Pathological Fractures of the Mandible: Our Department’s 15-Year Experience
Abstract
1. Introduction
2. Materials and Methods
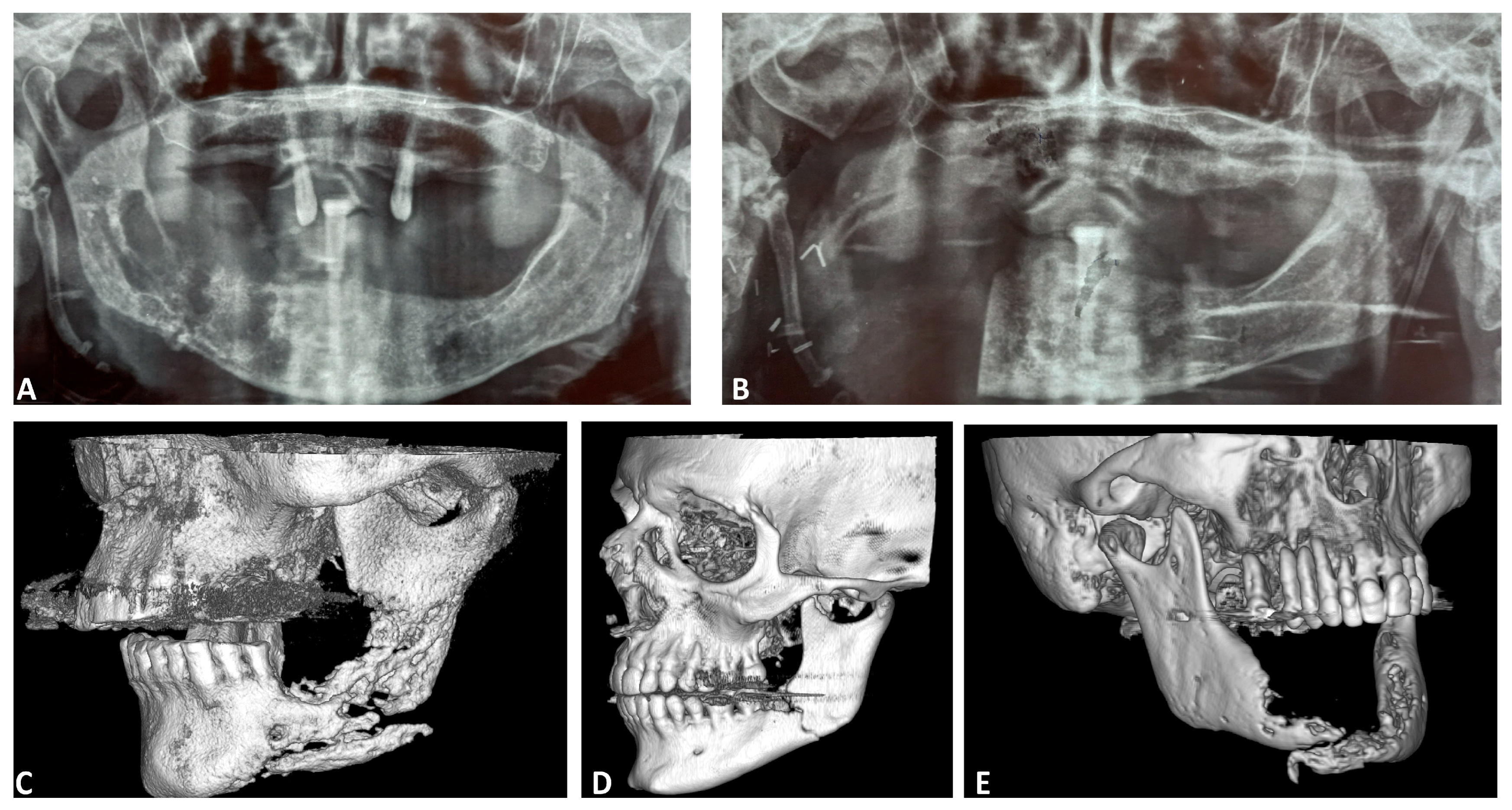
3. Results
3.1. Patient Characteristics
3.2. Fracture Characteristics
3.3. Intervention Characteristics
3.4. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRONJ | Medication-Related Osteonecrosis of the Jaws |
| OPG | Orthopantomogram |
| 3D | Three-Dimensional |
| CT | Computed Tomography |
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| MMF | Maxillo-Mandibular Fixation |
| CAD/CAM | Computer-Aided Design/Computer-Aided Manufacturing |
| COPD | Chronic Obstructive Pulmonary Disease |
References
- Ezsias, A.; Sugar, A.W. Pathological fractures of the mandible: A diagnostic and treatment dilemma. Br. J. Oral Maxillofac. Surg. 1994, 32, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, Y.; Akashi, M.; Wanifuchi, S.; Kusumoto, J.; Shigeoka, M.; Hasegawa, T.; Hashikawa, K.; Terashi, H.; Komori, T. Association between pain severity and clinicohistopathologic findings in the mandibular canal and inferior alveolar nerve of patients with advanced mandibular osteoradionecrosis. Oral. Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Khafif, A.; Posen, J.; Yagil, Y.; Beiser, M.; Gil, Z.; Ben-Yosef, R.; Landsberg, R.; Fliss, D.M. Quality of life in patients older than 75 years following major head and neck surgery. Head Neck 2007, 29, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Reuther, T.; Schuster, T.; Mende, U.; Kübler, A.C. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients—A report of a thirty year retrospective review. Int. J. Oral Maxillofac. Surg. 2003, 32, 289–295. [Google Scholar] [CrossRef]
- Niewald, M.; Fleckenstein, J.; Mang, K.; Holtmann, H.; Spitzer, W.J.; Rübe, C. Dental status, dental rehabilitation procedures, demographic and oncological data as potential risk factors for infected osteoradionecrosis of the lower jaw after radiotherapy for oral neoplasms: A retrospective evaluation. Radiat. Oncol. 2013, 8, 227. [Google Scholar] [CrossRef]
- Nelke, K.H.; Pawlak, W.; Gerber, H.; Leszczyszyn, J. Head and neck cancer patients’ quality of life. Adv. Clin. Exp. Med. 2014, 23, 1019–1027. [Google Scholar] [CrossRef]
- Reyes, I.M.; Arenilla, M.J.; Alarcón, D.; Jaenes, J.C.; Trujillo, M. Psychological impact after treatment in patients with head and neck cancer. Med. Oral Patol. Oral Cir. Bucal 2023, 28, e467. [Google Scholar] [CrossRef]
- Jimenez-Labaig, P.; Aymerich, C.; Braña, I.; Rullan, A.; Cacicedo, J.; González-Torres, M.Á.; Harrington, K.J.; Catalan, A. A comprehensive examination of mental health in patients with head and neck cancer: Systematic review and meta-analysis. JNCI Cancer Spectr. 2024, 8, pkae031. [Google Scholar] [CrossRef]
- Lin, L.; Lin, H.; Zhou, R.; Liu, B.; Liu, K.; Jiang, R. Surviving and thriving: Assessing quality of life and psychosocial interventions in mental health of head and neck cancer patients. Asian. J. Surg. 2025, 48, 1634–1642. [Google Scholar] [CrossRef]
- Rizzo, S.E.; Kenan, S. Pathologic Fractures; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Biermann, J.S.; Holt, G.E.; Lewis, V.O.; Schwartz, H.S.; Yaszemski, M.J. Metastatic bone disease: Diagnosis, evaluation, and treatment. J. Bone Jt. Surg. Am. 2009, 91, 1518–1530. [Google Scholar]
- Gerhards, F.; Kuffner, H.D.; Wagner, W. Pathological fractures of the mandible. A review of the etiology and treatment. Int. J. Oral Maxillofac. Surg. 1998, 27, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Ohori, H.; Iwata, E.; Takeda, D.; Kusumoto, J.; Hasegawa, T.; Akashi, M. Risk factors for pathological fracture in patients with mandibular osteoradionecrosis. Sci. Rep. 2023, 13, 5367. [Google Scholar] [CrossRef] [PubMed]
- Grau-Mancls, V.; Gargallo-Albiol, J.; Almendros-Marqus, N.; Gay-Escoda, C. Mandibular fractures related to the surgical extraction of impacted lower third molars: A report of 11 cases. J. Oral Maxillofac. Surg. 2011, 69, 1286–1290. [Google Scholar] [CrossRef]
- Bourhis, J.; Overgaard, J.; Audry, H.; Ang, K.K.; Saunders, M.; Bernier, J.; Horiot, J.C.; Le Maître, A.; Pajak, T.F.; Poulsen, M.G.; et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006, 368, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Boffano, P.; Roccia, F.; Gallesio, C.; Berrone, S. Pathological mandibular fractures: A review of the literature of the last two decades. Dent. Traumatol. 2013, 29, 185–196. [Google Scholar] [CrossRef]
- Coletti, D.; Ord, R.A. Treatment rationale for pathological fractures of the mandible: A series of 44 fractures. Int. J. Oral Maxillofac. Surg. 2008, 37, 215–222. [Google Scholar] [CrossRef]
- Joshi, A.; Goel, M.; Thorat, A. Identifying the risk factors causing iatrogenic mandibular fractures associated with exodontia: A systemic meta-analysis of 200 cases from 1953 to 2015. Oral Maxillofac. Surg. 2016, 20, 391–396. [Google Scholar] [CrossRef]
- Willaert, R.; Nevens, D.; Laenen, A.; Batstone, M.; Politis, C.; Nuyts, S. Does intensity-modulated radiation therapy lower the risk of osteoradionecrosis of the jaw? A long-term comparative analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 1387–1393. [Google Scholar] [CrossRef]
- Kubota, H.; Miyawaki, D.; Mukumoto, N.; Ishihara, T.; Matsumura, M.; Hasegawa, T.; Akashi, M.; Kiyota, N.; Shinomiya, H.; Teshima, M.; et al. Risk factors for osteoradionecrosis of the jaw in patients with head and neck squamous cell carcinoma. Radiat. Oncol. 2021, 16, 1. [Google Scholar] [CrossRef]
- Owosho, A.A.; Tsai, C.J.; Lee, R.S.; Freymiller, H.; Kadempour, A.; Varthis, S.; Sax, A.Z.; Rosen, E.B.; Yom, S.K.; Randazzo, J.; et al. The Prevalence and Risk Factors Associated with Osteoradionecrosis of the Jaw in Oral and Oropharyngeal Cancer Patients Treated with Intensity-Modulated Radiation Therapy (IMRT): The Memorial Sloan Kettering Cancer Center Experience. Oral Oncol. 2016, 64, 44. [Google Scholar] [CrossRef]
- Dhanda, J.; Pasquier, D.; Newman, L.; Shaw, R. Current Concepts in Osteoradionecrosis After Head and Neck Radiotherapy. Clin. Oncol. 2016, 28, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Huryn, J.M.; Kronstadt, K.L.; Yom, S.H.K.; Randazzo, J.R.; Estilo, C.L. Osteoradionecrosis of the jaw: A mini review. Front. Oral Heal. 2022, 3, 980786. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, R.F.; Sun, Y.F.; Liu, B.; Jia, J. Pathological Fractures of the Mandible: A Report of 27 Cases. Clin. Surg. 2017, 2, 1839. [Google Scholar]
- Carlsen, A.; Marcussen, M. Spontaneous fractures of the mandible concept & treatment strategy. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e88–e94. [Google Scholar]
- Sawhney, R.; Ducic, Y. Management of pathologic fractures of the mandible secondary to osteoradionecrosis. Otolaryngol. Head. Neck Surg. 2013, 148, 54–58. [Google Scholar] [CrossRef]
- Alam, D.S.; Nuara, M.; Christian, J. Analysis of outcomes of vascularized flap reconstruction in patients with advanced mandibular osteoradionecrosis. Otolaryngol. Head Neck Surg. 2009, 141, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Zaghi, S.; Danesh, J.; Hendizadeh, L.; Nabili, V.; Blackwell, K.E. Changing indications for maxillomandibular reconstruction with osseous free flaps: A 17-year experience with 620 consecutive cases at UCLA and the impact of osteoradionecrosis. Laryngoscope 2014, 124, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Tso, T.V.; Blackwell, K.E.; Sung, E.C. Predictive factors of osteoradionecrosis necessitating segmental mandibulectomy: A descriptive study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, e8–e13. [Google Scholar] [CrossRef]
- Hirsch, D.L.; Bell, R.B.; Dierks, E.J.; Potter, J.K.; Potter, B.E. Analysis of Microvascular Free Flaps for Reconstruction of Advanced Mandibular Osteoradionecrosis: A Retrospective Cohort Study. J. Oral Maxillofac. Surg. 2008, 66, 2545–2556. [Google Scholar] [CrossRef]
- Lee, M.; Chin, R.Y.; Eslick, G.D.; Sritharan, N.; Paramaesvaran, S. Outcomes of microvascular free flap reconstruction for mandibular osteoradionecrosis: A systematic review. J. Cranio-Maxillofac. Surg. 2015, 43, 2026–2033. [Google Scholar] [CrossRef]
- Cannady, S.B.; Dean, N.; Kroeker, A.; Albert, T.A.; Rosenthal, E.L.; Wax, M.K. Free flap reconstruction for osteoradionecrosis of the jaws-Outcomes and predictive factors for success. Head Neck 2011, 33, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Gao, W.; Liu, Z.; Zhu, D.; Zhu, F.; Li, X.; He, Y. Radiation-Induced Soft Tissue Injuries in Patients with Advanced Mandibular Osteoradionecrosis: A Preliminary Evaluation and Management of Various Soft Tissue Problems Around Radiation-Induced Osteonecrosis Lesions. Front. Oncol. 2021, 11, 641061. [Google Scholar] [CrossRef]
- D’Souza, J.; Lowe, D.; Rogers, S.N. Changing trends and the role of medical management on the outcome of patients treated for osteoradionecrosis of the mandible: Experience from a regional head and neck unit. Br. J. Oral Maxillofac. Surg. 2014, 52, 356–362. [Google Scholar] [CrossRef]
- Freiberger, J.J.; Yoo, D.S.; de Lisle Dear, G.; McGraw, T.A.; Blakey, G.H.; Padilla Burgos, R.; Kraft, K.; Nelson, J.W.; Moon, R.E.; Piantadosi, C.A. MultiModality Surgical and Hyperbaric Management of Mandibular Osteoradionecrosis. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 717–724. [Google Scholar] [CrossRef]
- Sweeny, L.; Lancaster, W.P.; Dean, N.R.; Magnuson, J.S.; Carroll, W.R.; Louis, P.J.; Rosenthal, E.L. Use of recombinant bone morphogenetic protein 2 in free flap reconstruction for osteonecrosis of the mandible. J. Oral Maxillofac. Surg. 2012, 70, 1991–1996. [Google Scholar] [CrossRef]
- Nabil, S.; Samman, N. Incidence and prevention of osteoradionecrosis after dental extraction in irradiated patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2011, 40, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.E.; Koyfman, S.A.; Yarom, N.; Lynggaard, C.D.; Ismaila, N.; Forner, L.E.; Fuller, C.D.; Mowery, Y.M.; Murphy, B.A.; Watson, E.; et al. Prevention and Management of Osteoradionecrosis in Patients with Head and Neck Cancer Treated with Radiation Therapy: ISOO-MASCC-ASCO Guideline. J. Clin. Oncol. 2024, 42, 1975–1996. [Google Scholar] [CrossRef] [PubMed]
- Contrera, K.J.; Chinn, S.B.; Weber, R.S.; Roberts, D.; Myers, J.N.; Lai, S.Y.; Lewis, C.M.; Hessel, A.C.; Gillenwater, A.M.; Mulcahy, C.F.; et al. Outcomes after definitive surgery for mandibular osteoradionecrosis. Head Neck 2022, 44, 1313–1323. [Google Scholar] [CrossRef]
- Gevorgyan, A.; Wong, K.; Poon, I.; Blanas, N.; Enepekides, D.J.; Higgins, K.M. Osteoradionecrosis of the mandible: A case series at a single institution. J. Otolaryngol. Head Neck Surg. 2013, 42, 46. [Google Scholar] [CrossRef]
- Mayland, E.; Curry, J.M.; Wax, M.K.; Thomas, C.M.; Swendseid, B.P.; Kejner, A.E.; Kain, J.J.; Cannady, S.B.; Miles, B.A.; DiLeo, M.; et al. Impact of preoperative and intraoperative management on outcomes in osteoradionecrosis requiring free flap reconstruction. Head Neck 2022, 44, 698–709. [Google Scholar] [CrossRef]
- Wu, R.T.; Lin, J.A.J.; Su, C.C.L.; Wei, F.C. Quality of Life for Osteoradionecrosis Reconstruction in the Head and Neck: A Longitudinal Framework and Risk Factors. Plast. Reconstr. Surg. 2025, 1, 55. [Google Scholar] [CrossRef] [PubMed]
- Diarra, D.; Chen, J.; Lin, H.; Zeng, B.; Man, Q.; Deng, W.; Nyimi, B.F.; Wu, T.; Liu, B. Retrospective study of morphological and functional analysis of mandibular reconstructions using iliac and fibular flaps. J. Stomatol. Oral Maxillofac. Surg. 2025, 102322. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, Z.; Tian, Z.; Dai, T.; Qiu, W.; Zhang, Z. Retrospective analysis of osteoradionecrosis of the mandible: Proposing a novel clinical classification and staging system. Int. J. Oral Maxillofac. Surg. 2015, 44, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, D.; Kowa, X.Y.; Sinha, D.; Kalavrezos, N. Does Combined Access Mandibulotomy, Rim Mandibulectomy and Neck Dissection Compound the Late Effects of Radiotherapy? Plast. Reconstr. Surg.–Glob. Open 2023, 11, E5081. [Google Scholar] [CrossRef]
- Frohwitter, G.; Rau, A.; Kesting, M.R.; Fichter, A. Microvascular reconstruction in the vessel depleted neck—A systematic review. J. Cranio. Maxillofac. Surg. 2018, 46, 1652–1658. [Google Scholar] [CrossRef]
- Prince, A.D.P.; Broderick, M.T.; Heft Neal, M.E.; Spector, M.E. Head and Neck Reconstruction in the Vessel Depleted Neck. Front. Oral Maxillofac. Med. 2020, 2, 13. [Google Scholar] [CrossRef]
- Toriumi, S.; Kobayashi, A.; Uesawa, Y. Comprehensive Study of the Risk Factors for Medication-Related Osteonecrosis of the Jaw Based on the Japanese Adverse Drug Event Report Database. Pharmaceuticals 2020, 13, 467. [Google Scholar] [CrossRef]
- Vahtsevanos, K.; Kyrgidis, A.; Verrou, E.; Katodritou, E.; Triaridis, S.; Andreadis, C.G.; Boukovinas, I.; Koloutsos, G.E.; Teleioudis, Z.; Kitikidou, K.; et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J. Clin. Oncol. 2009, 27, 5356–5362. [Google Scholar] [CrossRef]
- Pichardo, S.E.C.; ten Broek, F.W.; van Merkesteyn, M.J.P. Treatment of pathologic fractures of the mandible in stage III medication-related osteonecrosis of the jaw-an observational study. J. Craniomaxillofac. Surg. 2018, 46, 1241–1246. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, T.J.; Ahn, K.M. Bisphosphonate-related osteonecrosis of the jaw in metastatic breast cancer patients: A review of 25 cases. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 8. [Google Scholar] [CrossRef][Green Version]
- Nardi, C.; Vignoli, C.; Pietragalla, M.; Tonelli, P.; Calistri, L.; Franchi, L.; Preda, L.; Colagrande, S. Imaging of mandibular fractures: A pictorial review. Insights Imaging 2020, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Dreizin, D.; Nam, A.J.; Tirada, N.; Levin, M.D.; Stein, D.M.; Bodanapally, U.K.; Mirvis, S.E.; Munera, F. Multidetector CT of Mandibular Fractures, Reductions, and Complications: A Clinically Relevant Primer for the Radiologist. Radiography 2016, 36, 1539–1564. [Google Scholar] [CrossRef] [PubMed]
- May, M.M.; Howe, B.M.; O’Byrne, T.J.; Alexander, A.E.; Morris, J.M.; Moore, E.J.; Kasperbauer, J.L.; Janus, J.R.; Van Abel, K.M.; Dickens, H.J.; et al. Short and long-term outcomes of three-dimensional printed surgical guides and virtual surgical planning versus conventional methods for fibula free flap reconstruction of the mandible: Decreased nonunion and complication rates. Head Neck 2021, 43, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Castro-Núñez, J.; Shelton, J.M.; Snyder, S.; Sickels, J.V. Virtual Surgical Planning for the Management of Severe Atrophic Mandible Fractures. Craniomaxillofac. Trauma Reconstr. 2017, 11, 150. [Google Scholar] [CrossRef]
- Van Camp, N.; Verhelst, P.J.; Nicot, R.; Ferri, J.; Politis, C. Impaired Callus Formation in Pathological Mandibular Fractures in Medication-Related Osteonecrosis of the Jaw and Osteoradionecrosis. J. Oral Maxillofac. Surg. 2021, 79, 1892–1901. [Google Scholar] [CrossRef]
- Jonasson, G.; Sundh, V.; Hakeberg, M.; Hassani-Nejad, A.; Lissner, L.; Ahlqwist, M. Mandibular bone changes in 24 years and skeletal fracture prediction. Clin. Oral Investig. 2013, 17, 565–572. [Google Scholar] [CrossRef]
- Mohandas, R.; Mohapatra, S.; Narkhede, R.; Kheur, S. Effectiveness of Hyperbaric Oxygen Therapy in the Management of Osteoradionecrosis of the Jaw: A Systematic Review. J. Health Allied Sci. NU 2024, 14, 295–302. [Google Scholar] [CrossRef]
- Forner, L.E.; Dieleman, F.J.; Shaw, R.J.; Kanatas, A.; Butterworth, C.J.; Kjeller, G.; Alsner, J.; Overgaard, J.; Hillerup, S.; Hyldegaard, O.; et al. Hyperbaric oxygen treatment of mandibular osteoradionecrosis: Combined data from the two randomized clinical trials DAHANCA-21 and NWHHT2009-1. Radiother. Oncol. 2022, 166, 137–144. [Google Scholar] [CrossRef]
- Sultan, A.; Hanna, G.J.; Margalit, D.N.; Chau, N.; Goguen, L.A.; Marty, F.M.; Rabinowits, G.; Schoenfeld, J.D.; Sonis, S.T.; Thomas, T.; et al. The Use of Hyperbaric Oxygen for the Prevention and Management of Osteoradionecrosis of the Jaw: A Dana-Farber/Brigham and Women’s Cancer Center Multidisciplinary Guideline. Oncologist 2017, 22, 343. [Google Scholar] [CrossRef]
- Korambayil, P.M.; Ambookan, P.V.; Pillai, S.; Karangath, R.R.; George, D. Role of Hyperbaric Medicine for Osteoradionecrosis and Post Irradiation Wounds: An Institutional Experience. Indian J. Surg. Oncol. 2020, 11, 469. [Google Scholar] [CrossRef]
- Banjar, A.; Patel, V.; Abed, H. Pentoxifylline and tocopherol (vitamin E) with/without clodronate for the management of osteoradionecrosis: A scoping review. Oral Dis. 2023, 29, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Arqueros-Lemus, M.; Mariño-Recabarren, D.; Niklander, S.; Martínez-Flores, R.; Moraga, V. Pentoxifylline and tocopherol for the treatment of osteoradionecrosis of the jaws. A. systematic. review. Med. Oral Patol. Oral Cir. Bucal 2023, 28, e293. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.J.; Brennan, P.A. Pentoxifylline—A review of its use in osteoradionecrosis. Br. J. Oral Maxillofac. Surg. 2017, 55, 230–234. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | |
|---|---|
| Variable | Number of Patients (%) |
| Age | |
| <60 years | 21 (42%) |
| >60 years | 29 (58%) |
| Median Age | 66.4 years |
| Sex | |
| Male | 39 (78%) |
| Female | 11 (22%) |
| Major comorbidities | |
| Yes | 39 (78%) |
| No | 11 (22%) |
| Smokers | |
| Yes | 37 (74%) |
| No | 13 (26%) |
| Alcohol use | |
| Yes | 30 (60%) |
| No | 20 (40%) |
| Fracture Characteristics | |
|---|---|
| Variable | Number of Patients (%) |
| Etiology | |
| Medication-related osteonecrosis of the jaws | 8 (16%) |
| Osteoradionecrosis | 24 (48%) |
| Primary malignancy | 11 (22%) |
| Recurrence of malignancy | 7 (14%) |
| Benign tumours | 2 (4%) |
| Cysts | 1 (2%) |
| Osteoporosis | 1 (2%) |
| Mandibular atrophy | 7 (14%) |
| Osteomyelitis | 4 (8%) |
| Iatrogenic (after tooth extractions) | 1 (2%) |
| Fracture location | |
| Body only | 28 (56%) |
| Angle only | 6 (12%) |
| Ramus (as part of multiple locations) | 6 (12%) |
| Multiple (unilateral) | 10 (20%) |
| Multiple (bilateral) | 6 (12%) |
| Presence of fistula | |
| No | 22 (44%) |
| Yes, intraoral | 9 (18%) |
| Yes, extraoral | 10 (20%) |
| Yes, both | 9 (18%) |
| Imaging | |
| OPG | 37 (74%) |
| CT | 25 (50%) |
| Both | 14 (28%) |
| Biopsy | |
| No | 15 (30%) |
| Yes, inflammation | 17 (34%) |
| Yes, malignancy | 18 (36%) |
| Intervention Characteristics | |
|---|---|
| Variable | Number of Patients (%) |
| Surgical intervention | |
| Yes | 41 (82%) |
| No | 9 (18%) |
| Type of surgical intervention | |
| Mandibular resection | 25 (61%) |
| Mandibular resection and reconstruction | 14 (34%) |
| MMF | 2 (5%) |
| Adjunct Therapies | |
| Hyperbaric Oxygen Therapy | 5 (10%) |
| Pentoxifylline | 2 (4%) |
| Length of hospital stay (days) | 2–49 [median 11.5] |
| Postoperation imaging | |
| OPG | 36 (88%) |
| CT | 14 (34%) |
| Both | 6 (15%) |
| Outcomes | |
|---|---|
| Variable | Number of Patients (%) |
| Complications | |
| Yes | 27 (54%) |
| No | 23 (46%) |
| Overall survival (months) | 1–105 [median 27.6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatziantoniou, G.; Tatsis, D.; Politis, S.; Saramantos, A.; Koukolis, N.; Paraskevopoulos, K. Pathological Fractures of the Mandible: Our Department’s 15-Year Experience. Diagnostics 2025, 15, 1216. https://doi.org/10.3390/diagnostics15101216
Chatziantoniou G, Tatsis D, Politis S, Saramantos A, Koukolis N, Paraskevopoulos K. Pathological Fractures of the Mandible: Our Department’s 15-Year Experience. Diagnostics. 2025; 15(10):1216. https://doi.org/10.3390/diagnostics15101216
Chicago/Turabian StyleChatziantoniou, Georgios, Dimitris Tatsis, Solon Politis, Antonios Saramantos, Nikolaos Koukolis, and Konstantinos Paraskevopoulos. 2025. "Pathological Fractures of the Mandible: Our Department’s 15-Year Experience" Diagnostics 15, no. 10: 1216. https://doi.org/10.3390/diagnostics15101216
APA StyleChatziantoniou, G., Tatsis, D., Politis, S., Saramantos, A., Koukolis, N., & Paraskevopoulos, K. (2025). Pathological Fractures of the Mandible: Our Department’s 15-Year Experience. Diagnostics, 15(10), 1216. https://doi.org/10.3390/diagnostics15101216







