The Role of Lung Ultrasound Scan in Different Heart Failure Scenarios: Current Applications and Lacks of Evidences
Abstract
1. Introduction
2. Congestion Occurrence
3. Principle of LUS Scan
4. Role of LUS in the Emergency Department and Acute HF
| Study | Results | References |
|---|---|---|
| Coiro et al., 2015 | A B-line count ≥ 30 at discharge in patients hospitalized for AHF, is a strong predictor of all-cause death or HF hospitalization at 3 months | [21] |
| Pivetta et al., 2015 | The LUS-implemented approach had a significantly higher accuracy (sensitivity, 97% [95% CI, 95–98.3%]; specificity, 97.4% [95% CI, 95.7–98.6%]) in differentiating AHF from noncardiac causes of acute dyspnea than the initial clinical workup, chest radiography alone, and natriuretic peptides. | [11] |
| Platz et al., 2017 | In acute HF, ≥15 B-lines on 28-zone LUS at discharge identified patients at a more than five-fold risk for HF readmission or death | [5] |
| Palazzuoli et al., 2018 | ≥22 B-lines at discharge → cut-off for poor outcome at discharge in HFrEF. ≥18 B-lines at discharge → cut-off for poor outcome at discharge in HFpEF | [22] |
| Maw et al., 2019 | LUS is more sensitive than CXR in detecting pulmonary edema in AHF. The relative sensitivity ratio of LUS, compared with CXR, was 1.2 (95% CI, 1.08–1.34; p < 0.001). | [17] |
| Rivas-Lasarte et al., 2020 | The presence of subclinical pulmonary congestion at discharge (≥5 B-lines in LUS in absence of rales in the auscultation) was a risk factor for the occurrence of the primary combined outcome of rehospitalization or unexpected visit for HF worsening or death at 6- month follow-up (hazard ratio 2.63; 95% confidence interval: 1.08–6.41; p = 0.033). | [20] |
| Palazzuoli et al., 2024 | Adding echocardiographic and LUS features of congestion to a model than included age, sex, systolic blood pressure, clinical congestion and natriuretic peptides in AHF patients, improved risk stratification at 90 and 180 days. | [18] |
| Ruocco et al., 2024 | In AHF, the degree of congestion reduction assessed over the in-hospital stay period can stratify the subsequent event risk. Limited reduction in both clinical congestion and B-lines number are related to poor prognosis, irrespective of HF subtype. | [23] |
5. Role of LUS in Outpatients
6. LUS in HFpEF, HFmrEF, HFrEF
7. LUS and Body Mass Index (BMI)
8. Criticism of LUS
9. Future Applications of LUS
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef] [PubMed]
- Demi, L.; Wolfram, F.; Klersy, C.; De Silvestri, A.; Ferretti, V.V.; Muller, M.; Miller, D.; Feletti, F.; Wełnicki, M.; Buda, N.; et al. New International Guidelines and Consensus on the Use of Lung Ultrasound. J. Ultrasound Med. 2023, 42, 309–344. [Google Scholar] [CrossRef] [PubMed]
- Miglioranza, M.H.; Picano, E.; Badano, L.P.; Sant’Anna, R.; Rover, M.; Zaffaroni, F.; Sicari, R.; Kalil, R.K.; Leiria, T.L.; Gargani, L. Pulmonary congestion evaluated by lung ultrasound predicts decompensation in heart failure outpatients. Int. J. Cardiol. 2017, 240, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Nijst, P.; Verbrugge, F.H.; Grieten, L.; Dupont, M.; Steels, P.; Tang, W.W.; Mullens, W. The pathophysiological role of interstitial sodium in heart failure. J. Am. Coll. Cardiol. 2015, 65, 378–388. [Google Scholar] [CrossRef]
- Platz, E.; Merz, A.A.; Jhund, P.S.; Vazir, A.; Campbell, R.; McMurray, J.J. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: A systematic review. Eur. J. Heart Fail. 2017, 19, 1154–1163. [Google Scholar] [CrossRef]
- Boorsma, E.M.; ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in heart failure: A contemporary look at physiology, diagnosis and treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef]
- Gargani, L. Ultrasound of the Lungs: More than a Room with a View. Heart Fail. Clin. 2019, 15, 297–303. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Evangelista, I.; Beltrami, M.; Pirrotta, F.; Tavera, M.C.; Gennari, L.; Ruocco, G. Clinical, Laboratory and Lung Ultrasound Assessment of Congestion in Patients with Acute Heart Failure. J. Clin. Med. 2022, 11, 1642. [Google Scholar] [CrossRef]
- Coiro, S.; Rastogi, T.; Girerd, N. How and When to Use Lung Ultrasound in Patients with Heart Failure? Rev. Cardiovasc. Med. 2022, 23, 198. [Google Scholar] [CrossRef]
- Pellicori, P.; Platz, E.; Dauw, J.; ter Maaten, J.M.; Martens, P.; Pivetta, E.; Cleland, J.G.; McMurray, J.J.; Mullens, W.; Solomon, S.D.; et al. Ultrasound imaging of congestion in heart failure: Examinations beyond the heart. Eur. J. Heart Fail. 2021, 23, 703–712. [Google Scholar] [CrossRef]
- Pivetta, E.; Goffi, A.; Lupia, E.; Tizzani, M.; Porrino, G.; Ferreri, E.; Volpicelli, G.; Balzaretti, P.; Banderali, A.; Iacobucci, A.; et al. Lung Ultrasound-Implemented Diagnosis of Acute Decompensated Heart Failure in the ED: A SIMEU Multicenter Study. Chest 2015, 148, 202–210. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Beltrami, M.; Girerd, N.; Maw, A.; Ruocco, G.; Platz, E. The assessment, interpretation and implementation of lung ultrasound examinations in Heart Failure: Current evidence and gaps in knowledge. Eur. J. Intern. Med. 2024, 130, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, T.; Gargani, L.; Pellicori, P.; Lamiral, Z.; Ambrosio, G.; Bayés-Genis, A.; Domingo, M.; Lupon, J.; Simonovic, D.; Pugliese, N.R.; et al. Prognostic implication of lung ultrasound in heart failure: A pooled analysis of international cohorts. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Ruocco, G.; Pellicori, P.; Gargani, L.; Coiro, S.; Lamiral, Z.; Ambrosio, G.; Rastogi, T.; Girerd, N. Multi-modality assessment of congestion in acute heart failure: Associations with left ventricular ejection fraction and prognosis. Curr. Probl. Cardiol. 2024, 49, 102374. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.P.; Lindsell, C.J.; Storrow, A.B.; Abraham, W.T.; ADHERE Scientific Advisory Committee, Investigators and Study Group. Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann. Emerg. Med. 2006, 47, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Maw, A.M.; Hassanin, A.; Ho, P.M.; McInnes, M.D.F.; Moss, A.; Juarez-Colunga, E.; Soni, N.J.; Miglioranza, M.H.; Platz, E.; DeSanto, K.; et al. Diagnostic Accuracy of Point-of-Care Lung Ultrasonography and Chest Radiography in Adults With Symptoms Suggestive of Acute Decompensated Heart Failure: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e190703. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Cartocci, A.; Pirrotta, F.; Tavera, M.C.; Morrone, F.; Vannuccini, F.; Campora, A.; Ruocco, G. Usefulness of Combined Ultrasound Assessment of E/e’ Ratio, Pulmonary Pressure, and Cava Vein Status in Patients With Acute Heart Failure. Am. J. Cardiol. 2024, 213, 36–44. [Google Scholar] [CrossRef]
- Araiza-Garaygordobil, D.; Baeza-Herrera, L.A.; Gopar-Nieto, R.; Solis-Jimenez, F.; Cabello-López, A.; Martinez-Amezcua, P.; Sarabia-Chao, V.; González-Pacheco, H.; Martinez, D.S.-L.; la Cruz, J.L.B.-D.; et al. Pulmonary Congestion Assessed by Lung Ultrasound and Cardiovascular Outcomes in Patients With ST-Elevation Myocardial Infarction. Front. Physiol. 2022, 13, 881626. [Google Scholar] [CrossRef]
- Rivas-Lasarte, M.; Maestro, A.; Fernández-Martínez, J.; López-López, L.; Solé-González, E.; Vives-Borrás, M.; Montero, S.; Mesado, N.; Pirla, M.J.; Mirabet, S.; et al. Prevalence and prognostic impact of subclinical pulmonary congestion at discharge in patients with acute heart failure. ESC Heart Fail. 2020, 7, 2621–2628. [Google Scholar] [CrossRef]
- Coiro, S.; Rossignol, P.; Ambrosio, G.; Carluccio, E.; Alunni, G.; Murrone, A.; Tritto, I.; Zannad, F.; Girerd, N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur. J. Heart Fail. 2015, 17, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Ruocco, G.; Beltrami, M.; Nuti, R.; Cleland, J.G. Combined use of lung ultrasound, B-type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin. Res. Cardiol. 2018, 107, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, G.; Girerd, N.; Rastogi, T.; Lamiral, Z.; Palazzuoli, A. Poor in-hospital congestion improvement in acute heart failure patients classified according to left ventricular ejection fraction: Prognostic implications. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Filippatos, G.; De Luca, L.; Burnett, J. Congestion in acute heart failure syndromes: An essential target of evaluation and treatment. Am. J. Med. 2006, 119 (Suppl. 1), S3–S10. [Google Scholar] [CrossRef]
- Zile, M.R.; Bennett, T.D.; Sutton, M.S.J.; Cho, Y.K.; Adamson, P.B.; Aaron, M.F.; Aranda, J.J.M.; Abraham, W.T.; Smart, F.W.; Stevenson, L.W.; et al. Transition from chronic compensated to acute decompensated heart failure: Pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008, 118, 1433–1441. [Google Scholar] [CrossRef]
- Miglioranza, M.H.; Gargani, L.; Sant’Anna, R.T.; Rover, M.M.; Martins, V.M.; Mantovani, A.; Weber, C.; Moraes, M.A.; Feldman, C.J.; Kalil, R.A.K.; et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: A comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc. Imaging 2013, 6, 1141–1151. [Google Scholar] [CrossRef]
- Rivas-Lasarte, M.; Alvarez-Garcia, J.; Fernández-Martínez, J.; Maestro, A.; López-López, L.; Solé-González, E.; Pirla, M.J.; Mesado, N.; Mirabet, S.; Fluvià, P.; et al. Lung ultrasound-guided treatment in ambulatory patients with heart failure: A randomized controlled clinical trial (LUS-HF study). Eur. J. Heart Fail. 2019, 21, 1605–1613. [Google Scholar] [CrossRef]
- Marini, C.; Fragasso, G.; Italia, L.; Sisakian, H.; Tufaro, V.; Ingallina, G.; Stella, S.; Ancona, F.; Loiacono, F.; Innelli, P.; et al. Lung ultrasound-guided therapy reduces acute decompensation events in chronic heart failure. Heart 2020, 106, 1934–1939. [Google Scholar] [CrossRef]
- Sokolska, J.M.; Sokolski, M.; Zymliński, R.; Biegus, J.; Siwołowski, P.; Nawrocka-Millward, S.; Ponikowski, P. Distinct clinical phenotypes of congestion in acute heart failure: Characteristics, treatment response, and outcomes. ESC Heart Fail 2020, 7, 3830–3840. [Google Scholar] [CrossRef]
- Gargani, L.; Pang, P.S.; Frassi, F.; Miglioranza, M.; Dini, F.L.; Landi, P.; Picano, E. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: A lung ultrasound study. Cardiovasc. Ultrasound 2015, 13, 40. [Google Scholar] [CrossRef]
- Pang, P.S.; Russell, F.M.; Ehrman, R.; Ferre, R.; Gargani, L.; Levy, P.D.; Noble, V.; Lane, K.A.; Li, X.; Collins, S.P. Lung Ultrasound-Guided Emergency Department Management of Acute Heart Failure (BLUSHED-AHF): A Randomized Controlled Pilot Trial. JACC Heart Fail. 2021, 9, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Lillo, R.; Cangemi, S.; Graziani, F.; Locorotondo, G.; Pedicino, D.; Aurigemma, C.; Romagnoli, E.; Malara, S.; Meucci, M.C.; Iannaccone, G.; et al. Pulmonary congestion assessed by lung ultrasound in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation: Prevalence and prognostic implications. Eur. J. Heart Fail. 2024, 26, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Scali, M.C.; Zagatina, A.; Ciampi, Q.; Cortigiani, L.; D’Andrea, A.; Daros, C.B.; Zhuravskaya, N.; Kasprzak, J.D.; Wierzbowska-Drabik, K.; Pretto, J.L.d.C.e.S.; et al. Lung Ultrasound and Pulmonary Congestion During Stress Echocardiography. JACC Cardiovasc. Imaging. 2020, 13, 2085–2095. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.C.; Campora, A.; Mandoli, G.E.; Lisi, M.; Benfari, G.; Ilardi, F.; Malagoli, A.; Sperlongano, S.; Henein, M.Y.; Cameli, M.; et al. Stress echocardiography in heart failure patients: Additive value and caveats. Heart Fail. Rev. 2024, 29, 1117–1133. [Google Scholar] [CrossRef]
- Stassen, J.; Falter, M.; Herbots, L.; Timmermans, P.; Dendale, P.; Verwerft, J. Assessment of Venous Congestion Using Vascular Ultrasound. JACC Cardiovasc. Imaging 2023, 16, 426–431. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Pellicori, P.; Filidei, F.; Del Punta, L.; De Biase, N.; Balletti, A.; Di Fiore, V.; Mengozzi, A.; Taddei, S.; Gargani, L.; et al. The incremental value of multi-organ assessment of congestion using ultrasound in outpatients with heart failure. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 961–971. [Google Scholar] [CrossRef]
- Cuthbert, J.J.; Pellicori, P.; Flockton, R.; Kallvikbacka-Bennett, A.; Khan, J.; Rigby, A.S.; Girerd, N.; Zannad, F.; Cleland, J.G.F.; Clark, A.L. The prevalence and clinical associations of ultrasound measures of congestion in patients at risk of developing heart failure. Eur. J. Heart Fail 2021, 23, 1831–1840. [Google Scholar] [CrossRef]
- Di Fiore, V.; Del Punta, L.; De Biase, N.; Pellicori, P.; Gargani, L.; Dini, F.L.; Armenia, S.; Li Vigni, M.; Maremmani, D.; Masi, S.; et al. Integrative assessment of congestion in heart failure using ultrasound imaging. Intern. Emerg. Med. 2024; ahead of print. [Google Scholar]
- Burgos, L.M.; Baro Vila, R.C.; Ballari, F.N.; Goyeneche, A.; Costabel, J.P.; Muñoz, F.; Spaccavento, A.; Fasan, M.A.; Suárez, L.L.; Vivas, M.; et al. Inferior vena CAVA and lung ultraSound-guided therapy in acute heart failure: A randomized pilot study (CAVAL US-AHF study). Am. Heart J. 2024, 277, 47–57. [Google Scholar] [CrossRef]
- Pandhi, P.; ter Maaten, J.M.; Emmens, J.E.; Struck, J.; Bergmann, A.; Cleland, J.G.; Givertz, M.M.; Metra, M.; O’Connor, C.M.; Teerlink, J.R.; et al. Clinical value of pre-discharge bio-adrenomedullin as a marker of residual congestion and high risk of heart failure hospital readmission. Eur. J. Heart Fail. 2020, 22, 683–691. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Evangelista, I.; Nuti, R. Congestion occurrence and evaluation in acute heart failure scenario: Time to reconsider different pathways of volume overload. Heart Fail. Rev. 2020, 25, 119–131. [Google Scholar] [CrossRef]
- Brainin, P.; Claggett, B.; Lewis, E.F.; Dwyer, K.H.; Merz, A.A.; Silverman, M.B.; Swamy, V.; Biering-Sørensen, T.; Rivero, J.; Cheng, S.; et al. Body mass index and B-lines on lung ultrasonography in chronic and acute heart failure. ESC Heart Fail. 2020, 7, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Kotsis, V.; Stabouli, S.; Papakatsika, S.; Rizos, Z.; Parati, G. Mechanisms of obesity-induced hypertension. Hypertens. Res. 2010, 33, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Ruocco, G.; Franci, B.; Evangelista, I.; Lucani, B.; Nuti, R.; Pellicori, P. Ultrasound indices of congestion in patients with acute heart failure according to body mass index. Clin. Res. Cardiol. 2020, 109, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Gargani, L.; Girerd, N.; Platz, E.; Pellicori, P.; Stankovic, I.; Palazzuoli, A.; Pivetta, E.; Miglioranza, M.H.; Soliman-Aboumarie, H.; Agricola, E.; et al. Lung ultrasound in acute and chronic heart failure: A clinical consensus statement of the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1569–1582. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Kashoob, M.; Al-Busaidi, S.; Al-Maqbali, J.S.; Al-Badi, A.; Aalhamad, A.; Falahi, Z.A.; Huraizi, A.A.; Farhan, H.A.; Zeedy, K.A.; Hashim, A.H.A.; et al. LUDT-ADHF trial: Lung ultrasound-guided diuretic therapy for hospitalized patients with acute decompensated heart failure: An open-label clinical trial. Heart Lung 2024, 69, 155–162. [Google Scholar] [CrossRef]

| Study | Results | References |
|---|---|---|
| Miglioranza et al., 2013 | A B-line ≥ 15 cutoff could be considered for a quick and reliable assessment of decompensation in outpatients with HF | [26] |
| Platz et al., 2017 | in ambulatory patients with chronic HF, ≥3 B-lines on five- or eight-zone LUS marked those at a nearly four-fold risk for 6-month HF hospitalization or death. | [5] |
| Miglioranza et al., 2017 | An outpatients B-lines number ≥ 30 (HR 8.62; 95%CI: 1.8–40.1; p = 0.006) identified a group of patients at high risk for acute pulmonary edema admission at 120 days, and was the strongest predictor of events compared to other established clinical, laboratory and instrumental findings. | [3] |
| Rivas-Lasarte et al., 2019 | Tailored LUS-guided diuretic treatment of pulmonary congestion in this proof-of-concept study reduced the number of decompensations and improved walking capacity in patients with HF | [27] |
| Marini et al., 2019 | LUS-guided management reduces hospitalization for AHF at mid-term follow-up in outpatients with chronic HF. | [28] |
| Differential Diagnosis | LUS Pattern | Ecographic Image |
|---|---|---|
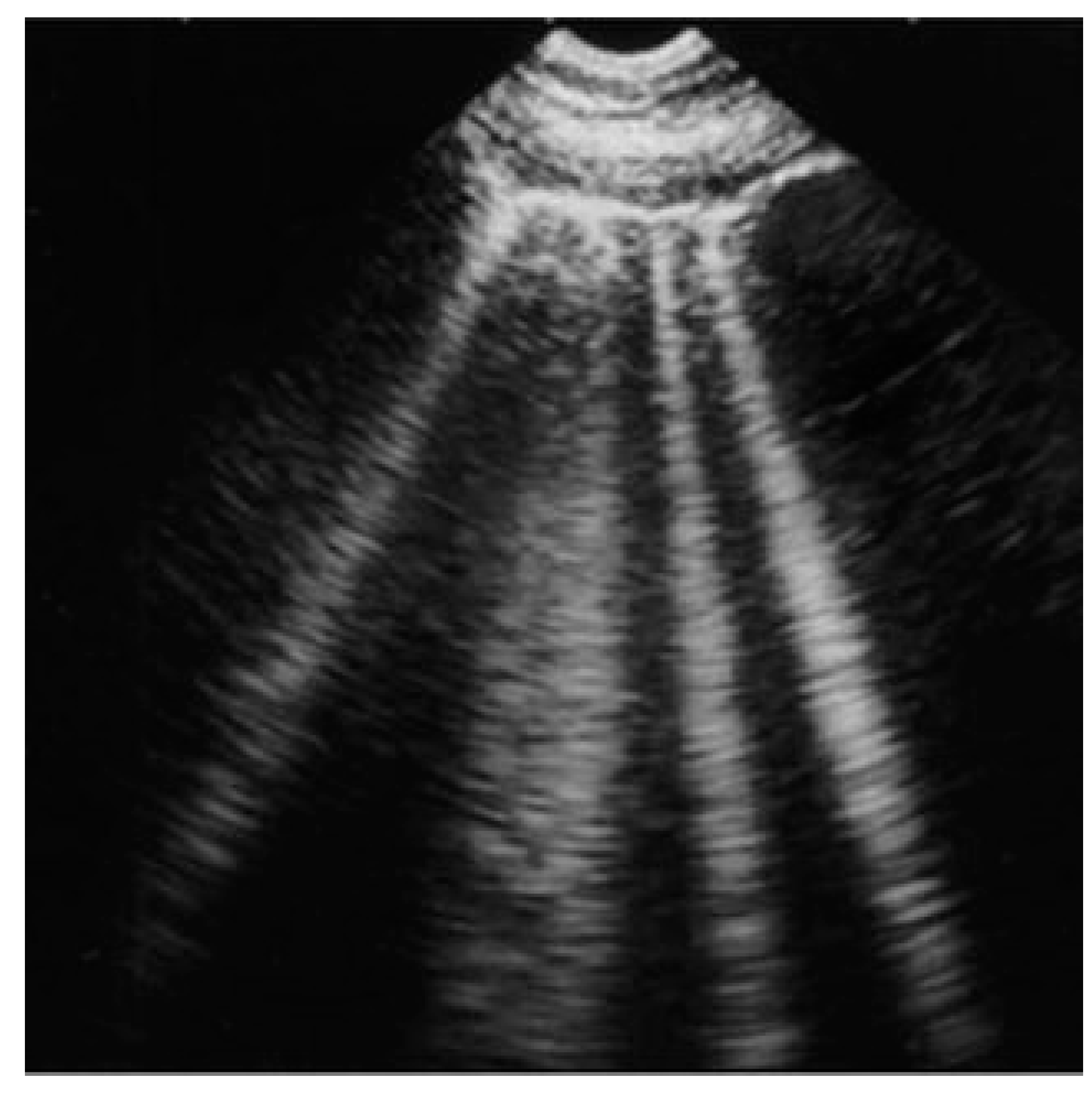
| Acute and chronic HF | Hyperechoic vertical lines extend from the pleural line (which is not thickened) and radiate towards the edge of the echocardiographic field. | 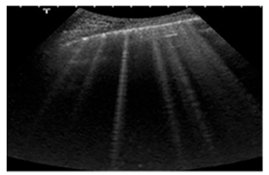 |
| Acute cardiogenic pulmonary oedema | Number of hyperechoic vertical lines increased, extending from the pleural line and radiating to the edge of the ultrasound field, associated with pleural effusion (without thickening of the pleural line). | 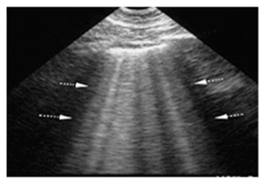 |
| ARDS | “Multiple non-homogeneous B-lines with a non-gravity-dependent distribution, potentially coexisting with spared areas, along with pleural thickening, reduced or absent lung sliding, and subpleural or trans-lobar consolidations.” | 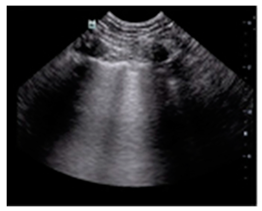 |
| Pneumonia/pulmonary consolidation | “Lung consolidation occurs due to a significant loss of aeration. It appears as a tissue-like echotexture, with a superficial boundary at the pleural line and a deep, irregular boundary with the aerated lung, known as the ’shred sign.’” | 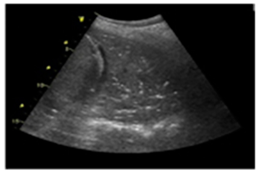 |
| Interstitial lung disease | “Confluent B-lines with discontinuity of the pleural line” | 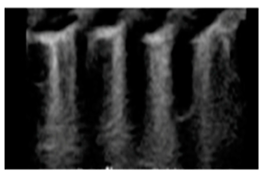 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campora, A.; Beltrami, M.; Di Renzo, A.; Petrini, A.; Palazzuoli, A. The Role of Lung Ultrasound Scan in Different Heart Failure Scenarios: Current Applications and Lacks of Evidences. Diagnostics 2025, 15, 45. https://doi.org/10.3390/diagnostics15010045
Campora A, Beltrami M, Di Renzo A, Petrini A, Palazzuoli A. The Role of Lung Ultrasound Scan in Different Heart Failure Scenarios: Current Applications and Lacks of Evidences. Diagnostics. 2025; 15(1):45. https://doi.org/10.3390/diagnostics15010045
Chicago/Turabian StyleCampora, Alessandro, Matteo Beltrami, Anita Di Renzo, Alessia Petrini, and Alberto Palazzuoli. 2025. "The Role of Lung Ultrasound Scan in Different Heart Failure Scenarios: Current Applications and Lacks of Evidences" Diagnostics 15, no. 1: 45. https://doi.org/10.3390/diagnostics15010045
APA StyleCampora, A., Beltrami, M., Di Renzo, A., Petrini, A., & Palazzuoli, A. (2025). The Role of Lung Ultrasound Scan in Different Heart Failure Scenarios: Current Applications and Lacks of Evidences. Diagnostics, 15(1), 45. https://doi.org/10.3390/diagnostics15010045






