Frequency and Demographic Analysis of Odontogenic Tumors in Three Tertiary Institutions: An 11-Year Retrospective Study
Abstract
1. Introduction
2. Methods and Materials
- Tumors with histopathological features compatible with an OT diagnosis.
- Patients with available demographic and clinical information, pathological reports, and histological slides.
- Patients with incomplete demographic and clinical data.
- Patients with missing histological slides.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vered, M.; Wright, J.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Odontogenic and Maxillofacial Bone Tumours. Head Neck Pathol. 2022, 16, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.A.C.; John, K.C.; Gale, N.; Helliwell, T.; Hyrcza, M.D.; Lewis, J.S., Jr.; Loney, E.L.; Mehortra, R.; Nete, O.; Muller, S.; et al. WHO Classification of Tumors of Head and Neck Tumours, 5th ed.; WHO Classification of Tumours Series; WHO: Lyon, France, 2022; Volume 9.
- Kokubun, K.; Yamamoto, K.; Nakajima, K.; Akashi, Y.; Chujo, T.; Takano, M.; Katakura, A.; Matsuzaka, K. Frequency of Odontogenic Tumors: A Single Center Study of 1089 Cases in Japan and Literature Review. Head Neck Pathol. 2022, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Siriwardena, B.; Tennakoon, T.; Tilakaratne, W. Relative frequency of odontogenic tumors in Sri Lanka: Analysis of 1677 cases. Pathol.-Res. Pract. 2012, 208, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Mamabolo, M.; Noffke, C.; Raubenheimer, E. Odontogenic tumours manifesting in the first two decades of life in a rural African population sample: A 26 year retrospective analysis. Dentomaxillofac. Radiol. 2011, 40, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Y.; Li, T.J. Odontogenic tumors: A study of 1309 cases in a Chinese population. Oral Oncol. 2009, 45, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Xuan, M.; Lin, Y.; Wu, L.; Liu, L.; Zheng, X.; Tang, W.; Qiao, J.; Tian, W. Odontogenic tumours: A retrospective study of 1642 cases in a Chinese population. Int. J. Oral Maxillofac. Surg. 2007, 36, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A. Biopsied jaw lesions in Kuwait: A six-year retrospective analysis. Med. Princ. Pract. 2011, 20, 550–555. [Google Scholar] [CrossRef]
- Al-Rawi, N.H.; Awad, M.; Al-Zuebi, I.E.; Hariri, R.A.; Salah, E.W. Prevalence of odontogenic cysts and tumors among UAE population. J. Orofac. Sci. 2013, 5, 95–100. [Google Scholar] [CrossRef]
- AlSheddi, M.A.; AlSenani, M.A.; AlDosarib, A.W. Odontogenic tumors: Analysis of 188 cases from Saudi Arabia. Ann. Saudi Med. 2015, 35, 146–150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnson, N.R.; Gannon, O.M.; Savage, N.W.; Batstone, M.D. Frequency of odontogenic cysts and tumors: A systematic review. J. Investig. Clin. Dent. 2014, 5, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Mosqueda-Taylor, A.; Pires, F.R.; Aguirre-Urízar, J.M.; Carlos-Bregni, R.; de la Piedra-Garza, J.M.; Martínez-Conde, R.; Martínez-Mata, G.; Carreño-Álvarez, S.J.; da Silveira, H.M.; Dias, B.S.d.B.; et al. Primordial odontogenic tumour: Clinicopathological analysis of six cases of a previously undescribed entity. Histopathology 2014, 65, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Almazyad, A.; Almutairi, M.; Almadan, N.; Alamro, M.; Maki, F.; AlQuwayz, T.S.; Alrumeh, A.S. Frequency and Demographic Profile of Odontogenic Cysts in Riyadh, Saudi Arabia: Retrospective Multicenter Study. Diagnostics 2023, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Gosavi, S.; Hazarey, V.K.; Gupta, V.; Bhadauria, U.S.; Kherde, P. Impact of changing classification systems on prevalence and frequency distribution of odontogenic tumors in tertiary care center of Nagpur. Braz. J. Otorhinolaryngol. 2022, 88 (Suppl. S1), S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Al-Aroomy, L.; Wali, M.; Alwadeai, M.; Desouky, E.; Amer, H. Odontogenic tumors: A Retrospective Study in Egyptian population using WHO 2017 classification. Med. Oral Patol. Oral Cir. Bucal 2022, 27, e198–e204. [Google Scholar] [CrossRef]
- Aregbesola, B.; Soyele, O.; Effiom, O.; Gbotolorun, O.; Taiwo, A.; Amole, I. Odontogenic tumours in Nigeria: A multicentre study of 582 cases and review of the literature. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e761–e766. [Google Scholar] [CrossRef] [PubMed]
- Mascitti, M.; Togni, L.; Troiano, G.; Caponio, V.C.A.; Sabatucci, A.; Balercia, A.; Rubini, C.; Muzio, L.L.; Santarelli, A. Odontogenic tumours: A 25-year epidemiological study in the Marche region of Italy. Eur. Arch. Otorhinolaryngol. 2020, 277, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Chrysomali, E.; Leventis, M.; Titsinides, S.; Kyriakopoulos, V.; Sklavounou, A. Odontogenic tumors. J. Craniofac. Surg. 2013, 24, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
- Bianco, B.C.F.; Sperandio, F.F.; Hanemann, J.A.C.; Pereira, A.A.C. New WHO odontogenic tumor classification: Impact on prevalence in a population. J. Appl. Oral Sci. 2020, 28, e20190067. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, W.K.; da Silva, L.P.; Pinto, L.P.; de Souza, L.B. Clinicopathological analysis of odontogenic tumors over 22 years period: Experience of a single center in northeastern Brazil. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e664–e671. [Google Scholar]
- Lima-Verde-Osterne, R.; Turatti, E.; Cordeiro-Teixeira, R.; Barroso-Cavalcante, R. The relative frequency of odontogenic tumors: A study of 376 cases in a Brazilian population. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e193–e200. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, F.; de Noronha, M.S.; Silva, M.L.V.; Amaral, M.B.F.; Grossmann, S.d.M.C.; Horta, M.C.R.; de Souza, P.E.A.; de Aguiar, M.C.F.; Mesquita, R.A. Prevalence profile of odontogenic cysts and tumors on Brazilian sample after the reclassification of odontogenic keratocyst. J. Craniomaxillofac. Surg. 2017, 45, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Izgi, E.; Mollaoglu, N.; Simsek, M. Prevalence of odontogenic cysts and tumors on turkish sample according to latest classification of world health organization: A 10-year retrospective study. Niger. J. Clin. Pract. 2021, 24, 355–361. [Google Scholar] [CrossRef]
- Mello, F.W.; Melo, G.; Kammer, P.V.; Speight, P.M.; Rivero, E.R.C. Prevalence of odontogenic cysts and tumors associated with impacted third molars: A systematic review and meta-analysis. J. Craniomaxillofac. Surg. 2019, 47, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, M.; Khiavi, M.M.; Ghazi, M.; Derakhshan, S. Primordial Odontogenic Tumor; Archival Review of 19380 Cases in a 55-Year Retrospective Study. Asian Pac. J. Cancer Prev. 2023, 24, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Poomsawat, S.; Ngamsom, S.; Nonpassopon, N. Primordial odontogenic tumor with prominent calcifications: A rare case report. J. Clin. Exp. Dent. 2019, 11, e952–e956. [Google Scholar] [CrossRef] [PubMed]
- Kebede, B.; Tare, D.; Bogale, B.; Alemseged, F. Odontogenic tumors in Ethiopia: Eight years retrospective study. BMC Oral Health 2017, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, M.; Zoller, J. Ameloblastoma-Clinical, radiological, and therapeutic findings. Oral Dis. 2018, 24, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Hendra, F.N.; Kalla, D.S.N.; Van Cann, E.M.; de Vet, H.C.W.; Helder, M.N.; Forouzanfar, T. Radical vs conservative treatment of intraosseous ameloblastoma: Systematic review and meta-analysis. Oral Dis. 2019, 25, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Saalim, M.; Sansare, K.; Karjodkar, F.; Farman, A.; Goyal, S.; Sharma, S. Recurrence rate of odontogenic myxoma after different treatments: A systematic review. Br. J. Oral Maxillofac. Surg. 2019, 57, 985–991. [Google Scholar] [CrossRef] [PubMed]
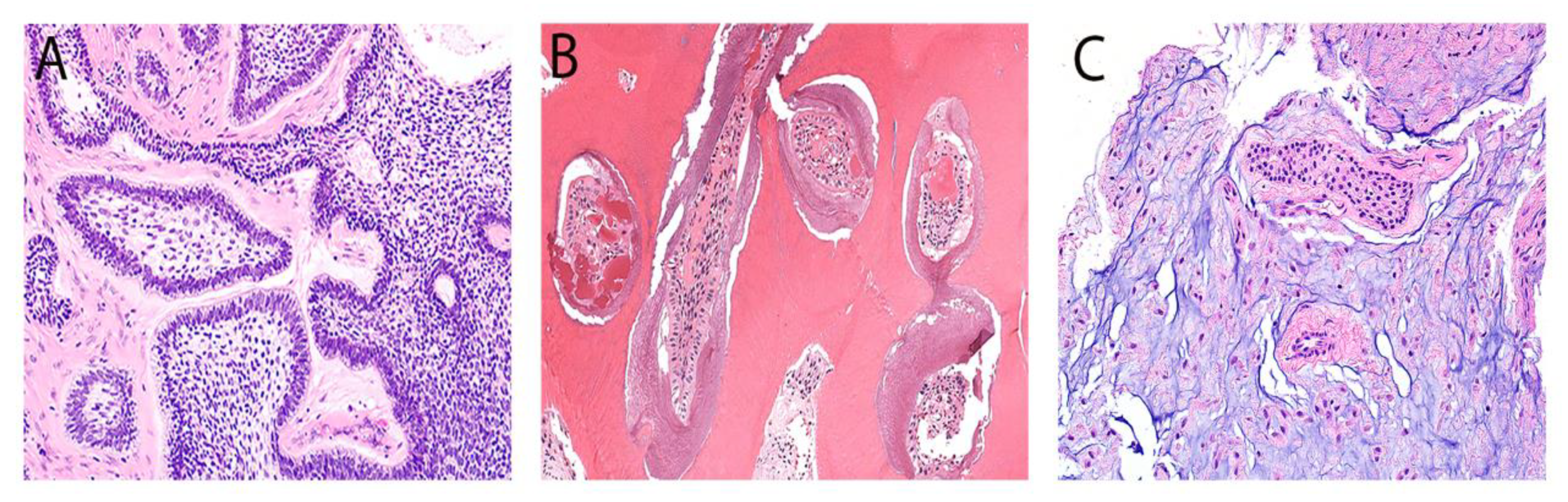


| Abbreviation | Definition | Clinical Presentation | Radiographic Presentation | |
|---|---|---|---|---|
| Epithelial Odontogenic Tumors | ||||
| Ameloblastoma | AM | |||
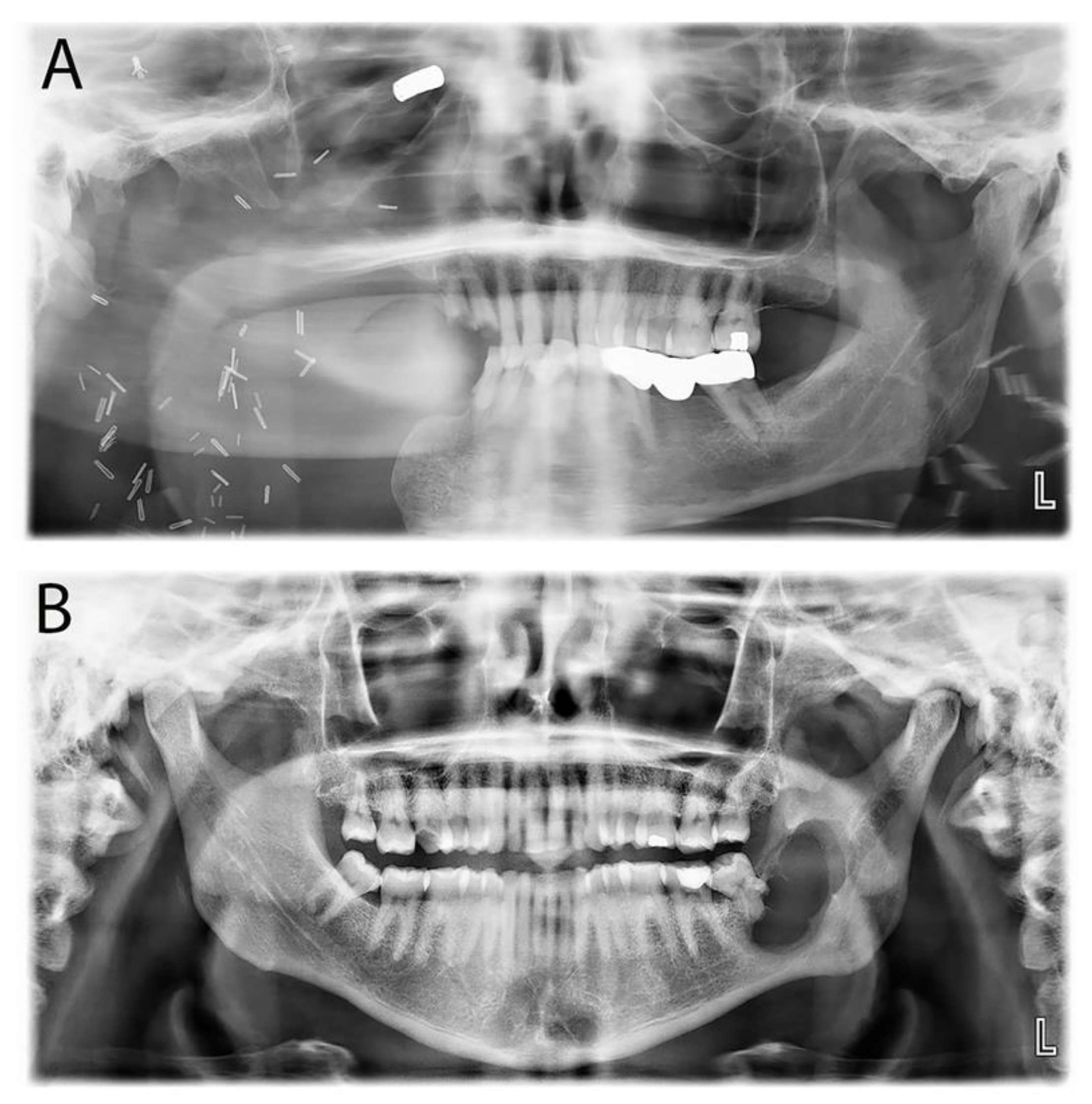
| Conventional ameloblastoma | cAM | A locally invasive and benign odontogenic tumor, most commonly in the posterior mandible. | Painless, slowly increasing swelling in the jaw, seen in the 4th to 5th decade, often asymptomatic with potential facial asymmetry and noticeable in cases in larger lesions. | Unilocular or multilocular radiolucency located in the posterior mandible. The multilocular type exhibits a soap bubble or honeycomb appearance, and root resorption of the adjacent teeth is frequent. |
| Unicystic ameloblastoma | UniAM | A distinct cystic variant constituting up to 25% of intraosseous AM. | Painless swelling is typically observed in the 2nd to 3rd decade, associated with the impacted 3rd molar in the posterior mandible. | Unilocular radiolucent area surrounding the crown of an impacted third molar. |
| Extraosseous/peripheral ameloblastoma | PeriAM | A rare variant comprising 1% of Ams; it arises either from the remnants of dental lamina within the oral mucosa or the basal cells of the surface epithelium. | Painless sessile nodule of the gingiva, occurring in the 5th to 6th decade and located in the premolar–molar region of the mandible. | N/A |
| Adenoid ameloblastoma | AdenoAM | Rare epithelial OT characterized by cribriform growth pattern, duct-like structures, and an occasional dentinoid, displaying aggressive behavior with a 70% recurrence rate. | Asymptomatic swelling with no site predilection; it may exhibit occasional pain and paresthesia. | The unilocular or multilocular with internal calcifications, cortical perforations, or root resorption. |
| Metastasizing ameloblastoma | MetAM | A histologically benign AM exhibiting metastasis to distant organs, commonly in the lungs. | Variable presentation and pulmonary metastasis may include a dry cough, hemoptysis, or dyspnea. | Similar to cAM in the jawbone |
| Adenomatoid odontogenic tumor | AOT | Uncommon, encapsulated OT with indolent behavior. | Known as two-thirds of a tumor because 2/3 occur in the 2nd decade, 2/3 occur in females, and 2/3 are associated with an impacted maxillary canine. | Unilocular radiolucency with variable radiopaque flecks (resembling snowflakes) is typically observed around the crown of an unerupted tooth, commonly the canine. |
| Squamous odontogenic tumor | SOT | Uncommon benign OT with squamous differentiation. | Painless swelling in the 4th decade, occasionally seen lateral to the roots of the teeth. Multiple or peripheral SOT has been reported. | Triangular or semicircular corticated radiolucency along the teeth roots. Tooth displacement occasionally seen. |
| Calcifying epithelial odontogenic tumor (Pindborg tumor) | CEOT | Uncommon benign epithelial OT with amyloid deposition and calcifications. | An asymptomatic, slowly growing mass occurring in the 4th decade and commonly in the posterior mandible. | Unilocular or multilocular radiolucency with variable radiodensity. Half of the cases were associated with an impacted tooth. |
| Epithelial and Mesenchymal Odontogenic Tumors | ||||
| Odontoma | OD | Hamartomatous growth exhibits different dental hard and soft tissues in various development stages. | Asymptomatic, slowly growing mass typically observed in the 2nd and 3rd decade, located in the anterior maxilla (compound) or posterior mandible (complex). | Compound: several tooth-like structures of varying sizes and shapes with a radiolucent rim. Complex: a calcified mass exhibiting radiodensity akin to the tooth structure, surrounded by a radiolucent rim. |
| Ameloblastic fibroma | AF | Benign-mixed OT without hard tissue deposition. | Asymptomatic, slowly growing lesion was seen in the second decade, most commonly in the mandible. | Unilocular or multilocular and corticated radiolucent lesion, 80% associated with an unerupted tooth. |
| Dentinogenic ghost cell tumor | DGCT | Rare benign OT displaying locally aggressive behavior, characterized by the abundance of ghost cells and dentinoid deposition. | Asymptomatic, slowly increasing swelling identified in the 3rd to 5th decade, typically localized in the posterior region of either jaw. | Well-defined, unilocular or multilocular-mixed radiolucent lesion. Tooth displacement or resorption is occasionally seen. |
| Primordial odontogenic tumor | POT | Recently described mixed POT exhibiting primitive dental tissue with occasional hard tissue deposition. | Slowly growing lesion in the first two decades and always associated with an unerupted tooth, commonly the third molar. | Well-demarcated, unilocular, bilocular, or multilocular radiolucency associated with an unerupted tooth. |
| Mesenchymal Odontogenic Tumors | ||||
| Odontogenic fibroma | OF | Rare OT consists mainly of mature fibrous tissue and inactive odontogenic epithelium with a peripheral variant (the most common peripheral odontogenic tumor). | Asymptomatic, slowly growing lesion seen in the fourth decade occurring commonly in the anterior maxilla and posterior mandible. Anterior maxillary lesions may cause soft tissue depression or dimpling. | Central OF: Unilocular or multilocular well-defined radiolucency is often seen intimately around the roots of teeth. |
| Cementoblastoma | CB | Benign neoplasm of cementoblasts, representing less than 3% of all OTs. | Slowly increasing painful swelling associated with teeth roots, most commonly the mandibular first molar. | The tumor appears as a radiopaque mass fused to one or more tooth roots and is surrounded by a thin radiolucent rim and resorption of the associated root is common. |
| Cemento-ossifying fibroma | COF | OT is derived from mesenchymal stem cells with differentiation towards periodontal structures, such as bone and cementum-like material. | Asymptomatic bony expansion in the posterior mandible mostly in the 3rd and 4th decades. | Well-demarcated radiolucency with a sclerotic rim in the tooth-bearing area of the jaws, accompanied by variable radiopacities. Bowing of the inferior border of the mandible may be evident. |
| Odontogenic myxoma | OM | The most common mesenchymal OT is composed of mainly myxoid stroma and occasional inactive odontogenic epithelium. | Painless swelling of the posterior mandible seen in the 2nd and 3rd decades. | Unilocular multilocular “honeycomb” or “tennis racket” radiolucency with diffuse borders and teeth displacement or resorption. |
| All Three Centers | KFMC | KAMC | PSMMC | p-Value | |
|---|---|---|---|---|---|
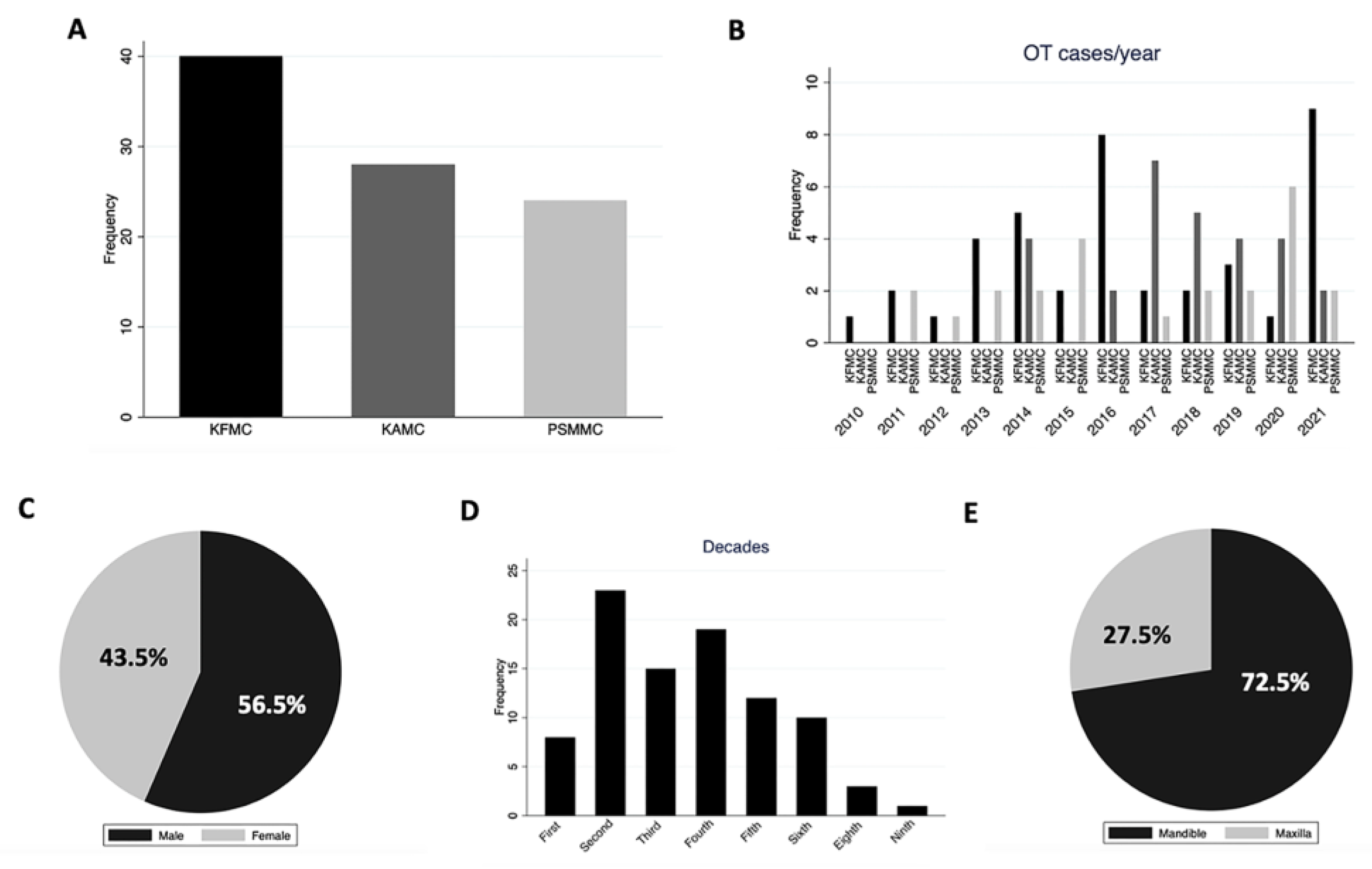
| Cases | 92 | 40 (43.5%) | 28 (30.4%) | 24 (26.1%) | |
| Age median (range) | 29 (5–83) | 33 (6–76) | 22 (5–83) | 26 (7–56) | 0.4020 |
| Gender | |||||
| Male | 52 | 23 | 17 | 12 | |
| Female | 40 | 17 | 11 | 12 | |
| Male/female ratio | 1.27:1 | 1.16:1 | 1.54:1 | 1:1 | 0.6031 |
| Mandible/maxilla ratio | 2.64:1 | 2.25:1 | 2.8:1 | 3:1 | 0.884 |
| All Three Centers | KFMC | KAMC | PSMMC | |
|---|---|---|---|---|
| Epithelial Odontogenic Tumors | ||||
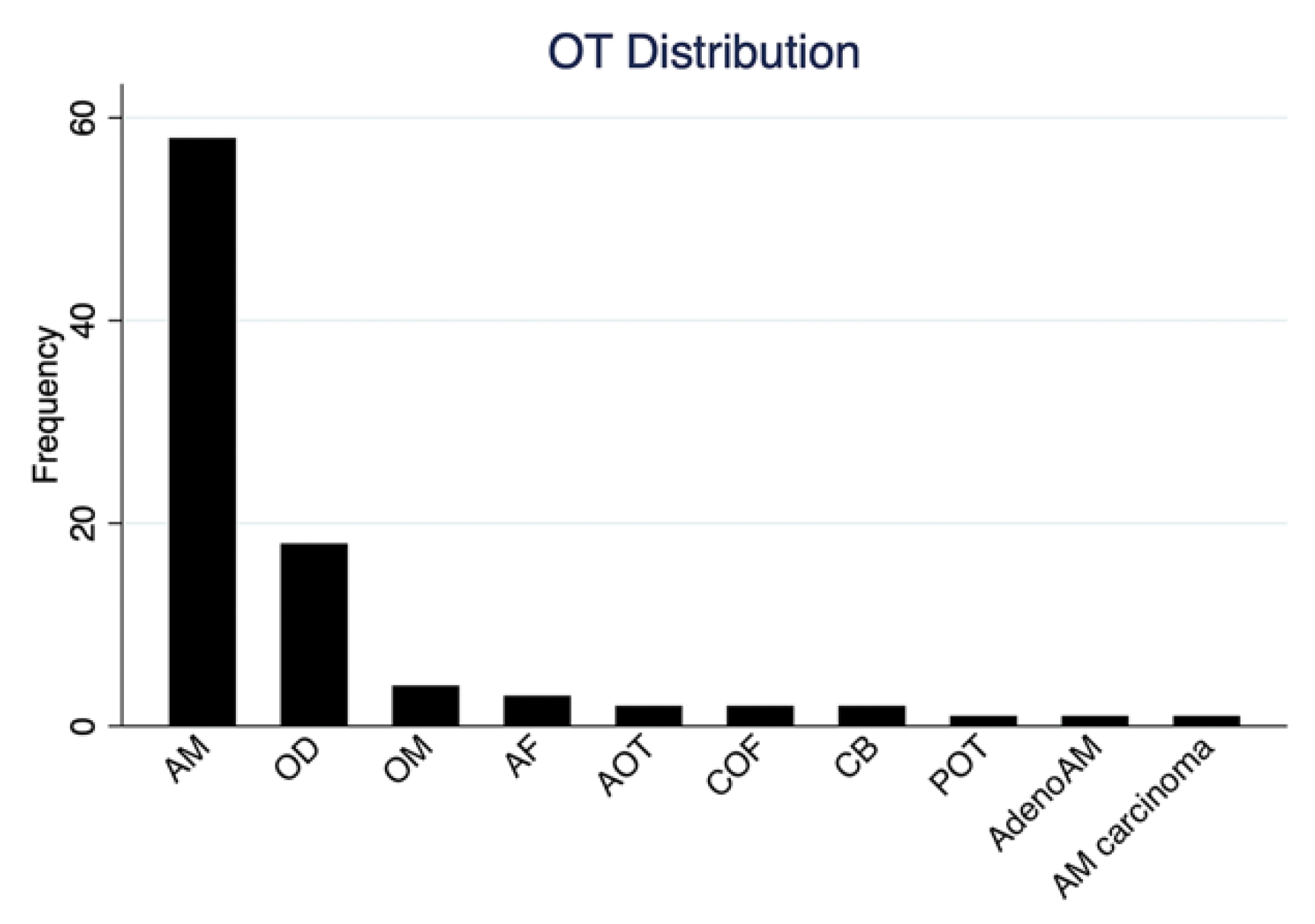
| Ameloblastoma | 58 (63.0%) | 27 (46.6%) | 12 (20.7%) | 19 (32.7%) |
| Adenoid ameloblastoma | 1 (1.1%) | 1 (100%) | 0 | 0 |
| Adenomatoid odontogenic tumor | 2 (2.2%) | 1 (50%) | 0 | 1 (50%) |
| Ameloblastic carcinoma | 1 (1.1%) | 0 | 0 | 1 (100%) |
| Mixed Epithelial–Mesenchymal Odontogenic Tumors | ||||
| Odontoma | 18 (19.5%) | 3 (16.7%) | 12 (66.6%) | 3 (16.7%) |
| Ameloblastic fibroma | 3 (3.3%) | 3 (100%) | 0 | 0 |
| Primordial odontogenic tumor | 1 (1.1%) | 1 (100%) | 0 | 0 |
| Mesenchymal Odontogenic Tumors | ||||
| Odontogenic myxoma | 4 (4.3%) | 3 (75.0%) | 0 | 1 (25.0%) |
| Central odontogenic fibroma | 2 (2.2%) | 1 (50%) | 1 (50%) | 0 |
| Cementoblastoma | 2 (2.2%) | 0 | 2 (100%) | 0 |
| Total | 92 (100%) | 40 (43.5%) | 28 (30.4%) | 24 (26.1%) |
| Number of Case | Age Median (Range) | Gender | Location * | |||
|---|---|---|---|---|---|---|
| M | F | Mandible | Maxilla | |||
| Epithelial Odontogenic Tumors | ||||||
| Ameloblastoma | 58 (63.0%) | 36 (6–83) | 34 (58.6%) | 24 (41.4%) | 47 (81.0%) | 11 (19.0%) |
| Conventional amelobastoma | 54 (93.1%) | 38 (6–83) | 33 (61.1%) | 21 (38.9%) | 44 (81.5%) | 10 (18.5%) |
| Unicystic ameloblastoma | 4 (6.9%) | 20 (16–51) | 1 (25.0%) | 3 (75.0%) | 3 (75.0%) | 1 (25.0%) |
| Adenoid ameloblastoma | 1 (1.1%) | 14 (N/A) | 0 | 1 (100%) | 0 | 1 (100%) |
| Adenomatoid odontogenic tumor | 2 (2.2%) | 17 (15–19) | 1 (50.0%) | 1 (50.0%) | 1 (50.0%) | 1 (50.0%) |
| Ameloblastic carcinoma | 1 (1.1%) | 31 (N/A) | 1 (100%) | 0 | 1 (100%) | 0 |
| Mixed Epithelial–Mesenchymal Odontogenic Tumors | ||||||
| Odontoma | 18 (19.5%) | 20 (5–50) | 10 (55.6%) | 8 (44.4%) | 8 (47.1%) | 9 (52.9%) |
| Complex | 10 (55.6%) | 17 (5–50) | 5 (50.0%) | 5 (50.0%) | 8 (80.0%) | 2 (20.0%) |
| Compound | 8 (44.4%) | 17 (9–37) | 5 (62.5%) | 3 (37.5%) | 0 | 7 (100%) |
| Ameloblastic fibroma | 3 (3.3%) | 13 (6–26) | 1 (25.0%) | 2 (27.5%) | 2 (75.0%) | 1 (25.0%) |
| Primordial odontogenic tumor | 1 (1.1%) | 16 (N/A) | 1 (100%) | 0 | 1 (100%) | 0 |
| Mesenchymal Odontogenic Tumors | ||||||
| Odontogenic myxoma | 4 (4.3%) | 31 (27–36) | 3 (75.0%) | 1 (25.0%) | 2 (50.0%) | 2 (50.0%) |
| Central odontogenic fibroma | 2 (2.2%) | 30 (15–45) | 1 (50.0%) | 1 (50.0%) | 2 (100%) | 0 |
| Cementoblastoma | 2 (2.2%) | 32 (17–48) | 0 | 2 (100%) | 2 (100%) | 0 |
| Total | 92 (100%) | 52 (56.5%) | 40 (43.5%) | 66 (72.5%) | 25 (27.5%) | |
| Number of Cases | Treatment | |||
|---|---|---|---|---|
| Enucleation | Excision | Resection | ||
| Epithelial Odontogenic Tumors | ||||
| Ameloblastoma | 52 (63.4%) | 6 (11.5%) | 12 (23.1%) | 34 (65.4%) |
| Conventional ameloblastoma | 48 (92.3%) | 4 (8.3%) | 12 (25.0%) | 32 (66.7%) |
| Unicystic ameloblastoma | 4 (7.7%) | 2 (50.0%) | 0 | 2 (50%) |
| Adenoid ameloblastoma | 1 (1.2%) | 0 | 0 | 1 (100%) |
| Adenomatoid odontogenic tumor | 1 (1.2%) | 1 (100%) | 0 | 0 |
| Ameloblastic carcinoma | 1 (1.2%) | 0 | 0 | 1 (100%) |
| Mixed Epithelial–Mesenchymal Odontogenic Tumors | ||||
| Odontoma | 17 (20.8%) | 12 (70.6%) | 4 (23.5%) | 1 (5.9%) |
| Ameloblastic fibroma | 2 (2.4%) | 1 (50.0%) | 1 (50.0%) | |
| Primordial odontogenic tumor | 1 (1.2%) | 0 | 0 | 1 (100%) |
| Mesenchymal Odontogenic Tumors | ||||
| Odontogenic myxoma | 4 (5.0%) | 0 | 1 (25.0%) | 3 (75.0%) |
| Central odontogenic fibroma | 1 (1.2%) | 1 (100%) | 0 | 0 |
| Cementoblastoma | 2 (2.4%) | 2 (100%) | 0 | 0 |
| Total | 82 (100%) | 23 (25.6%) | 17 (21.0%) | 42 (51.0%) |
| Number of Cases | Recurrence | No Recurrence | Follow-Up Period | |
|---|---|---|---|---|
| Ameloblastoma | 50 | 8 (16.0%) | 42 (84.0%) | 1 year–6 years |
| Odontoma | 17 | 1 (5.9%) | 16 (94.1%) | 1 year–3 years |
| Odontogenic myxoma | 4 | 1 (25.0%) | 3 (75.0%) | 7 months–4 years |
| Ameloblastic fibroma | 2 | 1 (50.0%) * | 1 (50.0%) | 1 year |
| Total | 73 | 11 (15.1%) | 62 (84.9%) | N/A |
| Current Study (Three Centers), Riyadh, SA | Ali MA et al., Kuwait University, Jabriya, Kuwait [8] | Alsheddi M et al., King Saud University, Riyadh, KSA [10] | Al-Rawi N et al., Tawam Hospital, Abu Dhabi, UAE [9] | |
|---|---|---|---|---|
| Sample size | 92 | 27 | 108 * | 22 |
| Period | 11 years | 6 years | 26 years | 20 years |
| Mean age | 30 | N/R | 29 | N/R |
| Male/female ratio | 1.3:1 | 1.25:1 | 1.4:1 | 1:1 |
| Mandible/maxilla ratio | 2.64:1 | 3.5:1 | 2.1:1 | 1.62:1 |
| Epithelial Odontogenic Tumors | ||||
| Ameloblastoma | 58 (63.0%) | 17 (63.0%) | 47 (43.5%) | 4 (18.1%) |
| Adenoid ameloblastoma | 1 (1.1%) | N/R | N/R | N/R |
| Adenomatoid odontogenic tumor | 2 (2.2%) | N/R | 8 (7.4%) | N/R |
| Calcifying epithelial odontogenic tumor | N/R | 1 (3.7%) | 2 (1.8%) | N/R |
| Ameloblastic carcinoma | 1 (1.1%) | N/R | 1 (0.9%) | 0 (0%) |
| Clear cell odontogenic carcinoma | N/R | N/R | 1 (0.9%) | N/R |
| Mixed Epithelial–Mesenchymal Odontogenic Tumors | ||||
| Odontoma | 18 (19.5%) | 9 (33.3%) | 28 (26.0%) | 17 (77.8%) |
| Ameloblastic fibroma | 3 (3.3%) | N/R | 4 (3.7%) | N/R |
| Primordial odontogenic tumor | 1 (1.1%) | N/R | N/R | N/R |
| Dentinogenic ghost cell tumor | N/R | N/R | N/R | N/R |
| Mesenchymal Odontogenic Tumors | ||||
| Odontogenic myxoma | 4 (4.3%) | N/R | 12 (11.1%) | N/R |
| Central odontogenic fibroma | 2 (2.2%) | N/R | 1 (0.9%) | N/R |
| Cementoblastoma | 2 (2.2%) | N/R | 4 (3.7%) | N/R |
| Total | 92 (100%) | 27 (100%) | 108 (100%) | 22 (100%) |
| Current Study (Three Centers), Riyadh, SA | Kokubun K et al. Tokyo, Japan [3] | de Medeiros WK et al. Natal, Northeastern Brazil [20] | Mascitti M et al. Ancona, Italy [17] | |
|---|---|---|---|---|
| Sample size | 92 | 1089 | 247 | 100 |
| Period | 11 years | 45 years | 22 years | 25 years |
| Mean age | 30 | 29 | 28 | 49.7 |
| Male/female ratio | 1.3:1 | 1.2:1 | 1:1.2 | 1.78:1 |
| Mandible/maxilla ratio | 2.64:1 | 2.1:1 | 2:1 | 2.1:1 |
| Epithelial Odontogenic Tumors | ||||
| Ameloblastoma | 58 (63.0%) | 456 (41.9%) | 112 (45.4%) | 56 (56%) |
| Adenoid ameloblastoma | 1 (1.1%) | N/R | N/R | N/R |
| Adenomatoid odontogenic tumor | 2 (2.2%) | 17 (1.6%) | 10 (4.0%) | 2 (2.0%) |
| Squamous odontogenic tumor | N/R | 2 (0.2%) | N/R | 1 (1.0%) |
| Calcifying epithelial odontogenic tumor | N/R | 8 (1.6%) | 5 (2.0%) | 4 (4.0%) |
| Ameloblastic carcinoma | 1 (1.1%) | 1 (0.1) | 1 (0.4%) | 2 (2.0%) |
| Primary intra-osseous carcinoma | N/R | 8 (0.7%) | N/R | N/R |
| Clear cell odontogenic carcinoma | N/R | N/R | 1 (0.4%) | 1 (1.0%) |
| Mixed Epithelial–Mesenchymal Odontogenic Tumors | ||||
| Odontoma | 18 (19.5%) | 463 (42.5%) | 89 (36.1%) | 17 (17.0%) |
| Ameloblastic fibroma | 3 (3.3%) | 17 (1.6%) | 4 (1.6%) | 3 (3.0%) |
| Primordial odontogenic tumor | 1 (1.1%) | N/R | N/R | N/R |
| Dentinogenic ghost cell tumor | N/R | 7 (0.6%) | 1 (0.4%) | 2 (2.0%) |
| Mesenchymal Odontogenic Tumors | ||||
| Odontogenic myxoma | 4 (4.3%) | 41 (3.8%) | 17 (6.9%) | 4 (4.0%) |
| Central odontogenic fibroma | 2 (2.2%) | 22 (2.0%) | 3 (1.2%) | 1 (1.0%) |
| Cementoblastoma | 2 (2.2%) | 8 (0.7%) | 4 (1.6%) | 2 (2.0%) |
| Cemento-ossifying fibroma | N/R | 38 (3.5%) | N/R | 4 (4.0%) |
| Odontogenic sarcoma | N/R | 1 (0.1%) | N/R | 1 (1.0%) |
| Total | 92 (100%) | 1089 (100%) | 247 (100%) | 100 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almazyad, A.; Alamro, M.; Almadan, N.; Almutairi, M.; AlQuwayz, T.S. Frequency and Demographic Analysis of Odontogenic Tumors in Three Tertiary Institutions: An 11-Year Retrospective Study. Diagnostics 2024, 14, 910. https://doi.org/10.3390/diagnostics14090910
Almazyad A, Alamro M, Almadan N, Almutairi M, AlQuwayz TS. Frequency and Demographic Analysis of Odontogenic Tumors in Three Tertiary Institutions: An 11-Year Retrospective Study. Diagnostics. 2024; 14(9):910. https://doi.org/10.3390/diagnostics14090910
Chicago/Turabian StyleAlmazyad, Asma, Mohammed Alamro, Nasser Almadan, Marzouq Almutairi, and Turki S. AlQuwayz. 2024. "Frequency and Demographic Analysis of Odontogenic Tumors in Three Tertiary Institutions: An 11-Year Retrospective Study" Diagnostics 14, no. 9: 910. https://doi.org/10.3390/diagnostics14090910
APA StyleAlmazyad, A., Alamro, M., Almadan, N., Almutairi, M., & AlQuwayz, T. S. (2024). Frequency and Demographic Analysis of Odontogenic Tumors in Three Tertiary Institutions: An 11-Year Retrospective Study. Diagnostics, 14(9), 910. https://doi.org/10.3390/diagnostics14090910






