.22 (1.23–4.43) | 7.15 (3.10–11.43) | <0.001 |
| SOFA | 5.00 (3.00–7.00) | 5.00 (3.00–7.00) | 7.00 (5.00–11.00) | <0.001 |
| APS III | 44.00 (33.00–56.00) | 42.00 (32.00–53.00) | 56.00 (48.50–74.00) | <0.001 |
| SAPS II | 36.00 (29.00–44.00) | 35.00 (27.00–42.00) | 45.00 (34.00–53.50) | <0.001 |
| OASIS | 30.00 (25.00–35.00) | 29.00 (25.00–34.00) | 34.00 (29.00–39.50) | <0.001 |
| Lymphocytes, K/µL | 1.32 (0.80–1.96) | 1.43 (0.89–2.18) | 0.76 (0.46–1.25) | <0.001 |
| Neutrophils, K/µL | 9.18 (5.50–12.65) | 9.00 (5.39–11.87) | 9.63 (5.81–14.09) | 0.349 |
| Monocytes, K/µL | 0.76 (0.49–1.10) | 0.75 (0.48–1.02) | 0.86 (0.59–1.25) | 0.093 |
| Hematocrit, % | 32.10 (27.60–37.50) | 31.90 (27.60–37.50) | 32.30 (26.95–38.25) | >0.999 |
| Hemoglobin, g/dL | 10.50 (8.80–12.00) | 10.50 (8.90–12.10) | 10.00 (8.20–12.00) | 0.473 |
| Platelet, K/µL | 174.00 (130.00–240.00) | 174.00 (130.00–236.00) | 180.50 (129.00–266.50) | 0.447 |
| Red blood cell, m/µL | 3.49 (2.98–4.08) | 3.49 (3.01–4.06) | 3.49 (2.96–4.40) | 0.966 |
| White blood cell, K/µL | 11.80 (8.10–15.70) | 11.70 (8.20–15.70) | 12.35 (7.80–15.75) | 0.861 |
| Anion gap, mmol/L | 13.00 (11.00–17.00) | 13.00 (11.00–16.00) | 16.50 (13.50–20.00) | <0.001 |
| Total calcium, mmol/L | 8.50 (8.00–8.90) | 8.50 (8.00–8.90) | 8.50 (7.80–9.15) | 0.985 |
| Chloride, mmol/L | 103.00 (99.00–107.00) | 104.00 (99.00–107.00) | 101.00 (95.00–103.50) | 0.002 |
| Glucose, mg/dL | 127.00 (109.00–155.00) | 124.00 (109.00–152.00) | 139.00 (111.50–184.00) | 0.044 |
| Potassium, mmol/L | 4.40 (4.00–4.90) | 4.40 (4.00–4.90) | 4.35 (4.00–5.05) | 0.283 |
| Sodium, mmol/L | 138.00 (135.00–140.00) | 138.00 (135.00–140.00) | 137.00 (132.00–141.50) | 0.261 |
| International normalized ratio | 1.50 (1.30–1.75) | 1.50 (1.30–1.75) | 1.75 (1.30–2.50) | 0.007 |
| Prothrombin time, s | 16.10 (13.80–19.05) | 15.80 (13.60–19.05) | 19.05 (14.50–27.10) | 0.007 |
| Partial thromboplastin time, s | 32.90 (28.20–39.91) | 32.50 (27.70–39.91) | 38.70 (29.85–48.65) | 0.009 |
| Blood urea nitrogen, mg/dL | 24.00 (16.00–37.00) | 21.00 (15.00–34.00) | 35.00 (24.00–60.50) | <0.001 |
| Creatinine, mg/dL | 1.20 (0.90–1.70) | 1.10 (0.80–1.69) | 1.69 (1.30–2.85) | <0.001 |
| NIBP, mmHg | 78.00 (69.00–91.00) | 80.00 (69.00–91.00) | 74.50 (64.50–91.50) | 0.085 |
| Respiratory rate, insp/min | 19.00 (16.00–24.00) | 19.00 (15.00–23.00) | 21.00 (18.00–25.00) | 0.013 |
| Heart rate, bpm | 85.00 (75.00–100.00) | 85.00 (74.00–100.00) | 85.50 (77.50–106.50) | 0.5 |
| SpO2, % | 98.00 (95.00–100.00) | 98.00 (95.00–100.00) | 97.00 (93.00–100.00) | 0.09 |
| ACEI, n (%) | 0.005 | |||
| No | 237.00 (74.76%) | 190.00 (71.70%) | 47.00 (90.38%) | |
| Yes | 80.00 (25.24%) | 75.00 (28.30%) | 5.00 (9.62%) | |
| ARB, n (%) | 0.055 | |||
| No | 289.00 (91.17%) | 238.00 (89.81%) | 51.00 (98.08%) | |
| Yes | 28.00 (8.83%) | 27.00 (10.19%) | 1.00 (1.92%) | |
| β-blockers, n (%) | <0.001 | |||
| No | 105.00 (33.12%) | 73.00 (27.55%) | 32.00 (61.54%) | |
| Yes | 212.00 (66.88%) | 192.00 (72.45%) | 20.00 (38.46%) | |
| Aldosterone antagonists, n (%) | 0.061 | |||
| No | 257.00 (81.07%) | 210.00 (79.25%) | 47.00 (90.38%) | |
| Yes | 60.00 (18.93%) | 55.00 (20.75%) | 5.00 (9.62%) | |
| Diuretics, n (%) | 0.85 | |||
| No | 70.00 (22.08%) | 58.00 (21.89%) | 12.00 (23.08%) | |
| Yes | 247.00 (77.92%) | 207.00 (78.11%) | 40.00 (76.92%) | |
| Inotropes, n (%) | <0.001 | |||
| No | 159.00 (50.16%) | 144.00 (54.34%) | 15.00 (28.85%) | |
| Yes | 158.00 (49.84%) | 121.00 (45.66%) | 37.00 (71.15%) | |
| Anticoagulants, n (%) | 0.133 | |||
| No | 53.00 (16.72%) | 48.00 (18.11%) | 5.00 (9.62%) | |
| Yes | 264.00 (83.28%) | 217.00 (81.89%) | 47.00 (90.38%) | |
| RDW, % | 14.70 (13.50–16.20) | 14.50 (13.30–15.80) | 16.80 (14.60–19.05) | <0.001 |
| SIRI | 4.18 (2.04–10.82) | 3.55 (1.81–8.55) | 10.92 (4.68–18.34) | <0.001 |
| AISI * | 7.98 (2.85–21.58) | 6.55 (2.70–18.44) | 17.74 (6.18–50.00) | <0.001 |
| SII * | 10.74 (5.14–21.56) | 9.60 (4.80–20.09) | 18.08 (9.27–56.02) | <0.001 |
| Variables | HR (95% CI) | p-Value |
|---|---|---|
| Age | 1.03 (1.01–1.06) | 0.02 |
| Male | 0.29 (0.15–0.58) | <0.001 |
| Myocardial infarction | 2.16 (1.03–4.53) | 0.04 |
| Stroke | 0.25 (0.05–1.26) | 0.09 |
| SOFA | 1.07 (0.93–1.22) | 0.37 |
| SAPS II | 1.03 (0.99–1.06) | 0.12 |
| White blood cell | 0.97 (0.92–1.03) | 0.38 |
| Anion gap | 1.02 (0.96–1.09) | 0.53 |
| Total calcium | 1.16 (0.84–1.59) | 0.37 |
| Chloride | 0.97 (0.92–1.02) | 0.20 |
| Prothrombin time | 1.02 (1–1.04) | 0.09 |
| Partial thromboplastin time | 1.02 (1–1.03) | 0.01 |
| Urea nitrogen | 1.01 (0.99–1.02) | 0.33 |
| Creatinine | 1.18 (0.98–1.41) | 0.08 |
| SpO2 | 0.97 (0.91–1.05) | 0.49 |
| Sepsis | 1.62 (0.69–3.79) | 0.27 |
| β-blockers | 0.2 (0.1–0.39) | <0.001 |
| Aldosterone antagonists | 0.33 (0.12–0.9) | 0.03 |
| Inotropes | 1.92 (0.8–4.65) | 0.15 |
| Anticoagulants | 1.84 (0.58–5.92) | 0.30 |
| RDW | 1.14 (1.01–1.29) | 0.03 |
| SII | 1.01 (1–1.01) | 0.03 |
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| RDW | 1.33 (1.19–1.50) | <0.0001 | 1.32 (1.18–1.50) | <0.0001 | 1.31 (1.10–1.60) | 0.004 | |
| RDW (quartile) | |||||||
| Q1 | 13.2 (12.8–13.6) | reference | reference | reference | |||
| Q2 | 14.8 (14.3–15.4) | 2.15 (0.84–5.93) | 0.12 | 1.85 (0.69–5.30) | 0.23 | 1.39 (0.38–5.32) | 0.62 |
| Q3 | 17.3 (16.3–19.2) | 6.70 (2.95–17.29) | <0.0001 | 5.60 (2.36–15.00) | 0.0002 | 4.59 (1.35–18.07) | 0.02 |
| p for trend | <0.0001 | 0.0001 | 0.0118 | ||||
| SIRI | 1.01 (1.00–1.03) | 0.025 | 1.01 (1.00–1.02) | 0.042 | 1.02 (1.00–1.03) | 0.074 | |
| SIRI (quartile) | |||||||
| Q1 | 1.41 (0.92–2.04) | reference | reference | reference | |||
| Q2 | 4.18 (3.16–5.76) | 1.43 (0.56–3.85) | 0.46 | 1.63 (0.59–4.71) | 0.35 | 0.82 (0.20–3.28) | 0.78 |
| Q3 | 15.97 (10.85–26.45) | 5.54 (2.52–13.53) | 0.0001 | 5.95 (2.51–15.71) | 0.0001 | 5.29 (1.46–21.12) | 0.014 |
| p for trend | <0.0001 | <0.0001 | 0.0059 | ||||
| AISI | 1.00 (1.00–1.01) | 0.008 | 1.00 (1.00–1.01) | 0.014 | 1.00 (1.00–1.01) | 0.087 | |
| AISI (quartile) | |||||||
| Q1 | 1.78 (1.11–2.84) | reference | reference | reference | |||
| Q2 | 7.98 (5.36–10.36) | 1.73 (0.70–4.55) | 0.25 | 1.87 (0.73–5.09) | 0.20 | 1.04 (0.29–3.75) | 0.95 |
| Q3 | 31.15 (21.58–60.9) | 5.06 (2.30–12.41) | 0.0001 | 4.79 (2.03–12.44) | 0.0006 | 3.64 (1.99–14.72) | 0.056 |
| p for trend | <0.0001 | 0.0003 | 0.038 | ||||
| SII | 1.01 (1.00–1.02) | 0.0005 | 1.01 (1.00–1.02) | 0.0052 | 1.01 (1.00–1.02) | 0.19 | |
| SII (quartile) | |||||||
| Q1 | 4.02 (3.04–5.14) | reference | reference | reference | |||
| Q2 | 10.74 (8.56–12.65) | 2.54 (1.03–6.88) | 0.05 | 2.60 (1.02–7.22) | 0.05 | 1.60 (0.43–6.33) | 0.49 |
| Q3 | 31.88 (21.62–58.55) | 5.33 (2.33–13.81) | 0.0002 | 4.94 (2.07–13.24) | 0.0006 | 3.58 (1.02–14.41) | 0.056 |
| p for trend | 0.0001 | 0.0004 | 0.0414 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Nie, R.; Wang, Y.; Guo, H.; Wang, Y.; Luan, H.; Zeng, X.; Yuan, H. Integrating Dynamic Red Blood Cell Distribution Width Monitoring and β-Blocker Therapy for Mortality Prediction in Intensive Care Unit Cardiomyopathy Patients: A Bayesian Multivariate Joint Model and Machine Learning Study. Diagnostics 2025, 15, 1236. https://doi.org/10.3390/diagnostics15101236
Chen S, Nie R, Wang Y, Guo H, Wang Y, Luan H, Zeng X, Yuan H. Integrating Dynamic Red Blood Cell Distribution Width Monitoring and β-Blocker Therapy for Mortality Prediction in Intensive Care Unit Cardiomyopathy Patients: A Bayesian Multivariate Joint Model and Machine Learning Study. Diagnostics. 2025; 15(10):1236. https://doi.org/10.3390/diagnostics15101236
Chicago/Turabian StyleChen, Si, Rui Nie, Yi Wang, Haoran Guo, Yan Wang, Haixia Luan, Xiaoli Zeng, and Hui Yuan. 2025. "Integrating Dynamic Red Blood Cell Distribution Width Monitoring and β-Blocker Therapy for Mortality Prediction in Intensive Care Unit Cardiomyopathy Patients: A Bayesian Multivariate Joint Model and Machine Learning Study" Diagnostics 15, no. 10: 1236. https://doi.org/10.3390/diagnostics15101236
APA StyleChen, S., Nie, R., Wang, Y., Guo, H., Wang, Y., Luan, H., Zeng, X., & Yuan, H. (2025). Integrating Dynamic Red Blood Cell Distribution Width Monitoring and β-Blocker Therapy for Mortality Prediction in Intensive Care Unit Cardiomyopathy Patients: A Bayesian Multivariate Joint Model and Machine Learning Study. Diagnostics, 15(10), 1236. https://doi.org/10.3390/diagnostics15101236





