Early-Onset Candidemia in Adult Intensive Care Units
Abstract
1. Introduction
2. Common Pathogens
3. Risk Factors
3.1. Administration of Broad-Spectrum Antibiotics
3.2. Surgical and Non-Surgical Disorders of Gastrointestinal Tract
| Risk Factors | Characteristics |
|---|---|
| Broad-spectrum antibiotics [36,37,38,39,40,41] | Piperacillin/tazobactam, vancomycin, cephalosporins, anti-anaerobic agents, glycopeptides, carbapenems, and tigecycline, aminoglycosides |
| Disorders of the gastrointestinal tract [43,44,45,46,47] | Perforation, bowel anastomosis leaks, peritonitis, reduced bowel function, and acute pancreatitis |
| Indwelling catheters [48,49,50,51] | Central venous catheters, arterial catheters, and dialysis catheters |
| Co-morbidities [52,53,54,55,56] | Chronic cardiovascular disease, chronic respiratory disease, kidney dysfunction, and diabetes mellitus |
| Immunosuppression [57,58,59,60,61,62,63] | Septic shock, corticosteroid therapy, solid organ transplantation, hematopoietic stem cell transplantation, and diabetes mellitus |
| Major trauma and burns [64,65,66,67] | High injury severity score, increased number of blood transfusions, numerous surgical interventions, and immunosuppression |
| Total parenteral nutrition [30,68,69] | Use of central venous catheters, rapid proliferation in TPN solutions |
| Mechanical ventilation [47,70,71] | Requirement for more than 48 h increases the risk of invasive candidiasis |
| Renal replacement therapy [40,72] | Central venous catheters, recurrent manipulations |
3.3. Indwelling Catheters
3.4. Co-Existing Medical Conditions
3.5. Immunosuppression
3.6. Major Trauma and Burns
3.7. Total Parenteral Nutrition
3.8. Mechanical Ventilation
3.9. Renal Replacement Therapy
4. Clinical Manifestations
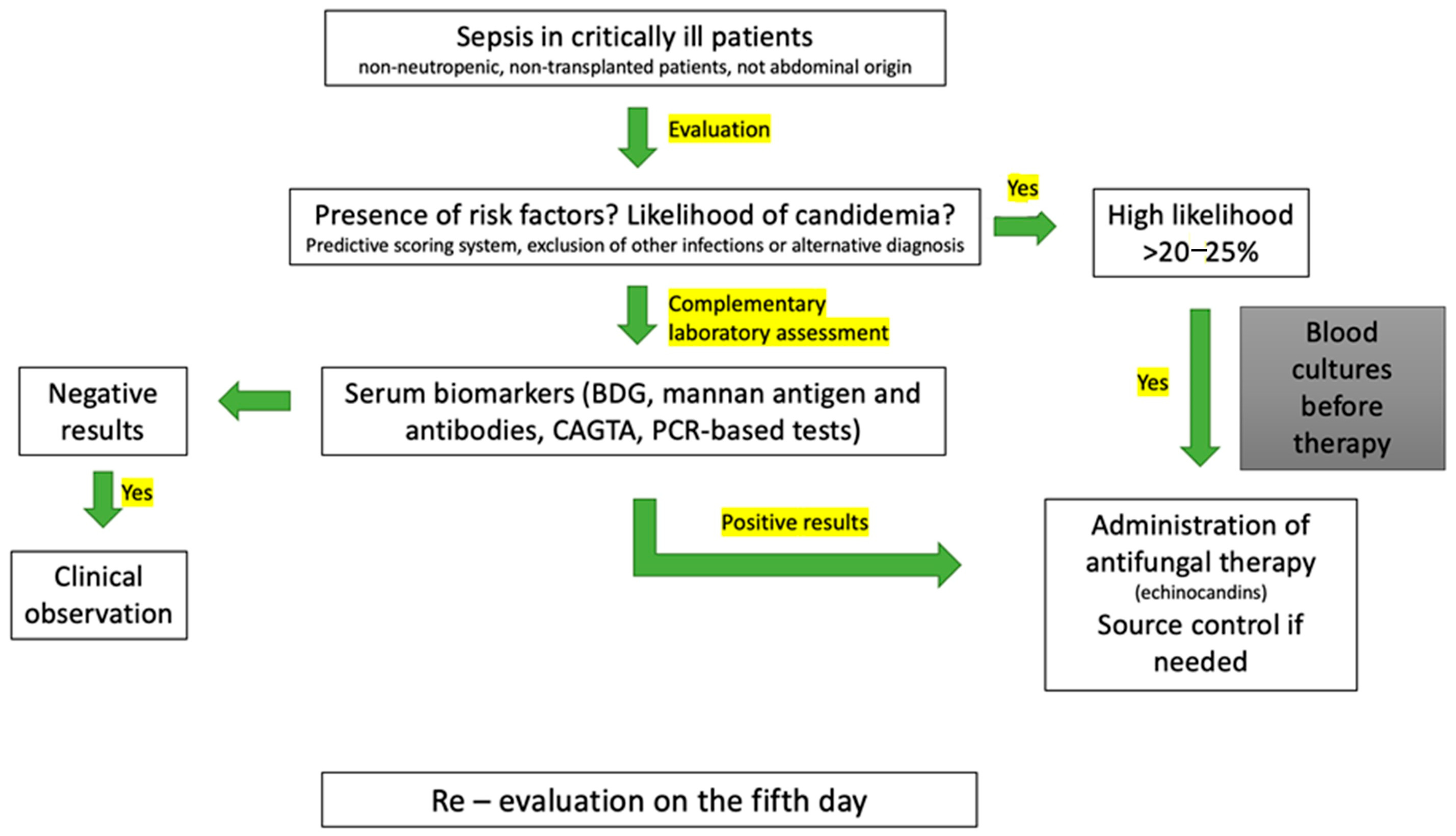
5. Diagnosis
5.1. Blood Cultures
5.2. Molecular Techniques
5.3. Serum Biomarkers
| Laboratory test | Characteristics |
|---|---|
| Blood cultures [88,89,90,91,92,93,94,95,96,97,98,99,100,101] | Definitive diagnosis Susceptibility testing Long turnaround time (reduced with MALDI-TOF-MS and PNA-FISH technology) |
| PCR-based tests [103,104,105,106,107,108,109,110,111] | Rapid turnaround time High sensitivity and specificity Multiple PCR panels High cost |
| T2Candida panel [112,113,114] | Combination of methods High sensitivity and specificity Rapid turnaround time No need for sample preparation High cost of purchasing equipment for the laboratory Not available in all laboratories |
| BDG assay [104,116,117,118,119,120,121,122] | High diagnostic sensitivity Rapid turnaround time High negative predictive value Poor species specificity |
| CAGTA [124,127,128,129,130] | Rapid turnaround time Better detection of candidemia and deep-seated candidiasis |
| Mannan antigen and anti-mannan antibody [51,123,124,125,126] | Rapid turnaround time Increased sensitivity and specificity when used together Suboptimal predictive accuracy |
5.4. Predictive Scoring Systems
6. Management
6.1. Antifungal Therapy
| Strategy | Antifungal Agents | Comments | References |
|---|---|---|---|
| Prophylaxis |
|
| Cornely, O.A. et al. [131] Cornely, O.A. et al. [142] Echeverria, P. et al. [143] Martin-Loeches, I. et al. [145] Einav, S. et al. [146] Cortegiani, A. et al. [147] Chen, S.C.A. et al. [148] |
| Pre-emptive therapy |
|
| Martin-Loeches, I. et al. [145] Sprute, R. et al. [151] Pham, H.T. et al. [153] |
| Empirical therapy |
|
| Pappas, P.G. et al. [73] Martin-Loeches, I. et al. [145] León, C. et al. [155] Klastersky, J. [156] Tang, B.H.E. et al. [157] Kanj, S.S. et al. [158] |
| Targeted therapy |
|
| Pappas, P.G. et al. [73] Martin-Loeches, I. et al. [145] Boutin, C.A. et al. [159] Chatelon, J. et al. [160] Garnacho-Montero, J. et al. [161] Yang, Q. et al. [162] |
6.2. Source Control
6.3. General Supportive Management
7. Prognosis and Prevention
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICU | Intensive Care Unit |
| BSI | Bloodstream Infection |
| AFS | Antifungal Stewardship |
| TPN | Total Parenteral Nutrition |
| MALDI-TOF-MS | Matrix-assisted laser desorption ionization time of flight mass spectrometry |
| PNA-FISH | Peptide nucleic acid—fluorescence in situ hybridization |
| PCR | Polymerase chain reaction |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| BDG | 1,3-β-D-glucan |
| CAGTA | Candida species germ tube antibody |
References
- Vincent, J.L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bajwa, S.; Kulshrestha, A. Fungal infections in intensive care unit: Challenges in diagnosis and management. Ann. Med. Health Sci. Res. 2013, 3, 238–244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cortegiani, A.; Russotto, V.; Graziano, G.; Saporito, L.; Raineri, S.M.; Mammina, C.; Giarratano, A. Bloodstream infections in intensive care unit patients: Distribution and antibiotic resistance of bacteria. Infect. Drug Resist. 2015, 8, 287–296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montagna, M.T.; Caggiano, G.; Lovero, G.; De Giglio, O.; Coretti, C.; Cuna, T.; Iatta, R.; Giglio, M.; Dalfino, L.; Bruno, F.; et al. Epidemiology of invasive fungal infections in the intensive care unit: Results of a multicenter Italian survey (AURORA Project). Infection 2013, 41, 645–653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kotey, F.C.; Dayie, N.T.; Tetteh-Uarcoo, P.B.; Donkor, E.S. Candida Bloodstream Infections: Changes in Epidemiology and Increase in Drug Resistance. Infect. Dis. Res. Treat. 2021, 14, 11786337211026927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- León, C.; Ruiz-Santana, S.; Saavedra, P.; Galván, B.; Blanco, A.; Castro, C.; Balasini, C.; Utande-Vázquez, A.; González de Molina, F.J.; Blasco-Navalproto, M.A.; et al. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: A prospective multicenter study. Crit. Care Med. 2009, 37, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Gupta, V.; Sun, X.; Johannes, R.S.; Spalding, J.; Tabak, Y.P. Burden of early-onset candidemia: Analysis of culture-positive bloodstream infections from a large U.S. database. Crit. Care Med. 2009, 37, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, F.G.; Trecarichi, E.M.; Montrucchio, C.; Losito, A.R.; Raviolo, S.; Posteraro, B.; Corcione, S.; Di Giambenedetto, S.; Fossati, L.; Sanguinetti, M.; et al. Mortality in patients with early- or late-onset candidaemia. J. Antimicrob. Chemother. 2013, 68, 927–935. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kidd, S.E.; Abdolrasouli, A.; Hagen, F. Fungal Nomenclature: Managing Change is the Name of the Game. Open Forum. Infect. Dis. 2023, 10, ofac559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The Virulence Factors and Clinical Manifestations of Infection. J. Fungi. 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Barata-Antunes, C.; Casal, M.; Brown, A.J.P.; Van Dijck, P.; Paiva, S. Adapting to survive: How Candida overcomes host-imposed constraints during human colonization. PLoS Pathog. 2020, 16, e1008478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Leary, R.A.; Einav, S.; Leone, M.; Madách, K.; Martin, C.; Martin-Loeches, I. Management of invasive candidiasis and candidaemia in critically ill adults: Expert opinion of the European Society of Anaesthesia Intensive Care Scientific Subcommittee. J. Hosp. Infect. 2018, 98, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Soulountsi, V.; Schizodimos, T.; Kotoulas, S.C. Deciphering the epidemiology of invasive candidiasis in the intensive care unit: Is it possible? Infection 2021, 49, 1107–1131. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Liu, Y.; Hu, K. Epidemiology and risk factors of candidemia due to Candida parapsilosis in an intensive care unit. Rev. Inst. Med. Trop. São Paulo 2021, 63, e20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, W.; Song, X.; Wu, H.; Zheng, R. Epidemiology, species distribution, and predictive factors for mortality of candidemia in adult surgical patients. BMC Infect. Dis. 2020, 20, 506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernández-Ruiz, M.; Puig-Asensio, M.; Guinea, J.; Almirante, B.; Padilla, B.; Almela, M.; Díaz-Martín, A.; Rodríguez-Baño, J.; Cuenca-Estrella, M.; Aguado, J.M.; et al. Candida tropicalis bloodstream infection: Incidence, risk factors and outcome in a population-based surveillance. J. Infect. 2015, 71, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Bouza, E.; Muñoz, P. Epidemiology of candidemia in intensive care units. Int. J. Antimicrob. Agents 2008, 32, S87–S91. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.Y.; Lee, L.N.; Jerng, J.S.; Yu, C.J.; Hsueh, P.R. Candida glabrata fungaemia in intensive care units. Clin. Microbiol. Infect. 2008, 14, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Lv, G.; Song, Y.H.; Zhao, J.T.; Liu, J.Y.; Wang, L.L.; Xiang, M.J. Antifungal susceptibility, molecular epidemiology, and clinical risk factors of Candida glabrata in intensive care unit in a Chinese Tertiary Hospital. Front. Cell. Infect. Microbiol. 2024, 14, 1455145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, Z.; Ding, Y.; Tian, G.; Yang, K.; Deng, J.; Li, G.; Liu, J. A seven-year surveillance study of the epidemiology, antifungal susceptibility, risk factors and mortality of candidaemia among paediatric and adult inpatients in a tertiary teaching hospital in China. Antimicrob. Resist. Infect. Control. 2020, 9, 133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonatti, H.; Stelzmueller, I.; Berger, N.; Lechner, M.; Grif, K.; Geltner, C.; Margreiter, R.; Lass-Flörl, C. Infections Caused by Candida krusei in Five Transplant and Two Surgical Patients. Surg. Infect. 2009, 10, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Wu, C.; Kothari, S. Outcomes with micafungin in patients with candidaemia or invasive candidiasis due to Candida glabrata and Candida krusei. J. Antimicrob. Chemother. 2011, 66, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Poulopoulou, A.; Sidiropoulou, A.; Sarmourli, T.; Zachrou, E.; Michailidou, C.; Zarras, C.; Vagdatli, E.; Massa, E.; Mouloudi, E.; Pyrpasopoulou, A.; et al. Candida auris: Outbreak, surveillance and epidemiological monitoring in Northern Greece. Med. Mycol. 2024, 62, myae062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cortegiani, A.; Misseri, G.; Giarratano, A.; Bassetti, M.; Eyre, D. The global challenge of Candida auris in the intensive care unit. Crit. Care 2019, 23, 150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhargava, A.; Klamer, K.; Sharma, M.; Ortiz, D.; Saravolatz, L. Candida auris: A Continuing Threat. Microorganisms 2025, 13, 652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rhodes, J.; Fisher, M.C. Global epidemiology of emerging Candida auris. Curr. Opin. Microbiol. 2019, 52, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Dire, O.; Ahmad, A.; Duze, S.; Patel, M. Survival of Candida auris on environmental surface materials and low-level resistance to disinfectant. J. Hosp. Infect. 2023, 137, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Roudbary, M.; Brás, S.; Tafaj, S.; Rodrigues, C.F. Candida auris: A Quick Review on Identification, Current Treatments, and Challenges. Int. J. Mol. Sci. 2021, 22, 4470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poissy, J.; Damonti, L.; Bignon, A.; Khanna, N.; Von Kietzell, M.; Boggian, K.; Neofytos, D.; Vuotto, F.; Coiteux, V.; Artru, F.; et al. Risk factors for candidemia: A prospective matched case-control study. Crit. Care 2020, 24, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bassetti, M.; Giacobbe, D.R.; Vena, A.; Wolff, M. Diagnosis and Treatment of Candidemia in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2019, 40, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R.; Ostrosky- Zeichner, L.; Govrins, M.A. Invasive candidiasis. Nat. Rev. Dis. Primer. 2024, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mason, K.L.; Erb Downward, J.R.; Mason, K.D.; Falkowski, N.R.; Eaton, K.A.; Kao, J.Y.; Young, V.B.; Huffnagle, G.B. Candida albicans and Bacterial Microbiota Interactions in the Cecum during Recolonization following Broad-Spectrum Antibiotic Therapy. Infect. Immun. 2012, 80, 3371–3380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rhee, C.; Chen, T.; Kadri, S.S.; Lawandi, A.; Yek, C.; Walker, M.; Warner, S.; Fram, D.; Chen, H.C.; Shappell, C.N.; et al. Trends in Empiric Broad-Spectrum Antibiotic Use for Suspected Community-Onset Sepsis in US Hospitals. JAMA Netw. Open. 2024, 7, e2418923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Breda, G.L.; Tuon, F.F.; Meis, J.F.; Herkert, P.F.; Hagen, F.; De Oliveira, L.Z.; Dias, V.C.; da Cunha, C.A.; Queiroz-Telles, F. Breakthrough candidemia after the introduction of broad spectrum antifungal agents: A 5-year retrospective study. Med. Mycol. 2018, 56, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Durmuş, M.; Kalkan, S.; Güzel Karahan, S.; Biçakcioğlu, M.; Özdemir, N.; Gün, Z.Ü.; Özer, A.B. Can antibiotics affect the clinical features of patients with candidemia? The retrospective evaluation of 5 years of data in an intensive care unit. Eur. J. Hosp. Pharm. 2024, 3, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Carmeli, Y.; Zumsteg, J.; Flores, E.L.; Tolentino, J.; Sreeramoju, P.; Weber, S.G. Prior Antimicrobial Therapy and Risk for Hospital-Acquired Candida glabrata and Candida krusei Fungemia: A Case-Case-Control Study. Antimicrob. Agents Chemother. 2005, 49, 4555–4560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.H.; Mun, S.J.; Kang, J.S.; Moon, C.; Kim, H.T.; Lee, H.Y. Multifaceted Evaluation of Antibiotic Therapy as a Factor Associated with Candidemia in Non-Neutropenic Patients. J. Fungi. 2023, 9, 270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas-Rüddel, D.O.; Schlattmann, P.; Pletz, M.; Kurzai, O.; Bloos, F. Risk Factors for Invasive Candida Infection in Critically Ill Patients. Chest 2022, 161, 345–355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eggimann, P.; Bille, J.; Marchetti, O. Diagnosis of invasive candidiasis in the ICU. Ann. Intensive Care. 2011, 1, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumamoto, C.A. Inflammation and gastrointestinal Candida colonization. Curr. Opin. Microbiol. 2011, 14, 386–391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schulze, J.; Sonnenborn, U. Yeasts in the gut: From commensals to infectious agents. Dtsch. Arztebl. Int. 2009, 106, 837–842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, L.; Yang, C.; Tang, J. Disruption of the intestinal mucosal barrier in Candida albicans infections. Microbiol. Res. 2013, 168, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Holzknecht, B.J.; Thorup, J.; Arendrup, M.C.; Andersen, S.E.; Steensen, M.; Hesselfeldt, P.; Nielsen, J.M.; Knudsen, J.D. Decreasing candidaemia rate in abdominal surgery patients after introduction of fluconazole prophylaxis. Clin. Microbiol. Infect. 2011, 17, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Tissot, F.; Lamoth, F.; Hauser, P.M.; Orasch, C.; Flückiger, U.; Siegemund, M.; Zimmerli, S.; Calandra, T.; Bille, J.; Eggimann, P.; et al. β-Glucan Antigenemia Anticipates Diagnosis of Blood Culture–Negative Intraabdominal Candidiasis. Am. J. Respir. Crit. Care Med. 2013, 188, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, P.; Pittet, D. Candida colonization index and subsequent infection in critically ill surgical patients: 20 years later. Intensive Care Med. 2014, 40, 1429–1448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Timsit, J.F.; Baleine, J.; Bernard, L.; Calvino-Gunther, S.; Darmon, M.; Dellamonica, J.; Desruennes, E.; Leone, M.; Lepape, A.; Leroy, O.; et al. Expert consensus-based clinical practice guidelines management of intravascular catheters in the intensive care unit. Ann. Intensive Care. 2020, 10, 118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kato, H.; Yoshimura, Y.; Suido, Y.; Shimizu, H.; Ide, K.; Sugiyama, Y.; Matsuno, K.; Nakajima, H. Mortality and risk factor analysis for Candida blood stream infection: A multicenter study. J. Infect. Chemother. 2019, 25, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, M.; Halleyantoro, R.; Kalumpiu, J.F. Biofilm: The invisible culprit in catheter-induced candidemia. AIMS Microbiol. 2023, 9, 467–485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alves, J.; Alonso-Tarrés, C.; Rello, J. How to Identify Invasive Candidemia in ICU—A Narrative Review. Antibiotics 2022, 11, 1804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lausch, K.R.; Søgaard, M.; Rosenvinge, F.S.; Johansen, H.K.; Boysen, T.; Røder, B.; Mortensen, K.L.; Nielsen, L.; Lemming, L.; Olesen, B.; et al. High incidence of candidaemia in a nationwide cohort: Underlying diseases, risk factors and mortality. Int. J. Infect. Dis. 2018, 76, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Keighley, C.; Chen, S.C.A.; Marriott, D.; Pope, A.; Chapman, B.; Kennedy, K.; Bak, N.; Underwood, N.; Wilson, H.L.; McDonald, K.; et al. Candidaemia and a risk predictive model for overall mortality: A prospective multicentre study. BMC Infect. Dis. 2019, 19, 445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dowey, R.; Iqbal, A.; Heller, S.R.; Sabroe, I.; Prince, L.R. A Bittersweet Response to Infection in Diabetes; Targeting Neutrophils to Modify Inflammation and Improve Host Immunity. Front. Immunol. 2021, 12, 678771. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodrigues, C.; Rodrigues, M.; Henriques, M. Candida sp. Infections in Patients with Diabetes Mellitus. J. Clin. Med. 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kojic, E.M.; Darouiche, R.O. Candida Infections of Medical Devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hankovszky, P.; Társy, D.; Öveges, N.; Molnár, Z. Invasive Candida Infections in the ICU: Diagnosis and Therapy. J. Crit. Care Med. 2015, 1, 129–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ortega-Loubon, C.; Cano-Hernández, B.; Poves-Alvarez, R.; Muñoz-Moreno, M.F.; Román-García, P.; Balbás-Alvarez, S.; de la Varga-Martínez, O.; Gómez-Sánchez, E.; Gómez-Pesquera, E.; Lorenzo-López, M.; et al. The Overlooked Immune State in Candidemia: A Risk Factor for Mortality. J. Clin. Med. 2019, 8, 1512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, G.; Karabinis, A.; Samonis, G.; Falagas, M.E. Candidemia in immunocompromised and immunocompetent critically ill patients: A prospective comparative study. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 377–384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Hal, S.J.; Marriott, D.J.E.; Chen, S.C.A.; Nguyen, Q.; Sorrell, T.C.; Ellis, D.H.; Slavin, M.A. Candidemia following solid organ transplantation in the era of antifungal prophylaxis: The Australian experience. Transpl. Infect. Dis. 2009, 11, 122–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, S.; Tridello, G.; Knelange, N.S.; Blijlevens, N.; Martin, M.; Snowden, J.A.; Malladi, R.; Ljungman, P.; Deconinck, E.; Gedde-Dahl, T.; et al. Impact of early candidemia on the long-term outcome of allogeneic hematopoietic stem cell transplant in non-leukemic patients: An outcome analysis on behalf of IDWP–EBMT. Bone Marrow Transplant. 2021, 56, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Javidnia, J.; Abastabar, M.; Mobayen, M.R.; Moslemi, A.; Rahimzadeh, G.; Yazdani Charati, J.; Mirzaei Tirabadi, N.; Nouranibaladezaei, S.; Asghari, H.; et al. Multi-state evaluation of Candida infections in burn patients. Mycoses 2024, 67, e13788. [Google Scholar] [CrossRef] [PubMed]
- Manolakaki, D.; Velmahos, G.; Kourkoumpetis, T.; Chang, Y.; Alam, H.B.; De Moya, M.M.; Mylonakis, E. Candida infection and colonization among trauma patients. Virulence 2010, 1, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.; Gregson, D.B.; Kirkpatrick, A.W.; Kortbeek, J.B.; Zygun, D.A.; Findlay, C.; Hameed, S.M. Bloodstream infection complicating trauma. Clin. Investig. Med. Med. Clin. Exp. 2004, 27, 253–258. [Google Scholar] [PubMed]
- Haltmeier, T.; Inaba, K.; Effron, Z.; Dollbaum, R.; Shulman, I.A.; Benjamin, E.; Lam, L.; Demetriades, D. Candida Score as a Predictor of Worse Outcomes and Mortality in Severely Injured Trauma Patients with Positive Candida Cultures. Am. Surg. 2015, 81, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Lay, C.J.; Wang, C.L.; Lin, M.L.; Yang, S.P. Prognostic factors of candidemia among nonneutropenic adults with total parenteral nutrition. J. Microbiol. Immunol. Infect. 2011, 44, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Shimono, K.; Kaneda, S.; Tamura, T.; Ichihara, M.; Nakashima, Y. Growth of Microorganisms in Total Parenteral Nutrition Solutions Containing Lipid. Int. J. Med. Sci. 2010, 7, 101–109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azoulay, E.; Timsit, J.F.; Tafflet, M.; De Lassence, A.; Darmon, M.; Zahar, J.R.; Adrie, C.; Garrouste-Orgeas, M.; Cohen, Y.; Mourvillier, B.; et al. Candida Colonization of the Respiratory Tract and Subsequent Pseudomonas Ventilator-Associated Pneumonia. Chest 2006, 129, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.F.; Kabir, M.; Chen, S.C.A.; Playford, E.G.; Marriott, D.J.; Jones, M.; Lipman, J.; McBryde, E.; Gottlieb, T.; Cheung, W.; et al. Candida colonization as a risk marker for invasive candidiasis in mixed medical-surgical intensive care units: Development and evaluation of a simple, standard protocol. J. Clin. Microbiol. 2015, 53, 1324–1330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hohmann, F.B.; Chaves, R.C.D.F.; Olivato, G.B.; Souza, G.M.D.; Galindo, V.B.; Silva Jr, M.; Martino, M.D.V.; Menezes, F.G.; Corrêa, T.D. Characteristics, risk factors, and outcomes of bloodstream Candida infections in the intensive care unit: A retrospective cohort study. J. Int. Med. Res. 2023, 51, 03000605221131122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mikulska, M.; Del Bono, V.; Ratto, S.; Viscoli, C. Occurrence, presentation and treatment of candidemia. Expert. Rev. Clin. Immunol. 2012, 8, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Honore, P.M.; Puerta-Alcalde, P.; Garcia-Vidal, C.; Pagotto, A.; Gonçalves-Bradley, D.C.; Verweij, P.E. Invasive candidiasis: Current clinical challenges and unmet needs in adult populations. J. Antimicrob. Chemother. 2023, 78, 1569–1585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Almeida, B.L.; Agnelli, C.; Guimarães, T.; Sukiennik, T.; Lima, P.R.P.; Salles, M.J.C.; Breda, G.L.; Queiroz-Telles, F.; Mendes, A.V.A.; Camargo, L.F.A.; et al. Candidemia in ICU Patients: What Are the Real Game-Changers for Survival? J. Fungi. 2025, 11, 152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, S.Y.; Kumar, A. Empiric Antimicrobial Therapy in Severe Sepsis and Septic Shock: Optimizing Pathogen Clearance. Curr. Infect. Dis. Rep. 2015, 17, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clancy, C.J.; Hong Nguyen, M. Systemic Candidiasis: Candidemia and Deep-Organ Infections. In Candida and Candidiasis; Wiley: Hoboken, NJ, USA, 2011; pp. 429–441. [Google Scholar] [CrossRef]
- Danielescu, C.; Stanca, H.T.; Iorga, R.E.; Darabus, D.M.; Potop, V. The Diagnosis and Treatment of Fungal Endophthalmitis: An Update. Diagnostics 2022, 12, 679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foong, K.S.; Sung, A.; Burnham, J.P.; Kronen, R.; Lian, Q.; Salazar Zetina, A.; Hsueh, K.; Lin, C.; Powderly, W.G.; Spec, A. Risk factors predicting Candida infective endocarditis in patients with candidemia. Med. Mycol. 2020, 58, 593–599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thompson, G.R.; Jenks, J.D.; Baddley, J.W.; Lewis, J.S.; Egger, M.; Schwartz, I.S.; Boyer, J.; Patterson, T.F.; Chen, S.C.; Pappas, P.G.; et al. Fungal Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management. Clin. Microbiol. Rev. 2023, 36, e00019-23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cornely, O.A.; Bangard, C.; Jaspers, N.I. Hepatosplenic candidiasis. Clin. Liver Dis. 2015, 6, 47–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sobel, J.D.; Vazquez, J. Candidiasis in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2003, 24, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Fennelly, A.M.; Slenker, A.K.; Murphy, L.C.; Moussouttas, M.; DeSimone, J.A. Candida cerebral abscesses: A case report and review of the literature. Med. Mycol. 2013, 51, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.; Walsh, J.; Skally, M.; Dinesh, B.; Burns, K.; O’Connell, K.; MacNally, S.; Humphreys, H.; Fitzpatrick, F. Candida meningitis/ventriculitis over a decade. Increased morbidity and length of stay a concern. Br. J. Neurosurg. 2023, 37, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.; Micek, S.; Hampton, N.; Doherty, J.A.; Kumar, A. Septic Shock Attributed to Candida Infection: Importance of Empiric Therapy and Source Control. Clin. Infect. Dis. 2012, 54, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Morrell, M.; Fraser, V.J.; Kollef, M.H. Delaying the Empiric Treatment of Candida Bloodstream Infection until Positive Blood Culture Results Are Obtained: A Potential Risk Factor for Hospital Mortality. Antimicrob. Agents Chemother. 2005, 49, 3640–3645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clancy, C.J.; Nguyen, M.H. Finding the “Missing 50%” of Invasive Candidiasis: How Nonculture Diagnostics Will Improve Understanding of Disease Spectrum and Transform Patient Care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Calandra, T.; Roberts, J.A.; Antonelli, M.; Bassetti, M.; Vincent, J.L. Diagnosis and management of invasive candidiasis in the ICU: An updated approach to an old enemy. Crit. Care 2016, 20, 125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pfeiffer, C.D.; Samsa, G.P.; Schell, W.A.; Reller, L.B.; Perfect, J.R.; Alexander, B.D. Quantitation of Candida CFU in Initial Positive Blood Cultures. J. Clin. Microbiol. 2011, 49, 2879–2883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soriano, A.; Honore, P.M.; Cornely, O.A.; Chayakulkeeree, M.; Bassetti, M.; Haihui, H.; Dupont, H.; Kim, Y.K.; Kollef, M.; Kullberg, B.J.; et al. Treatment Outcomes Among Patients With a Positive Candida Culture Close to Randomization Receiving Rezafungin or Caspofungin in the ReSTORE Study. Clin. Infect. Dis. 2024, 79, 672–681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandez, J.; Erstad, B.L.; Petty, W.; Nix, D.E. Time to positive culture and identification for Candida blood stream infections. Diagn. Microbiol. Infect. Dis. 2009, 64, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Bal, A.; McGill, M. Rapid Species Identification of Candida Directly from Blood Culture Broths by Sepsityper-Maldi-Tof Mass Spectrometry: Impact on Antifungal Therapy. J. R. Coll. Physicians Edinb. 2018, 48, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Spanu, T.; Posteraro, B.; Fiori, B.; D’Inzeo, T.; Campoli, S.; Ruggeri, A.; Tumbarello, M.; Canu, G.; Trecarichi, E.M.; Parisi, G.; et al. Direct MALDI-TOF Mass Spectrometry Assay of Blood Culture Broths for Rapid Identification of Candida Species Causing Bloodstream Infections: An Observational Study in Two Large Microbiology Laboratories. J. Clin. Microbiol. 2012, 50, 176–179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delavy, M.; Dos Santos, A.R.; Heiman, C.M.; Coste, A.T. Investigating Antifungal Susceptibility in Candida Species With MALDI-TOF MS-Based Assays. Front. Cell. Infect. Microbiol. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alexander, B.D.; Ashley, E.D.; Reller, L.B.; Reed, S.D. Cost savings with implementation of PNA FISH testing for identification of Candida albicans in blood cultures. Diagn. Microbiol. Infect. Dis. 2006, 54, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Klingspor, L.; Lindbäck, E.; Ullberg, M.; Özenci, V. Seven years of clinical experience with the Yeast Traffic Light PNA FISH: Assay performance and possible implications on antifungal therapy. Mycoses 2018, 61, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.R.H.; Gorton, R.L.; Barker, K.; Ramnarain, P.; Kibbler, C.C. Evaluation of PNA-FISH Yeast Traffic Light for Rapid Identification of Yeast Directly from Positive Blood Cultures and Assessment of Clinical Impact. J. Clin. Microbiol. 2013, 51, 1301–1302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mermutluoglu, C.; Deveci, O.; Dayan, S.; Aslan, E.; Bozkurt, F.; Tekin, R. Antifungal Susceptibility and Risk Factors in Patients with Candidemia. Eurasian J. Med. 2017, 48, 199–203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Kollef, M.H.; Arnold, H.; Labelle, A.; Micek, S.T.; Kothari, S.; Shorr, A.F. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: A retrospective cohort study. BMC Infect. Dis. 2010, 10, 150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camp, I.; Spettel, K.; Willinger, B. Molecular Methods for the Diagnosis of Invasive Candidiasis. J. Fungi. 2020, 6, 101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avni, T.; Leibovici, L.; Paul, M. PCR Diagnosis of Invasive Candidiasis: Systematic Review and Meta-Analysis. J. Clin. Microbiol. 2011, 49, 665–670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- León, C.; Ruiz-Santana, S.; Saavedra, P.; Castro, C.; Loza, A.; Zakariya, I.; Úbeda, A.; Parra, M.; Macías, D.; Tomás, J.I.; et al. Contribution of Candida biomarkers and DNA detection for the diagnosis of invasive candidiasis in ICU patients with severe abdominal conditions. Crit. Care 2016, 20, 149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klingspor, L.; Jalal, S. Molecular detection and identification of Candida and Aspergillus spp. from clinical samples using real-time PCR. Clin. Microbiol. Infect. 2006, 12, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Arafa, S.H.; Elbanna, K.; Osman, G.E.H.; Abulreesh, H.H. Candida diagnostic techniques: A review. J. Umm Al-Qura Univ. Appl. Sci. 2023, 9, 360–377. [Google Scholar] [CrossRef]
- Fricke, S.; Fricke, C.; Schimmelpfennig, C.; Oelkrug, C.; Schönfelder, U.; Blatz, R.; Zilch, C.; Faber, S.; Hilger, N.; Ruhnke, M.; et al. A real-time PCR assay for the differentiation of Candida species: Real-time PCR of Candida species. J. Appl. Microbiol. 2010, 109, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, J.; Dorn, C.; Hebart, H.; Cox, P.; Magga, S.; Einsele, H. Development and evaluation of the nuclisens basic kit NASBA for the detection of RNA from Candida species frequently resistant to antifungal drugs. Diagn. Microbiol. Infect. Dis. 2003, 45, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Berlau, A.; Stoll, S.; Edel, B.; Löffler, B.; Rödel, J. Evaluation of the Eazyplex® Candida ID LAMP Assay for the Rapid Diagnosis of Positive Blood Cultures. Diagnostics 2024, 14, 2125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uthayakumar, D.; Sharma, J.; Wensing, L.; Shapiro, R.S. CRISPR-Based Genetic Manipulation of Candida Species: Historical Perspectives and Current Approaches. Front. Genome Ed. 2021, 2, 606281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, R.; Ji, W.; Jiang, M.; Shen, J. CRISPR technology combined with isothermal amplification methods for the diagnosis of Candida albicans infection. Clin. Chim. Acta. 2025, 567, 120106. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Wolk, D.M.; Lowery, T.J. T2MR and T2Candida: Novel technology for the rapid diagnosis of candidemia and invasive candidiasis. Future Microbiol. 2016, 11, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Clancy, C.J.; Ostrosky-Zeichner, L.; Garey, K.W.; Alangaden, G.J.; Vazquez, J.A.; Groeger, J.S.; Judson, M.A.; Vinagre, Y.M.; Heard, S.O.; et al. T2 Magnetic Resonance Assay for the Rapid Diagnosis of Candidemia in Whole Blood: A Clinical Trial. Clin. Infect. Dis. 2015, 60, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Neely, L.A.; Audeh, M.; Phung, N.A.; Min, M.; Suchocki, A.; Plourde, D.; Blanco, M.; Demas, V.; Skewis, L.R.; Anagnostou, T.; et al. T2 Magnetic Resonance Enables Nanoparticle-Mediated Rapid Detection of Candidemia in Whole Blood. Sci. Transl. Med. 2013, 5, 182ra54. [Google Scholar] [CrossRef] [PubMed]
- Kinet-Poleur, A.; Deckers, C.; Saad Albichr, I.; Bogaerts, P.; Honoré, P.M.; Bulpa, P.; Ausselet, N.; Foret, F.; Kidd, F.; Huang, T.D.; et al. Evaluation of Serum Biomarkers for Improved Diagnosis of Candidemia. J. Fungi. 2025, 11, 224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Persat, F.; Ranque, S.; Derouin, F.; Michel-Nguyen, A.; Picot, S.; Sulahian, A. Contribution of the (1→3)-β- d -Glucan Assay for Diagnosis of Invasive Fungal Infections. J. Clin. Microbiol. 2008, 46, 1009–1013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forster, J.; Dichtl, K.; Wagener, J. Lower beta-1,3-D-glucan testing cut-offs increase sensitivity for non-albicans Candida species bloodstream infections. Mycoses 2022, 65, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Lim, S.Y.; Jin, S.; Park, H.J.; Sung, H.; Kim, M.N.; Bae, S.; Jung, J.; Kim, M.J.; Kim, S.H.; et al. Clinical Sensitivity of the (1–3)-β-D-glucan Test for Predicting Candidemia. Ann. Lab. Med. 2023, 43, 381–385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez-Jiménez, M.C.; Muñoz, P.; Valerio, M.; Alonso, R.; Martos, C.; Guinea, J.; Bouza, E. Candida biomarkers in patients with candidaemia and bacteraemia. J. Antimicrob. Chemother. 2015, 70, 2354–2361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nucci, M.; Nouér, S.A.; Esteves, P.; Guimarães, T.; Breda, G.; De Miranda, B.G.; Queiroz-Telles, F.; Colombo, A.L. Discontinuation of empirical antifungal therapy in ICU patients using 1,3-β-d-glucan. J. Antimicrob. Chemother. 2016, 71, 2628–2633. [Google Scholar] [CrossRef] [PubMed]
- Theel, E.S.; Doern, C.D. Point-Counterpoint: β- d -Glucan Testing Is Important for Diagnosis of Invasive Fungal Infections. J. Clin. Microbiol. 2013, 51, 3478–3483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cuenca-Estrella, M.; Verweij, P.E.; Arendrup, M.C.; Arikan-Akdagli, S.; Bille, J.; Donnelly, J.P.; Jensen, H.E.; Lass-Flörl, C.; Richardson, M.D.; Akova, M.; et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: Diagnostic procedures. Clin. Microbiol. Infect. 2012, 18, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Chumpitazi, B.F.F.; Lebeau, B.; Faure-Cognet, O.; Hamidfar-Roy, R.; Timsit, J.F.; Pavese, P.; Thiebaut-Bertrand, A.; Quesada, J.L.; Pelloux, H.; Pinel, C. Characteristic and clinical relevance of Candida mannan test in the diagnosis of probable invasive candidiasis. Med. Mycol. 2014, 52, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, M.; Calandra, T.; Sanguinetti, M.; Poulain, D.; Viscoli, C. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: Recommendations from the Third European Conference on Infections in Leukemia. Crit. Care 2010, 14, R222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dupuis, C.; Le Bihan, C.; Maubon, D.; Calvet, L.; Ruckly, S.; Schwebel, C.; Bouadma, L.; Azoulay, E.; Cornet, M.; Timsit, J.F. Performance of Repeated Measures of (1–3)-β-D-Glucan, Mannan Antigen, and Antimannan Antibodies for the Diagnosis of Invasive Candidiasis in ICU Patients: A Preplanned Ancillary Analysis of the EMPIRICUS Randomized Clinical Trial. Open Forum Infect. Dis. 2021, 8, ofab080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Träger, J.; Dräger, S.; Mihai, S.; Cipa, F.; Busse Grawitz, A.; Epting, T.; Meyer, R.; Rappold, E.; Held, J. Detailed β-(1→3)-D-glucan and mannan antigen kinetics in patients with candidemia. J. Clin. Microbiol. 2023, 61, e00598-23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bregón-Villahoz, M.; Menéndez-Manjón, P.; Carrano, G.; Díez-Villalba, A.; Arrieta-Aguirre, I.; Fernandez-de-Larrinoa, I.; Moragues, M.D. Candida albicans cDNA library screening reveals novel potential diagnostic targets for invasive candidiasis. Diagn. Microbiol. Infect. Dis. 2024, 109, 116311. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, R.; Pemán, J.; Quindos, G.; Iruretagoyena, J.R.; Cuétara, M.S.; Ramírez, P.; Gómez, M.D.; Camarena, J.J.; Viudes, A.; Pontón, J. Clinical significance of the detection of Candida albicans germ tube-specific antibodies in critically ill patients. Clin. Microbiol. Infect. 2009, 15, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Díez, A.; Carrano, G.; Bregón-Villahoz, M.; Cuétara, M.S.; García-Ruiz, J.C.; Fernandez-de-Larrinoa, I.; Moragues, M.D. Biomarkers for the diagnosis of invasive candidiasis in immunocompetent and immunocompromised patients. Diagn. Microbiol. Infect. Dis. 2021, 101, 115509. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wu, T.; Wu, Y.; Ming, D.; Zhu, X. Diagnostic accuracy of Candida albicans germ tube antibody for invasive candidiasis: Systematic review and meta-analysis. Diagn. Microbiol. Infect. Dis. 2019, 93, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Sprute, R.; Bassetti, M.; Chen, S.C.A.; Groll, A.H.; Kurzai, O.; Lass-Flörl, C.; Ostrosky-Zeichner, L.; Rautemaa-Richardson, R.; Revathi, G.; et al. Global guideline for the diagnosis and management of candidiasis: An initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 2025, 25, e280–e293. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, G.; Lin, F.; Yang, H.; Cui, Y.; Lu, R.; Song, C.; Li, H.; Li, Y.; Pan, P. A scoring system based on novel biomarkers and clinical risk factors to predict invasive candidiasis in immunocompetent critically ill patients. Front. Microbiol. 2023, 14, 1097574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- León, C.; Ruiz-Santana, S.; Saavedra, P.; Almirante, B.; Nolla-Salas, J.; Álvarez-Lerma, F.; Garnacho-Montero, J.; León, M.A. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit. Care Med. 2006, 34, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Pittet, D.; Monod, M.; Suter, P.M.; Frenk, E.; Auckenthaler, R. Candida colonization and subsequent infections in critically ill surgical patients. Ann. Surg. 1994, 220, 751–758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caggiano, G.; Puntillo, F.; Coretti, C.; Giglio, M.; Alicino, I.; Manca, F.; Bruno, F.; Montagna, M.T. Candida Colonization Index in Patients Admitted to an ICU. Int. J. Mol. Sci. 2011, 12, 7038–7047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostrosky-Zeichner, L.; Pappas, P.G.; Shoham, S.; Reboli, A.; Barron, M.A.; Sims, C.; Wood, C.; Sobel, J.D. Improvement of a clinical prediction rule for clinical trials on prophylaxis for invasive candidiasis in the intensive care unit: Prediction rule for Candida in ICU. Mycoses 2011, 54, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, E.D.; Zapapas, M.K.; Maiefski, M.; Rupp, M.E.; Freifeld, A.G.; Kalil, A.C. Validation and comparison of clinical prediction rules for invasive candidiasis in intensive care unit patients: A matched case-control study. Crit. Care 2011, 15, R198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostrosky-Zeichner, L.; Sable, C.; Sobel, J.; Alexander, B.D.; Donowitz, G.; Kan, V.; Kauffman, C.A.; Kett, D.; Larsen, R.A.; Morrison, V.; et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Delaloye, J.; Calandra, T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2014, 5, 161–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bassetti, M.; Vena, A.; Meroi, M.; Cardozo, C.; Cuervo, G.; Giacobbe, D.R.; Salavert, M.; Merino, P.; Gioia, F.; Fernández-Ruiz, M.; et al. Factors associated with the development of septic shock in patients with candidemia: A post hoc analysis from two prospective cohorts. Crit. Care 2020, 24, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azim, A.; Ahmed, A. Diagnosis and management of invasive fungal diseases in non-neutropenic ICU patients, with focus on candidiasis and aspergillosis: A comprehensive review. Front. Cell. Infect. Microbiol. 2024, 14, 1256158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cornely, O.A.; Hoenigl, M.; Lass-Flörl, C.; Chen, S.C.-A.; Kontoyiannis, D.P.; Morrissey, C.O.; Thompson, G.R. 3rd. Defining breakthrough invasive fungal infection–Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 2019, 62, 716–729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Echeverria, P.; Kett, D.; Azoulay, E. Candida Prophylaxis and Therapy in the ICU. Semin. Respir. Crit. Care Med. 2011, 32, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Playford, E.G.; Webster, A.C.; Sorrell, T.C.; Craig, J.C. Antifungal agents for preventing fungal infections in non-neutropenic critically ill and surgical patients: Systematic review and meta-analysis of randomized clinical trials. J. Antimicrob. Chemother. 2006, 57, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019, 45, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Einav, S.; Raveh, D.; Lachish, T.; Baumstarck, K.; Martin, C.; Martin-Loeches, I.; Leone, M. Candida Prophylaxis and Treatment in Critically Ill Patients after Abdominal Surgery: A Survey of Practice. Surg. Infect. 2019, 20, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Russotto, V.; Maggiore, A.; Attanasio, M.; Naro, A.R.; Raineri, S.M.; Giarratano, A. Antifungal agents for preventing fungal infections in non-neutropenic critically ill patients. Cochrane Database Syst. Rev. 2016, 2016, CD004920. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.C.A.; Slavin, M.A.; Sorrell, T.C. Echinocandin Antifungal Drugs in Fungal Infections: A Comparison. Drugs 2011, 71, 11–41. [Google Scholar] [CrossRef] [PubMed]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grau, S.; Pozo, J.C.; Romá, E.; Salavert, M.; Barrueta, J.A.; Peral, C.; Rodriguez, I.; Rubio-Rodríguez, D.; Rubio-Terrés, C. Cost-effectiveness of three echinocandins and fluconazole in the treatment of candidemia and/or invasive candidiasis in nonneutropenic adult patients. Clin. Outcomes Res. CEOR 2015, 7, 527–535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sprute, R.; Nacov, J.A.; Neofytos, D.; Oliverio, M.; Prattes, J.; Reinhold, I.; Cornely, O.A.; Stemler, J. Antifungal prophylaxis and pre-emptive therapy: When and how? Mol. Asp. Med. 2023, 92, 101190. [Google Scholar] [CrossRef] [PubMed]
- Viscoli, C. Antifungal Prophylaxis and Pre-Emptive Therapy. Drugs 2009, 69, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.T.; Castelino, R.L.; Kiser, T.H.; Truong-Nguyen, K.H.; Tran, M.H. Empirical versus pre-emptive antifungal therapies for invasive fungal infections in critically ill patients. BMC Infect. Dis. 2025, 25, 395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, Y.; Ip, M.; You, J.H.S. Potential clinical and economic outcomes of active beta-D-glucan surveillance with preemptive therapy for invasive candidiasis at intensive care units: A decision model analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 187–194. [Google Scholar] [CrossRef] [PubMed]
- León, C.; Ostrosky-Zeichner, L.; Schuster, M. What’s new in the clinical and diagnostic management of invasive candidiasis in critically ill patients. Intensive Care Med. 2014, 40, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Klastersky, J. Empirical antifungal therapy. Int. J. Antimicrob. Agents 2004, 23, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.H.E.; Bay, J.W.; Yeong, F.M.; Samuel, M. Efficacy and safety of echinocandin monotherapy and combination therapy for immunocompromised patients with systemic candidiasis: A systematic review and meta-analysis. J. Med. Mycol. 2023, 33, 101362. [Google Scholar] [CrossRef] [PubMed]
- Kanj, S.S.; Omrani, A.S.; Al-Abdely, H.M.; Subhi, A.; Fakih, R.E.; Abosoudah, I.; Kanj, H.; Dimopoulos, G. Survival Outcome of Empirical Antifungal Therapy and the Value of Early Initiation: A Review of the Last Decade. J. Fungi. 2022, 8, 1146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boutin, C.A.; Luong, M.L. Update on therapeutic approaches for invasive fungal infections in adults. Ther. Adv. Infect. Dis. 2024, 11, 20499361231224980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatelon, J.; Cortegiani, A.; Hammad, E.; Cassir, N.; Leone, M. Choosing the Right Antifungal Agent in ICU Patients. Adv. Ther. 2019, 36, 3308–3320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garnacho-Montero, J.; Díaz-Martín, A.; Cantón-Bulnes, L.; Ramírez, P.; Sierra, R.; Arias-Verdú, D.; Rodríguez-Delgado, M.; Loza-Vázquez, A.; Rodriguez-Gomez, J.; Gordón, M.; et al. Initial Antifungal Strategy Reduces Mortality in Critically Ill Patients With Candidemia: A Propensity Score–Adjusted Analysis of a Multicenter Study. Crit. Care Med. 2018, 46, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xie, J.; Cai, Y.; Wang, N.; Wang, Y.; Zhang, L.; Li, Y.; Yu, J.; Li, Y.; Wang, H.; et al. Efficacy and Safety of Combination Antifungals as Empirical, Preemptive, and Targeted Therapies for Invasive Fungal Infections in Intensive-Care Units. Infect. Drug Resist. 2022, 15, 5331–5344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khanina, A.; Tio, S.Y.; Ananda-Rajah, M.R.; Kidd, S.E.; Williams, E.; Chee, L.; Urbancic, K.; Thursky, K.A. Consensus guidelines for antifungal stewardship, surveillance and infection prevention, 2021. Intern. Med. J. 2021, 51, 18–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ye, F.; Cai, S.; Cao, B.; Cheng, L.; Fan, H.; Huang, Y.; Jiang, S.; Lai, G.; Li, Y.; Shi, Y.; et al. Consensus for antifungal stewardship in China (2024 edition). J. Thorac. Dis. 2024, 16, 4016–4029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hare, D.; Coates, C.; Kelly, M.; Cottrell, E.; Connolly, E.; Muldoon, E.G.; O’ Connell, B.; Rogers, T.R.; Talento, A. Antifungal stewardship in critical care: Implementing a diagnostics-driven care pathway in the management of invasive candidiasis. Infect. Prev. Pract. 2020, 2, 100047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Pascale, G.; Martin-Loeches, I.; Nseir, S. Antifungal stewardship in critically ill patients. Intensive Care Med. 2023, 49, 681–684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andruszko, B.; Dodds Ashley, E. Antifungal Stewardship: An Emerging Practice in Antimicrobial Stewardship. Curr. Clin. Microbiol. Rep. 2016, 3, 111–119. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Battistolo, J.; Poissy, J.; Coste, A.; Bochud, P.Y.; Calandra, T.; Senn, L.; Lamoth, F. Κey Role of Early Source Control in Candidemic Patients With Sepsis or Septic Shock. Open Forum Infect. Dis. 2022, 9, ofac383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marangos, M.; Ioannou, P.; Senn, L.; Spiliopoulou, A.; Tzalis, S.; Kolonitsiou, F.; Valta, M.; Kokkini, S.; Pagani, J.L.; Stafylaki, D.; et al. Role of source control in critically ill candidemic patients: A multicenter retrospective study. Infection 2024, 52, 1733–1743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raad, I.; Hanna, H.; Boktour, M.; Girgawy, E.; Danawi, H.; Mardani, M.; Kontoyiannis, D.; Darouiche, R.; Hachem, R.; Bodey, G.P. Management of Central Venous Catheters in Patients with Cancer and Candidemia. Clin. Infect. Dis. 2004, 38, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and Invasive Candidiasis. Infect. Dis. Clin. 2021, 35, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Narui, R.; Holmes, B.; Norton, C.; Kim, E.; Nakajima, I.; Stevenson, W.G.; Greene, M.H.; John, R.M.; Ellis, C.R.; et al. Candidemia in patients with cardiovascular implantable electronic devices. J. Interv. Card. Electrophysiol. 2021, 60, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans biofilms and polymicrobial interactions. Crit. Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baman, J.R.; Medhekar, A.N.; Jain, S.K.; Knight, B.P.; Harrison, L.H.; Smith, B.; Saba, S. Management of systemic fungal infections in the presence of a cardiac implantable electronic device: A systematic review. Pacing Clin. Electrophysiol. 2021, 44, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Vena, A.; Russo, A. Management of patients with septic shock due to Candida infection. Hosp. Pract. 2018, 46, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Marriott, D.J.; Playford, E.G.; Chen, S.; Slavin, M.; Nguyen, Q.; Ellis, D.; Sorrell, T.C. Determinants of mortality in non-neutropenic ICU patients with candidaemia. Crit. Care 2009, 13, R115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohki, S.; Shime, N.; Kosaka, T.; Fujita, N. Impact of host- and early treatment-related factors on mortality in ICU patients with candidemia: A bicentric retrospective observational study. J. Intensive Care 2020, 8, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Epelbaum, O.; Chasan, R. Candidemia in the Intensive Care Unit. Clin. Chest Med. 2017, 38, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Asai, N.; Ohashi, W.; Sakanashi, D.; Suematsu, H.; Kato, H.; Hagihara, M.; Watanabe, H.; Shiota, A.; Koizumi, Y.; Yamagishi, Y.; et al. Combination of Sequential Organ Failure Assessment (SOFA) score and Charlson Comorbidity Index (CCI) could predict the severity and prognosis of candidemia more accurately than the Acute Physiology, Age, Chronic Health Evaluation II (APACHE II) score. BMC Infect. Dis. 2021, 21, 77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bassetti, M.; Righi, E.; Ansaldi, F.; Merelli, M.; Cecilia, T.; De Pascale, G.; Diaz-Martin, A.; Luzzati, R.; Rosin, C.; Lagunes, L.; et al. A multicenter study of septic shock due to candidemia: Outcomes and predictors of mortality. Intensive Care Med. 2014, 40, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, H.; Yin, M.; Han, H.; Yue, J.F.; Zhang, F.; Shan, T.C.; Guo, H.P.; Wu, D.W. The Differences in the Epidemiology and Predictors of Death between Candidemia Acquired in Intensive Care Units and Other Hospital Settings. Intern. Med. 2015, 54, 3009–3016. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dupont, B.F.; Lortholary, O.; Ostrosky-Zeichner, L.; Stucker, F.; Yeldandi, V. Treatment of candidemia and invasive candidiasis in the intensive care unit: Post hoc analysis of a randomized, controlled trial comparing micafungin and liposomal amphotericin B. Crit. Care 2009, 13, R159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwon, Y.J.; Won, E.J.; Jeong, S.H.; Shin, K.S.; Shin, J.H.; Kim, Y.R.; Kim, H.; Kim, Y.A.; Uh, Y.; Kim, T.S.; et al. Dynamics and Predictors of Mortality Due to Candidemia Caused by Different Candida Species: Comparison of Intensive Care Unit-Associated Candidemia (ICUAC) and Non-ICUAC. J. Fungi. 2021, 7, 597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Madec, Y.; Denoeud-Ndam, L.; Wolff, M.; Fontanet, A.; Bretagne, S.; Dromer, F. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med. 2014, 40, 1303–1312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puig-Asensio, M.; Pemán, J.; Zaragoza, R.; Garnacho-Montero, J.; Martín-Mazuelos, E.; Cuenca-Estrella, M.; Almirante, B. Impact of Therapeutic Strategies on the Prognosis of Candidemia in the ICU. Crit. Care Med. 2014, 42, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, C.; Cuervo, G.; Salavert, M.; Merino, P.; Gioia, F.; Fernández-Ruiz, M.; López-Cortés, L.E.; Escolá-Vergé, L.; Montejo, M.; Muñoz, P.; et al. An evidence-based bundle improves the quality of care and outcomes of patients with candidaemia. J. Antimicrob. Chemother. 2020, 75, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; Whitaker, L.; Zubovskaia, A. Invasive Candidiasis in the Intensive Care Unit: Where Are We Now? J. Fungi 2025, 11, 258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agnelli, C.; Guimarães, T.; Sukiennik, T.; Lima, P.R.P.; Salles, M.J.; Breda, G.L.; Queiroz-Telles, F.; Chaves Magri, M.M.; Mendes, A.V.; Camargo, L.F.A.; et al. Prognostic Trends and Current Challenges in Candidemia: A Comparative Analysis of Two Multicenter Cohorts within the Past Decade. J. Fungi 2023, 9, 468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salmanton-García, J.; Cornely, O.A.; Stemler, J.; Barać, A.; Steinmann, J.; Siváková, A.; Akalin, E.H.; Arikan-Akdagli, S.; Loughlin, L.; Toscano, C.; et al. Attributable mortality of candidemia—Results from the ECMM Candida III multinational European Observational Cohort Study. J. Infect. 2024, 89, 106229. [Google Scholar] [CrossRef] [PubMed]
- Hirano, R.; Sakamoto, Y.; Kudo, K.; Ohnishi, M. Retrospective analysis of mortality and Candida isolates of 75 patients with candidemia: A single hospital experience. Infect. Drug Resist. 2015, 8, 199–205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin. Infect. Dis. 2011, 2, e162–e193. [Google Scholar] [CrossRef]
- Drummond, R.A.; Desai, J.V.; Ricotta, E.E.; Swamydas, M.; Deming, C.; Conlan, S.; Quinones, M.; Matei-Rascu, V.; Sherif, L.; Lecky, D.; et al. Long-term antibiotic exposure promotes mortality after systemic fungal infection by driving lymphocyte dysfunction and systemic escape of commensal bacteria. Cell Host Microbe 2022, 30, 1020–1033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Species | Age Predisposition | Prevalence in ICU | Virulence Factors | Common Risk Factors | Antifungal Resistance | Notable Clinical Associations |
|---|---|---|---|---|---|---|
| C.albicans [13,14] | None | Common | Biofilm formation, tissue invasion | Broad-spectrum antibiotics, central venous catheters | Generally susceptible | High dissemination risk, deep organ invasion |
| C. parapsilosis [15,16] | Younger | Increasing | Biofilm formation | Central venous catheters, TPN, surgical procedures | Reduced echinocandin susceptibility, Azole-sensitive | TPN, central lines, indwelling devices |
| C. tropicalis [17] | None | Common in neutropenic and cancer patients | Hyphal formation, deep tissue invasion | Neutropenia, malignancies, advanced age, chronic respiratory co-morbidity | Variable azole resistance, echinocandin-sensitive | Aggressive course, organ dissemination |
| C. glabrata [18,19,20,21] | Advanced | Increasing | Adherence | Advances in age, prior azole exposure, diabetes, immunosuppression | Often resistant to fluconazole, variable to echinocandins | High mortality requires susceptibility-guided therapy |
| C. krusei [22,23] | None | Less common | Moderate | Immunosuppression | Fluconazole-resistant, echinocandin-sensitive | Requires susceptibility-guided therapy |
| C. auris [24,25,26,27,28,29] | None | Increasing | Biofilm formation, hyphal formation, adherence | Antibiotic use, medical devices, immunosuppression, frequent hospitalization in healthcare facilities | Often resistant to fluconazole, variable to Amphotericin B | Nosocomial outbreaks, invasive infections, requires susceptibility-guided therapy |
| Scoring System | Characteristics | References |
|---|---|---|
Candida Score
| >2.5 points: predictor of invasive candidiasis Sensitivity 81%, specificity of 74% | León, C. et al. [133] |
Candida Colonization Index
| A score> 0.5 is considered positive Sensitivity 100%, specificity 69% | Eggimann, P. et al. [47] Pittet, D. et al. [134] Caggiano, G. et al. [135] |
Ostrosky-Zeichner score
| High negative predictive value Sensitivity 70%, specificity 60% | Ostrosky-Zeichner, L. et al. [136] Hermsen, E.D. et al. [137] Ostrosky-Zeichner, L. et al. [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouratidou, C.; Tsakiri, K.; Dourliou, V.; Marneri, A.; Stougianni, M.; Pavlidis, E. Early-Onset Candidemia in Adult Intensive Care Units. Diagnostics 2025, 15, 1402. https://doi.org/10.3390/diagnostics15111402
Mouratidou C, Tsakiri K, Dourliou V, Marneri A, Stougianni M, Pavlidis E. Early-Onset Candidemia in Adult Intensive Care Units. Diagnostics. 2025; 15(11):1402. https://doi.org/10.3390/diagnostics15111402
Chicago/Turabian StyleMouratidou, Christina, Kalliopi Tsakiri, Vasiliki Dourliou, Alexandra Marneri, Maria Stougianni, and Efstathios Pavlidis. 2025. "Early-Onset Candidemia in Adult Intensive Care Units" Diagnostics 15, no. 11: 1402. https://doi.org/10.3390/diagnostics15111402
APA StyleMouratidou, C., Tsakiri, K., Dourliou, V., Marneri, A., Stougianni, M., & Pavlidis, E. (2025). Early-Onset Candidemia in Adult Intensive Care Units. Diagnostics, 15(11), 1402. https://doi.org/10.3390/diagnostics15111402






