Diabetes Mellitus and Multidrug-Resistant Gram-Negative Bacterial Infections in Critically Ill COVID-19 Patients: A Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Laboratory Methods
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khunti, K.; Chudasama, Y.V.; Gregg, E.W.; Kamkuemah, M.; Misra, S.; Suls, J.; Venkateshmurthy, N.S.; Valabhji, J. Diabetes and Multiple Long-term Conditions: A Review of Our Current Global Health Challenge. Diabetes Care 2023, 46, 2092–2101. [Google Scholar] [CrossRef]
- Emami, A.; Javanmardi, F.; Pirbonyeh, N.; Akbari, A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2020, 8, e35. [Google Scholar] [PubMed]
- Collard, D.; Nurmohamed, N.S.; Kaiser, Y.; Reeskamp, L.F.; Dormans, T.; Moeniralam, H.; Simsek, S.; Douma, R.; Eerens, A.; Reidinga, A.C.; et al. Cardiovascular risk factors and COVID-19 outcomes in hospitalised patients: A prospective cohort study. BMJ Open 2021, 11, e045482. [Google Scholar] [CrossRef]
- National Center for Immunization and Respiratory Diseases (NCIRD) Division of Viral Diseases (DOVD). Evidence Used to Update the List of Underlying Medical Conditions That Increase a Person’s Risk of Severe Illness from COVID-19 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html (accessed on 19 June 2024).
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020. China CDC Wkly. 2020, 2, 113–122. [Google Scholar] [CrossRef]
- Huang, I.; Lim, M.A.; Pranata, R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—A systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. 2020, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, G.; Grassi, G.; Borghi, C.; Ferri, C.; Salvetti, M.; Volpe, M. Age and Multimorbidity Predict Death Among COVID-19 Patients: Results of the SARS-RAS Study of the Italian Society of Hypertension. Hypertension 2020, 76, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Codo, A.C.; Davanzo, G.G.; Monteiro, L.d.B.; de Souza, G.F.; Muraro, S.P.; Virgilio-Da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; Junior, C.A.O.d.B.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 498–499. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar]
- Sheetz, M.J.; King, G.L. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 2002, 288, 2579–2588. [Google Scholar] [CrossRef]
- Jafar, N.; Edriss, H.; Nugent, K. The Effect of Short-Term Hyperglycemia on the Innate Immune System. Am. J. Med. Sci. 2016, 351, 201–211. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008, 14, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Tomás, E.; Lin, Y.; Dagher, Z.; Saha, A.; Luo, Z.; Ido, Y.; Ruderman, N.B. Hyperglycemia and insulin resistance: Possible mechanisms. Ann. N. Y. Acad. Sci. 2002, 967, 43–51. [Google Scholar] [CrossRef]
- Tonetti, T.; Grasselli, G.; Zanella, A.; Pizzilli, G.; Fumagalli, R.; Piva, S.; Lorini, L.; Iotti, G.; Foti, G.; Colombo, S.; et al. Use of critical care resources during the first 2 weeks (February 24-March 8, 2020) of the Covid-19 outbreak in Italy. Ann. Intensive Care 2020, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020, 46, 1339–1348. [Google Scholar] [CrossRef]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Bhatla, A.; Mayer, M.M.; Adusumalli, S.; Hyman, M.C.; Oh, E.; Tierney, A.; Moss, J.; Chahal, A.A.; Anesi, G.; Denduluri, S.; et al. COVID-19 and cardiac arrhythmias. Heart Rhythm 2020, 17, 1439–1444. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA 2020, 323, 1545–1546. [Google Scholar] [CrossRef]
- CDC. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/antimicrobial-resistance/media/pdfs/covid19-impact-report-508.pdf (accessed on 14 June 2024).
- WHONET. Greek System for the Surveillance of Antimicrobial Resistance. Available online: http://www.mednet.gr/ (accessed on 12 June 2024).
- EARSS. European Antimicrobial Resistance Surveillance System. Available online: https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data (accessed on 12 June 2024).
- Plachouras, D.; Lepape, A.; Suetens, C. ECDC definitions and methods for the surveillance of healthcare-associated infections in intensive care units. Intensive Care Med. 2018, 44, 2216–2218. [Google Scholar] [CrossRef]
- Control ECfDPa. Surveillance of Healthcare-Associated Infections and Prevention Indicators in European Intensive Care Units Stockholm. 2017. Available online: https://ecdc.europa.eu/sites/portal/files/documents/HAI-Net-ICU-protocol-v2.2_0.pdf (accessed on 14 June 2024).
- Sobczak, M.; Pawliczak, R. Factors That Affect the COVID-19 Pandemic in Summer 2022 Compared to Summer 2021. Int. J. Environ. Res. Public Health 2022, 19, 12561. [Google Scholar] [CrossRef]
- Cavalieri, S.J.; Kwon, S.; Vivekanandan, R.; Ased, S.; Carroll, C.; Anthone, J.; Schmidt, D.; Baysden, M.; Destache, C.J. Effect of antimicrobial stewardship with rapid MALDI-TOF identification and Vitek 2 antimicrobial susceptibility testing on hospitalization outcome. Diagn. Microbiol. Infect. Dis. 2019, 95, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Halvatsiotis, P.; Kotanidou, A.; Tzannis, K.; Jahaj, E.; Magira, E.; Theodorakopoulou, M.; Konstandopoulou, G.; Gkeka, E.; Pourzitaki, C.; Kapravelos, N.; et al. Demographic and clinical features of critically ill patients with COVID-19 in Greece: The burden of diabetes and obesity. Diabetes Res. Clin. Pract. 2020, 166, 108331. [Google Scholar] [CrossRef]
- Harbuwono, D.S.; Handayani, D.O.; Wahyuningsih, E.S.; Supraptowati, N.; Ananda; Kurniawan, F.; Wafa, S.; Kristanti, M.; Pantoro, N.I.; Sinto, R.; et al. Impact of diabetes mellitus on COVID-19 clinical symptoms and mortality: Jakarta’s COVID-19 epidemiological registry. Prim. Care Diabetes 2022, 16, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wang, Q.; Zhang, H.; Wang, X.; Wan, J.; Yan, Y.; Gao, Y.; Cheng, J.; Li, Z.; Lin, J. The Relationship Between Diabetes Mellitus and COVID-19 Prognosis: A Retrospective Cohort Study in Wuhan, China. Am. J. Med. 2021, 134, e6–e14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Y.; Shen, M.; Zhang, J.; Liu, B.; Dai, M.; Chen, L.; Han, D.; Fan, Y.; Zeng, Y.; et al. Association of diabetes mellitus with disease severity and prognosis in COVID-19: A retrospective cohort study. Diabetes Res. Clin. Pract. 2020, 165, 108227. [Google Scholar] [CrossRef]
- Kumar, A.; Arora, A.; Sharma, P.; Anikhindi, S.A.; Bansal, N.; Singla, V.; Khare, S.; Srivastava, A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. 2020, 14, 535–545. [Google Scholar] [CrossRef]
- Al-Salameh, A.; Lanoix, J.; Bennis, Y.; Andrejak, C.; Brochot, E.; Deschasse, G.; Dupont, H.; Goeb, V.; Jaureguy, M.; Lion, S.; et al. Characteristics and outcomes of COVID-19 in hospitalized patients with and without diabetes. Diabetes Metab. Res. Rev. 2021, 37, e3388. [Google Scholar] [CrossRef]
- Orioli, L.; Servais, T.; Belkhir, L.; Laterre, P.-F.; Thissen, J.-P.; Vandeleene, B.; Maiter, D.; Yombi, J.C.; Hermans, M.P. Clinical characteristics and short-term prognosis of in-patients with diabetes and COVID-19: A retrospective study from an academic center in Belgium. Diabetes Metab. Syndr. 2021, 15, 149–157. [Google Scholar] [CrossRef]
- Başı, N.B.; Metin, S.; Sevinç, S.A.; Peker, N.; Çınar, A.S.; Salkaya, A.; Altuntaş, Y.; Özdemir, H.M. The Effect of Diabetes Mellitus on Mortality in Patients Hospitalized Intensive Care Unit in COVID-19 Pandemic. Acta Biomed. 2022, 93, e2022068. [Google Scholar]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Apicella, M.; Campopiano, M.C.; Mantuano, M.; Mazoni, L.; Coppelli, A.; Del Prato, S. COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020, 8, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, R.; Lu, Z.; Huang, Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging 2020, 12, 6049–6057. [Google Scholar] [CrossRef]
- Alfano, G.; Ferrari, A.; Fontana, F.; Mori, G.; Magistroni, R.; Meschiari, M.; Franceschini, E.; Menozzi, M.; Cuomo, G.; Orlando, G.; et al. Incidence, risk factors and outcome of acute kidney injury (AKI) in patients with COVID-19. Clin. Exp. Nephrol. 2021, 25, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Piovani, D.; Brunetta, E.; Aghemo, A.; Greco, M.; Ciccarelli, M.; Angelini, C.; Voza, A.; Omodei, P.; Vespa, E.; et al. Early Predictors of Clinical Deterioration in a Cohort of 239 Patients Hospitalized for Covid-19 Infection in Lombardy, Italy. J. Clin. Med. 2020, 9, 1548. [Google Scholar] [CrossRef]
- Agarwal, S.; Mathew, J.; Davis, G.M.; Shephardson, A.; Levine, A.; Louard, R.; Urrutia, A.; Perez-Guzman, C.; Umpierrez, G.E.; Peng, L.; et al. Continuous Glucose Monitoring in the Intensive Care Unit During the COVID-19 Pandemic. Diabetes Care 2021, 44, 847–849. [Google Scholar] [CrossRef]
- Fadini, G.P.; Morieri, M.L.; Boscari, F.; Fioretto, P.; Maran, A.; Busetto, L.; Bonora, B.M.; Selmin, E.; Arcidiacono, G.; Pinelli, S.; et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res. Clin. Pract. 2020, 168, 108374. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Vitale, M.; Resi, V.; Orsi, E. Is diabetes mellitus a risk factor for COronaVIrus Disease 19 (COVID-19)? Acta Diabetol. 2020, 57, 1275–1285. [Google Scholar] [CrossRef]
- Mostaza, J.M.; Barbado, F.J.; Fernandez-Martin, J.; Peña-Yañez, J.; Vazquez-Rodriguez, J.J. Cutaneoarticular mucormycosis due to Cunninghamella bertholletiae in a patient with AIDS. Rev. Infect. Dis. 1989, 11, 316–318. [Google Scholar] [CrossRef]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. 2020, 14, 303–310. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Z.; Zhang, J. Insulin Treatment May Increase Adverse Outcomes in Patients With COVID-19 and Diabetes: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 696087. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020, 36, e3319. [Google Scholar] [CrossRef]
- Feldman, E.L.; Savelieff, M.G.; Hayek, S.S.; Pennathur, S.; Kretzler, M.; Pop-Busui, R. COVID-19 and Diabetes: A Collision and Collusion of Two Diseases. Diabetes 2020, 69, 2549–2565. [Google Scholar] [CrossRef] [PubMed]
- McGurnaghan, S.J.; Weir, A.; Bishop, J.; Kennedy, S.; Blackbourn, L.A.K.; A McAllister, D.; Hutchinson, S.; Caparrotta, T.M.; Mellor, J.; Jeyam, A.; et al. Risks of and risk factors for COVID-19 disease in people with diabetes: A cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021, 9, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef]
- Li, X.C.; Zhang, J.; Zhuo, J.L. The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol. Res. 2017, 125 (Pt A), 21–38. [Google Scholar] [CrossRef]
- Daniels, L.B.; Sitapati, A.M.; Zhang, J.; Zou, J.; Bui, Q.M.; Ren, J.; Longhurst, C.A.; Criqui, M.H.; Messer, K. Relation of Statin Use Prior to Admission to Severity and Recovery Among COVID-19 Inpatients. Am. J. Cardiol. 2020, 136, 149–155. [Google Scholar] [CrossRef]
- Lavinio, A.; Ercole, A.; Battaglini, D.; Magnoni, S.; Badenes, R.; Taccone, F.S.; Helbok, R.; Thomas, W.; Pelosi, P.; Robba, C. Safety profile of enhanced thromboprophylaxis strategies for critically ill COVID-19 patients during the first wave of the pandemic: Observational report from 28 European intensive care units. Crit. Care 2021, 25, 155. [Google Scholar] [CrossRef]
- Leão, A.C.; Menezes, P.R.; Oliveira, M.S.; Levin, A.S. Acinetobacter spp. are associated with a higher mortality in intensive care patients with bacteremia: A survival analysis. BMC Infect. Dis. 2016, 16, 386. [Google Scholar] [CrossRef]
- Leung, C.H.; Liu, C.P. Diabetic status and the relationship of blood glucose to mortality in adults with carbapenem-resistant Acinetobacter baumannii complex bacteremia. J. Microbiol. Immunol. Infect. 2019, 52, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Furniss, D.; Gore, S.; Azadian, B.; Myers, S.R. Acinetobacter infection is associated with acquired glucose intolerance in burn patients. J. Burn Care Rehabil. 2005, 26, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, N.; Nakanouchi, J.; Yüzen, D.I.; Fung, S.; Fernandez, J.S.; Barberis, C.; Tuchscherr, L.; Ramirez, M.S. A Study on Acinetobacter baumannii and Staphylococcus aureus Strains Recovered from the Same Infection Site of a Diabetic Patient. Curr. Microbiol. 2019, 76, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Henig, O.; Pogue, J.M.; Martin, E.; Hayat, U.; Ja’ara, M.; E Kilgore, P.; Cha, R.; Dhar, S.; Kaye, K.S. The Impact of Multidrug-Resistant Organisms on Outcomes in Patients with Diabetic Foot Infections. Open Forum Infect. Dis. 2020, 7, ofaa161. [Google Scholar] [CrossRef]
- Qiu, H.; KuoLee, R.; Harris, G.; Chen, W. High susceptibility to respiratory Acinetobacter baumannii infection in A/J mice is associated with a delay in early pulmonary recruitment of neutrophils. Microbes Infect. 2009, 11, 946–955. [Google Scholar] [CrossRef]
- Kleinstein, S.E.; McCorrison, J.; Ahmed, A.; Hasturk, H.; Van Dyke, T.E.; Freire, M. Transcriptomics of type 2 diabetic and healthy human neutrophils. BMC Immunol. 2021, 22, 37. [Google Scholar] [CrossRef]
- Perera, D.; Kleinstein, S.E.; Hanson, B.; Hasturk, H.; Eveloff, R.; Freire, M.; Ramsey, M. Impaired host response and the presence of type-II diabetic patients. iScience 2021, 24, 101941. [Google Scholar] [CrossRef]
- Polemis, M.; Mandilara, G.; Pappa, O.; Argyropoulou, A.; Perivolioti, E.; Koudoumnakis, N.; Pournaras, S.; Vasilakopoulou, A.; Vourli, S.; Katsifa, H.; et al. COVID-19 and Antimicrobial Resistance: Data from the Greek Electronic System for the Surveillance of Antimicrobial Resistance-WHONET-Greece (January 2018–March 2021). Life 2021, 11, 996. [Google Scholar] [CrossRef]
- da Costa, R.L.; Lamas, C.d.C.; Simvoulidis, L.F.N.; Espanha, C.A.; Moreira, L.P.M.; Bonancim, R.A.B.; Weber, J.V.L.A.; Ramos, M.R.F.; Silva, E.C.d.F.; de Oliveira, L.P. Secondary infections in a cohort of patients with COVID-19 admitted to an intensive care unit: Impact of gram-negative bacterial resistance. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e6. [Google Scholar] [CrossRef]
- Dezza, F.C.; Arcari, G.; Alessi, F.; Valeri, S.; Curtolo, A.; Sacco, F.; Ceccarelli, G.; Raponi, G.; Alessandri, F.; Mastroianni, C.M.; et al. Clinical Impact of COVID-19 on Multi-Drug-Resistant Gram-Negative Bacilli Bloodstream Infections in an Intensive Care Unit Setting: Two Pandemics Compared. Antibiotics 2022, 11, 926. [Google Scholar] [CrossRef]
- Polly, M.; de Almeida, B.L.; Lennon, R.P.; Cortês, M.F.; Costa, S.F.; Guimarães, T. Impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil. Am. J. Infect. Control 2022, 50, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Chamieh, A.; Zgheib, R.; El-Sawalhi, S.; Yammine, L.; El-Hajj, G.; Zmerli, O.; Afif, C.; Rolain, J.-M.; Azar, E. Trends of Multidrug-Resistant Pathogens, Difficult to Treat Bloodstream Infections, and Antimicrobial Consumption at a Tertiary Care Center in Lebanon from 2015-2020: COVID-19 Aftermath. Antibiotics 2021, 10, 1016. [Google Scholar] [CrossRef]
- Magnasco, L.; Mikulska, M.; Giacobbe, D.R.; Taramasso, L.; Vena, A.; Dentone, C.; Dettori, S.; Tutino, S.; Labate, L.; Di Pilato, V.; et al. Spread of Carbapenem-Resistant Gram-Negatives and Candida auris during the COVID-19 Pandemic in Critically Ill Patients: One Step Back in Antimicrobial Stewardship? Microorganisms 2021, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Baccolini, V.; Migliara, G.; Isonne, C.; Dorelli, B.; Barone, L.C.; Giannini, D.; Marotta, D.; Marte, M.; Mazzalai, E.; Alessandri, F.; et al. The impact of the COVID-19 pandemic on healthcare-associated infections in intensive care unit patients: A retrospective cohort study. Antimicrob. Resist. Infect. Control 2021, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Serapide, F.; Quirino, A.; Scaglione, V.; Morrone, H.L.; Longhini, F.; Bruni, A.; Garofalo, E.; Matera, G.; Marascio, N.; Scarlata, G.G.M.; et al. Is the Pendulum of Antimicrobial Drug Resistance Swinging Back after COVID-19? Microorganisms 2022, 10, 957. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Gavaruzzi, F.; Ceccarelli, G.; Borrazzo, C.; Oliva, A.; Alessandri, F.; Magnanimi, E.; Pugliese, F.; Venditti, M. Multidrug-resistant Acinetobacter baumannii infections in COVID-19 patients hospitalized in intensive care unit. Infection 2022, 50, 83–92. [Google Scholar] [CrossRef]
- Pascale, R.; Bussini, L.; Gaibani, P.; Bovo, F.; Fornaro, G.; Lombardo, D.; Ambretti, S.; Pensalfine, G.; Appolloni, L.; Bartoletti, M.; et al. Carbapenem-resistant bacteria in an intensive care unit during the coronavirus disease 2019 (COVID-19) pandemic: A multicenter before-and-after cross-sectional study. Infect. Control. Hosp. Epidemiol. 2022, 43, 461–466. [Google Scholar] [CrossRef]
- Thoma, R.; Seneghini, M.; Seiffert, S.N.; Gysin, D.V.; Scanferla, G.; Haller, S.; Flury, D.; Boggian, K.; Kleger, G.-R.; Filipovic, M.; et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: Report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob. Resist. Infect. Control 2022, 11, 12. [Google Scholar]
- Ricciardiello, F.; Falco, M.; Tortoriello, G.; Riccardi, F.; Pellini, R.; Iorio, B.; Russo, G.; Longo, G.; Coppola, C.; Takeuchi, T.; et al. Poorly Differentiated Neuroendocrine Larynx Carcinoma: Clinical Features and miRNAs Signature-A New Goal for Early Diagnosis and Therapy? J. Clin. Med. 2021, 10, 2019. [Google Scholar] [CrossRef]
- Nebreda-Mayoral, T.; Miguel-Gómez, M.A.; March-Rosselló, G.A.; Puente-Fuertes, L.; Cantón-Benito, E.; Martínez-García, A.M.; Muñoz-Martín, A.B.; Orduña-Domingo, A. Bacterial/fungal infection in hospitalized patients with COVID-19 in a tertiary hospital in the Community of Castilla y León, Spain. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2020, 40, 158–165. [Google Scholar] [CrossRef]
- Merola, R.; Iacovazzo, C.; Troise, S.; Marra, A.; Formichella, A.; Servillo, G.; Vargas, M. Timing of Tracheostomy in ICU Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Life 2024, 14, 1165. [Google Scholar] [CrossRef] [PubMed]
- Merola, R.; Vargas, M.; Sanfilippo, F.; Vergano, M.; Mistraletti, G.; Vetrugno, L.; De Pascale, G.; Bignami, E.G.; Servillo, G.; Battaglini, D. Tracheostomy Practice in the Italian Intensive Care Units: A Point-Prevalence Survey. Medicina 2025, 61, 87. [Google Scholar] [CrossRef] [PubMed]
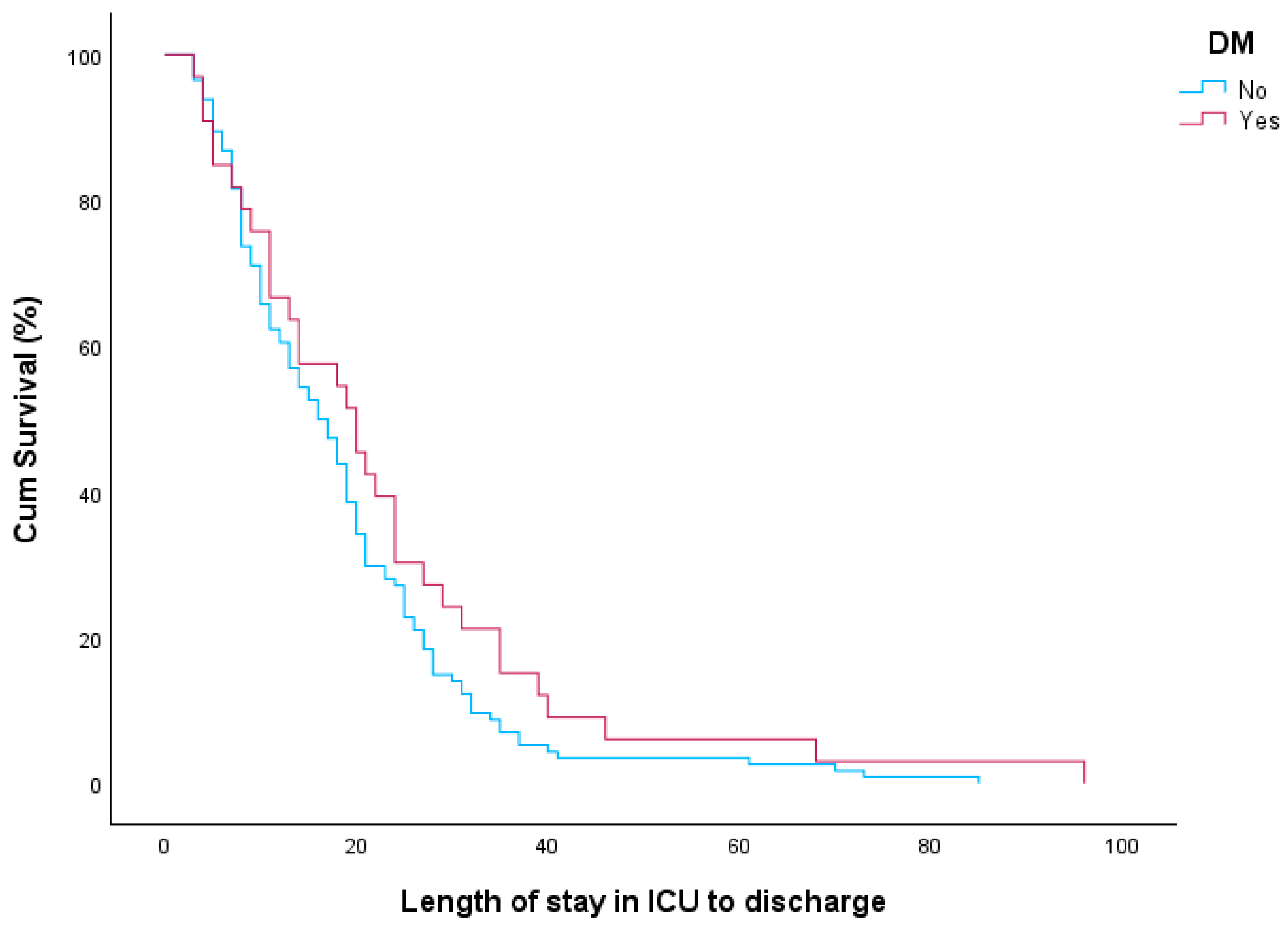
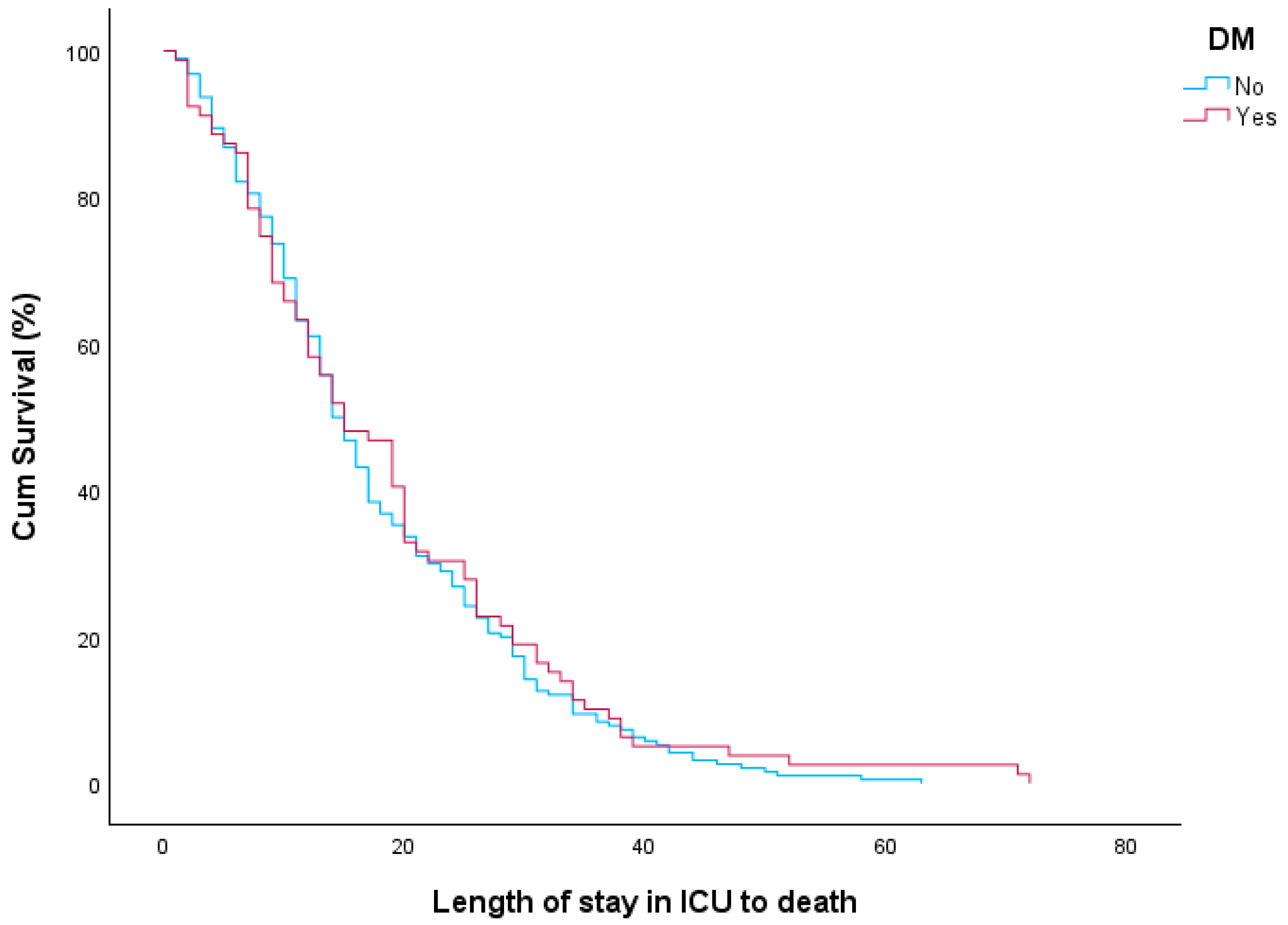
| Total (n = 86) | Deceased (n = 62) | Survived (n = 24) | p-Value | |
|---|---|---|---|---|
| Age (years), Mean ± SD | 69.1 (10.2) | 71.6 (6.6) | 62.8 (14.5) | 0.002 |
| Gender (male), n(%) | 43 (50.0%) | 34 (54.8%) | 9 (37.5%) | 0.149 |
| Pandemic Wave, n(%) | ||||
| 2nd | 21 (24.4%) | 14 (22.6%) | 7 (29.2%) | 0.487 |
| 3rd | 39 (45.3%) | 27 (43.5%) | 12 (50.0%) | |
| 4th | 26 (30.2%) | 21 (33.9%) | 5 (20.8%) | |
| LOS (days), mean ± SD | 13.3 (7.4) | 12.8 (7.2) | 14.5 (8.0) | 0.396 |
| ICU Readmission, n(%) | 5 (5.8%) | 3 (4.8%) | 2 (8.3%) | 0.534 |
| DLP, n(%) | 67 (77.9%) | 48 (77.4%) | 19 (79.2%) | 0.861 |
| HTN, n(%) | 61 (70.9%) | 46 (74.2%) | 15 (62.5%) | 0.284 |
| CAD, n(%) | 19 (22.1%) | 16 (25.8%) | 3 (12.5%) | 0.182 |
| Arrhythmia, n(%) | 10 (11.6%) | 6 (9.7%) | 4 (16.7%) | 0.364 |
| COPD, n(%) | 5 (5.8%) | 4 (6.5%) | 1 (4.2%) | 0.685 |
| Cancer, n(%) | 2 (2.3%) | 1 (1.6%) | 1 (4.2%) | 0.481 |
| Hypothyroidism, n(%) | 11 (12.8%) | 7 (11.3%) | 4 (16.7%) | 0.503 |
| Hyperuricemia, n(%) | 11 (12.8%) | 9 (14.5%) | 2 (8.3%) | 0.441 |
| Anemia n(%) | 10 (11.6%) | 8 (12.9%) | 2 (8.3%) | 0.553 |
| BPH, n(%) | 7 (8.1%) | 6 (9.7%) | 1 (4.2%) | 0.402 |
| Psychiatric Disorder, n(%) | 16 (18.6%) | 13 (21.0%) | 3 (12.5%) | 0.365 |
| Statin, n(%) | 59 (68.6%) | 43 (69.4%) | 16 (66.7%) | 0.810 |
| ASA, n(%) | 32 (37.2%) | 22 (35.5%) | 10 (41.7%) | 0.595 |
| Clopidogrel, n(%) | 15 (17.4%) | 13 (21.0%) | 2 (8.3%) | 0.166 |
| NOACs, n(%) | 9 (10.5%) | 7 (11.3%) | 2 (8.3%) | 0.688 |
| Acenocoumarol, n(%) | 1 (1.2%) | 0 (0.0%) | 1 (4.2%) | 0.106 |
| ARBs, n(%) | 44 (51.2%) | 32 (51.6%) | 12 (50.0%) | 0.893 |
| Diuretics, n(%) | 43 (50.0%) | 31 (50.0%) | 12 (50.0%) | 1.000 |
| CCBs, n(%) | 34 (39.5%) | 26 (41.9%) | 8 (33.3%) | 0.464 |
| Beta-blockers n(%) | 31 (36.0%) | 22 (35.5%) | 9 (37.5%) | 0.861 |
| ACEi, n(%) | 9 (10.5%) | 8 (12.9%) | 1 (4.2%) | 0.235 |
| Aldosterone Antagonists, n(%) | 4 (4.7%) | 4 (6.5%) | 0 (0.0%) | 0.203 |
| Central α-agonists, n(%) | 4 (4.7%) | 3 (4.8%) | 1 (4.2%) | 0.894 |
| No Antidiabetic Treatment, n(%) | 7 (8.1%) | 2 (3.2%) | 5 (20.8%) | 0.021 |
| OAD Monotherapy, n(%) | 54 (62.8%) | 44 (71.0%) | 10 (41.7%) | |
| Insulin Monotherapy, n(%) | 14 (16.3%) | 9 (14.5%) | 5 (20.8%) | |
| Combination OADs/Insulin, n(%) | 11 (12.8%) | 7 (11.3%) | 4 (16.7%) | |
| MET, n(%) | 54 (62.8%) | 40 (64.5%) | 14 (58.3%) | 0.595 |
| DPP-4i, n(%) | 31 (36.0%) | 24 (38.7%) | 7 (29.2%) | 0.408 |
| SGLT-2i, n(%) | 20 (23.3%) | 13 (21.0%) | 7 (29.2%) | 0.420 |
| SU n(%) | 16 (18.6%) | 12 (19.4%) | 4 (16.7%) | 0.774 |
| GLP-1 RA, n(%) | 9 (10.5%) | 7 (11.3%) | 2 (8.3%) | 0.688 |
| PIOn(%) | 2 (2.3%) | 2 (3.2%) | 0 (0.0%) | 0.517 |
| APACHE II on Admission, Median (IQR) | 14 (6) | 14.5 (7) | 13 (4) | 0.010 |
| AKI on Admission, n(%) | 25 (29.1%) | 22 (35.5%) | 3 (12.5%) | 0.035 |
| Admission Glucose Value (mg/dL) mean ± SD | 219.1 (84.8) | 211.0 (75.8) | 240.1 (103.4) | 0.277 |
| Mean Fasting Glucose (mg/dL) Mean ± SD | 200.2 (48.8) | 199.7 (45.4) | 201.2 (57.8) | 0.725 |
| WBC (K/μL), Mean ± SD | 14.4 (6.4) | 15.3 (6.5) | 12.1 (5.3) | 0.033 |
| Hct (%), Mean ± SD | 36.1 (5.5) | 36.3 (5.6) | 35.7 (5.3) | 0.473 |
| Cr serum (mg/dL), Mean ± SD | 1.4 (1.3) | 1.5 (1.4) | 1.1 (0.6) | 0.045 |
| eGFR (mL/min), Mean ± SD | 62.1 (28.5) | 58.2 (28.0) | 72.1 (27.7) | 0.053 |
| Troponin (pg/mL), Mean ± SD | 59.2 (124.3) | 70.7 (152.1) | 36.3 (21.6) | 0.371 |
| CRP (mg/L), Mean ±SD | 108.3 (79.3) | 109.5 (82.4) | 104.5 (70.7) | 0.949 |
| Ferritin (ng/mL), Mean ± SD | 1203.5 (1848.1) | 1280.4 (1684.2) | 955.7 (2339.2) | 0.005 |
| PCT (ng/mL), Mean ± SD | 1.5 (4.3) | 1.8 (4.7) | 0.2 (0.3) | 0.030 |
| D-dimer (μg/dL), Mean ± SD | 6048.8 (8382.9) | 6963.2 (9451.5) | 3402.1 (2656.0) | 0.634 |
| All Patients (LOS ≤ 28 Days) Multivariate OR (95% CI) | p-Value | DM Patients (LOS ≤ 28 Days) Multivariate OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Age (years) | 1.01 (0.98–1.04) | 0.418 | 1.10 (1.02–1.18) | 0.011 |
| DM | 1.12 (0.52–2.41) | 0.769 | - | - |
| HTN | 1.01 (0.55–1.85) | 0.980 | - | - |
| CAD | 1.82 (0.75–4.43) | 0.184 | 1.97 (0.69–3.51) | 0.497 |
| COPD | 2.85 (1.06–7.68) | 0.038 | - | - |
| APACHE II | 1.14 (1.02–1.26) | 0.017 | 1.02 (0.88–1.20) | 0.763 |
| AKI | 1.56 (0.69–3.51) | 0.283 | 4.63 (1.02–20.94) | 0.047 |
| Admission glucose value (mg/dL) | 1.03 (0.99–1.07) | 0.213 | 1.01 (0.99–1.12) | 0.904 |
| WBC (K/μL) | 1.05 (1.00–1.09) | 0.035 | 1.09 (0.98–1.20) | 0.084 |
| Ferritin (ng/mL) | 1.00 (1.00–1.00) | 0.058 | - | - |
| DM vs. Non-DM | 95% CI | ||||
|---|---|---|---|---|---|
| Culture Type | Pathogen | OR | Lower 95% | Upper 95% | p-Value |
| Bronchial Secretion | Acinetobacter baumannii | 2.179 | 1.397 | 3.399 | <0.001 |
| Klebsiella pneumoniae | 0.968 | 0.544 | 1.72 | 0.911 | |
| Pseudomonas aeruginosa | 0.661 | 0.328 | 1.332 | 0.247 | |
| Stenotrophomonas maltophilia | 0.986 | 0.426 | 2.284 | 0.974 | |
| Enterobacter cloacae | 2.761 | 0.549 | 13.888 | 0.218 | |
| Enterobacter aerogenes | 0.904 | 0.093 | 8.781 | 0.931 | |
| Providencia stuartii | 0.904 | 0.093 | 8.781 | 0.931 | |
| Blood | Acinetobacter baumannii | 1.226 | 0.737 | 2.037 | 0.432 |
| Klebsiella pneumoniae | 0.989 | 0.579 | 1.689 | 0.967 | |
| Pseudomonas aeruginosa | 0.556 | 0.185 | 1.67 | 0.295 | |
| Providencia stuartii | 0.382 | 0.046 | 3.142 | 0.371 | |
| Stenotrophomonas maltophilia | 0.382 | 0.046 | 3.142 | 0.371 | |
| CVC Tip | Acinetobacter baumannii | 0.718 | 0.332 | 1.552 | 0.399 |
| Klebsiella pneumoniae | 0.558 | 0.225 | 1.386 | 0.209 | |
| Pseudomonas aeruginosa | 1.168 | 0.297 | 4.596 | 0.824 | |
| Providencia stuartii | 0.673 | 0.141 | 3.217 | 0.620 | |
| Urine | Klebsiella pneumoniae | 0.956 | 0.367 | 2.488 | 0.926 |
| Acinetobacter baumannii | 0.789 | 0.284 | 2.191 | 0.649 | |
| Providencia stuartii | 1.087 | 0.208 | 5.686 | 0.921 | |
| Acinetobacter baumannii (Bronchial Secretions) Multivariate OR | 95% CI (Lower–Upper) | p-Value | |
|---|---|---|---|
| DM | 2.046 | 1.256–3.333 | 0.004 |
| DLP | 1.15 | 0.733–1.803 | 0.543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dourliou, V.; Kakaletsis, N.; Stamou, D.; Champla, A.; Tsakiri, K.; Agapakis, D.; Didangelos, T. Diabetes Mellitus and Multidrug-Resistant Gram-Negative Bacterial Infections in Critically Ill COVID-19 Patients: A Retrospective Observational Study. Diagnostics 2025, 15, 1190. https://doi.org/10.3390/diagnostics15101190
Dourliou V, Kakaletsis N, Stamou D, Champla A, Tsakiri K, Agapakis D, Didangelos T. Diabetes Mellitus and Multidrug-Resistant Gram-Negative Bacterial Infections in Critically Ill COVID-19 Patients: A Retrospective Observational Study. Diagnostics. 2025; 15(10):1190. https://doi.org/10.3390/diagnostics15101190
Chicago/Turabian StyleDourliou, Vasiliki, Nikolaos Kakaletsis, Dafni Stamou, Antigoni Champla, Kalliopi Tsakiri, Dimitrios Agapakis, and Triantafyllos Didangelos. 2025. "Diabetes Mellitus and Multidrug-Resistant Gram-Negative Bacterial Infections in Critically Ill COVID-19 Patients: A Retrospective Observational Study" Diagnostics 15, no. 10: 1190. https://doi.org/10.3390/diagnostics15101190
APA StyleDourliou, V., Kakaletsis, N., Stamou, D., Champla, A., Tsakiri, K., Agapakis, D., & Didangelos, T. (2025). Diabetes Mellitus and Multidrug-Resistant Gram-Negative Bacterial Infections in Critically Ill COVID-19 Patients: A Retrospective Observational Study. Diagnostics, 15(10), 1190. https://doi.org/10.3390/diagnostics15101190







