Abstract
Background: The relationship between respiratory function and craniofacial morphology has garnered significant attention due to its implications for upper airway and stomatognathic development. Nasal breathing plays a key role in craniofacial growth and dental positioning. This study investigated upper airway morphology and volume differences among individuals with class I, II, and III skeletal anomalies. Methods: Ninety orthodontic patients’ CBCT scans were analyzed to assess the oropharynx and hypopharynx volumes. Skeletal diagnosis was established based on the cephalometric analysis. Results: A significant volume change in the oropharynx and pharynx was demonstrated when comparing class II with class III anomalies (p = 0.0414, p = 0.0313). The total volume of the pharynx was increased in class III anomalies. The area of the narrowest part of the pharynx (MIN-CSA) significantly decreased in classes I and II compared to class III (p = 0.0289, p = 0.0003). Patients with Angle class III anomalies exhibited higher values in the narrowest pharyngeal segment. Gender differences were significant in pharyngeal volumes and morphologies across malocclusion classes. Conclusions: The narrowest segment of the pharynx had the highest values in patients with Angle class III. The volume of the oropharynx was found to be greater in patients with Angle class III versus patients with Angle class II.
1. Introduction
The link between respiratory function and craniofacial morphology has been a topic of interest in recent years. Previous research has shown the connection between upper airway and stomatognathic development [1,2,3,4,5,6]. As a result of upper airway restriction or obstruction, changes in breathing may occur, directly influencing normal craniofacial development and dental positions [1]. The ideal upper airway involves nasal breathing [7,8,9,10]. Normal nasal breathing involves air circulation through filtration [11,12]. Studies demonstrate multiple advantages of nasal breathing [13]. These functions can be compromised when upper airway obstruction occurs. Obstruction can be correlated with the functional activity and volume of the surrounding soft tissues and has been shown to be frequently encountered from an early age [14,15,16].
Mouth breathing can negatively affect growth and development, predisposing the subject to a lowered position of the mandible and tongue and changing the growth direction of the facial structures [15]. It may determine a clockwise rotation of the mandible and a vertical type of growth [17]. Depending on the position of the tongue and its action on the floor of the mouth, two types of anomalies can develop: Angle class II, with the tongue in a lower and posterior position, and Angle class III, with the tongue in a lower and anterior position [17,18,19,20,21]. An enlarged lower floor, dental crowding, difficulties in swallowing and mastication, the upper incisors proclinated and lower ones lingualized, and a deep palatal vault are also mentioned as complications of the altered tongue position [8,17]. Today, radiography is an indispensable tool in orthodontic practice. Studies show that more than a quarter of radiographs in the European Union are made in dental medicine [9]. However, two-dimensional (2D) imaging techniques have proven to be ineffective in reproducing three-dimensional (3D) structures and associated pathologies [9,22,23,24,25]. With the appearance of CBCT in the late 1990s, dental radiography was revolutionized [10]. CBCT has the great advantage of reconstructing inaccessible images from previous orthodontic practice, allowing the orthodontist to analyze multiple planes, such as axial, sagittal, and coronal. Studying tissues, facial soft structures, and dentition from infinite incidences is also possible. The second significant advantage is the possibility of extracting conventional radiographs, such as panoramic and lateral cephalograms, from a single CBCT scan [11,25,26,27]. Some studies [17,18,19] have investigated the correlation between the morphology of the upper airways and different growth patterns in sagittal and vertical planes. Di Carlo et al. studied [19] the upper airway’s morphology and dimensions through 3D radiological measurements in 90 young adult patients. The sagittal plane was assessed, and the patients were divided into three groups according to the value of the ANB angle. In their recent study, Sfondrini et al. [17] evaluated upper airways in adult Caucasian subjects without previous orthodontic treatment. Their main objective was to measure the upper airway dimensions in adult skeletal class I, II, and III patients [17]. However, their measurements were performed two-dimensionally in lateral radiographs [17]. The morphology of the pharyngeal airway in growing and nongrowing cleft lip and palate patients was assessed in Abdelkarim et al.’s study [22]. Their sample consisted of 36 cleft lip and palate subjects and 30 subjects without cleft lip and palate in the control group [22]. A meta-analysis [20] in 2022 aimed to evaluate scientific evidence related to the effects of different orthodontic treatment possibilities on the airways. The authors included in their meta-analysis 66 eligible articles about the CT and CBCT airway evaluation after orthodontic therapy [20]. The orthodontist has a vital role in recognizing a patient’s respiratory problem and correlating it with the type of malocclusion to prescribe the correct treatment.
The primary purpose of this study was to evaluate the following parameters: upper airway morphology and volume in class I, II, and III Angles. The secondary objective was to compare and correlate these parameters.
2. Materials and Methods
2.1. Study Design, Participants, Measurements, and Variables
The present retrospective observational study was approved by the Ethical Committee of the “George Emil Palade” University of Medicine, Pharmacy, Science, and Technology of Targu Mures (approval no. 2904/8 March 2024).
The sample size for this study was determined using G*Power version 3.1.9.6 software (Franz Faul, Universität Kiel, Kiel, Germany). The calculations indicated that a minimum of 16 patients per group (total sample size of 42) would be necessary; this size would provide greater than 95% power to detect significant differences, with an effect size of 0.80 at a significance level of α = 0.05. Thus, 90 preorthodontic CBCT scans of orthodontic patients (38 males, 52 females, mean age 42.29, SD = 10.23) were included in this study.
Based on the cephalometric values, the skeletal diagnosis of each patient was established: class I (ANB = 0–4°), 29 patients (11 males, 18 females, mean age 45.38); class II (ANB > 4°), 33 patients (15 males, 18 females, mean age 40.67); and class III (ANB < 0°), 28 patients (11 males, 16 females, mean age 41.00).
Each patient signed an informed consent agreeing to the use of these records. The following inclusion criteria were used: no previous orthodontic treatment, no surgical interventions on the upper/lower airway, no general illness with respiratory symptoms, age between 25 and 64 years. CBCTs were performed as part of the diagnostic process, thus ensuring that this study abides by good clinical practice. CBCTs (FOV 13 × 15) were performed using a KaVo OP 3D machine (Kavo LTD., Charlotte, NC, USA).
The skeletal sagittal patterns (ANB angle) were established using Steiner cephalometric analysis, an essential tool in orthodontic assessment and treatment planning, on the CBCT cross section representing the lateral cephalometric image. The images were uploaded using digital cephalometric software Romexis 3.6.0 (Romexis, Planmeca, Helsinki, Finland). The landmarks, angles, and planes for the cephalometric tracing were as follows:
- Nasion (N): most anterior point on the frontonasal suture in the midsagittal plane.
- Sella (S): center of the pituitary fossa of the sphenoid bone.
- Point A (A): deepest point of the curve of the anterior border of the maxilla.
- Point B (B): most posterior point in the concavity along the anterior border of the symphysis.
- SNA: angle between the Sella, Nasion, and A point; this angle measures the position of the upper jaw relative to the base of the skull.
- SNB: angle between the Sella, Nasion, and B point; this angle measures the lower jaw’s position relative to the skull’s base.
- ANB: angle between point A, the Nasion, and point B, indicating the relationship between the upper and lower jaws.
- SN: plane between the Sella and Nasion.
- Anterior nasal spine (ANS): anterior tip of the sharp bony process of the maxilla at the lower margin of the anterior nasal aperture.
- Posterior nasal spine (PNS): posterior limit of the palatine bone.
Based on the cephalometric values, the skeletal diagnosis of each patient was established: class I (ANB = 0–4°), class II (ANB > 4°), and class III (ANB < 0°).
For the CBCT measurements, Hounsfield units were set between −1000 and −350. By setting the Hounsfield unit window to −1000 to −350, the CBCT images may be optimized to highlight structures such as bone, teeth, and soft tissues within the desired radiodensity range while minimizing interference from air or other low-density materials that fall below −1000 HU. Each case was analyzed in several sections within the 3D OnDemand program, especially the sagittal and axial sections.
The anatomic landmarks and planes related to upper airway analysis are presented in Table 1.

Table 1.
Anatomic landmarks of the upper airways.
The volume of the pharynx (V-PA) was divided into the volume of the oropharynx (V-OA) and the volume of the hypopharynx (V-HA), which were summed up, resulting in the volume of the pharynx (V-PA) (Figure 1a). The upper limit of the oropharynx was the posterior nasal spine, and the lower limit was the antero-inferior border of the C2 vertebra. The upper limit of the hypopharynx was the antero-inferior border of the C2 vertebra, and the lower limit was the superior border of the hyoid bone. In addition, for calculating the volumes, the vertical length of the oropharynx (L-OA) and the vertical length of the hypopharynx (L-HA) were also calculated, which were summed up, generating the vertical length of the pharynx (L-PA) (Figure 1b).
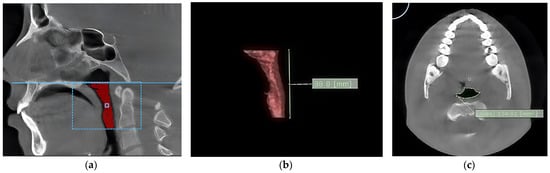
Figure 1.
The aspect of the oropharynx. (a) The volume of the oropharynx; (b) the length of the oropharynx; (c) the area of the narrowest segment of the pharynx.
After measuring the volumes of interest, editing tool functions were used to eliminate the unwanted hollow structures. The methodology used was similar to the one described by Meehan [28]: the interest zone was threshold-segmented, and the slice was edited by hand to remove any artifacts. After segmentation, the software automatically computed pharyngeal airway volumes in cubic millimeters, and the cross-sectional area (in square millimeters) was displayed on the axial image.
The area of the narrowest part of the pharynx (MIN-CSA) was also calculated (Figure 1c). Scrolling through all cross-sectional images determined the most constricted cross-sectional area (Min-CSA) of the pharynx. The vertical length of the Min-CSA (L-CSA) was measured with linear measurement tools. It was defined as the distance between the upper border of the oropharynx and the Min-CSA in the midsagittal view. The volume calculation was performed in cubic millimeters (mm3).
2.2. Data Measurement
Two experienced orthodontists performed the cephalometric analysis. After two weeks, reliability was assessed by reperforming 50% of the cephalometric analyses.
2.3. Statistical Methods
The recorded values were analyzed statistically. The statistical analysis was performed using GraphPad Prism 8 for macOS version 10.2.1 (GraphPad Software, Boston, MA, USA). The mean (M), median (Me), and standard deviation (SD) were calculated. The statistical significance was set at p < 0.05. Given the non-normal distribution of the data, as confirmed by the Shapiro–Wilk test, and the heterogeneity of variances indicated by Levene’s test, we employed the Kruskal–Wallis test for the initial analysis. This choice was guided by the test’s suitability for nonparametric data. After identifying significant differences, Dunn’s post hoc test was proposed to pinpoint specific group disparities. The Mann–Whitney U test explored gender differences in pharyngeal volume and morphology among class I, II, and III individuals.
3. Results
The Kruskal–Wallis test revealed significant differences in the distribution of the V-OA (p = 0.0429) and V-PA (p = 0.0259) across the classes, suggesting variability in these parameters’ behaviors (Table 2). Conversely, the V-HA did not exhibit statistically significant differences (p = 0.4015), indicating similar distributions across the groups. Significant differences were found across the classes regarding the MIN-CS (p = 0.0004). A significant difference was also found in the distribution of the L-OA across the classes (p = 0.0180). No statistical difference was found between the L-HA and L-PA across classes.

Table 2.
Pharyngeal airway spaces in the skeletal patterns using the Kruskal–Wallis test with Dunn’s post hoc test.
A significant change in the volume of the oropharynx and pharynx was demonstrated when comparing class II with class III anomalies (p = 0.0414, respectively, p = 0.0313). The total volume of the pharynx was increased in patients with class III anomalies. The area of the narrowest part of the pharynx (MIN-CSA) significantly decreased in classes I and II compared to class III (p = 0.0289, p = 0.0003). Between classes I and II, one parameter was modified, L-OA, with a p-value (p = 0.0138) being statistically significant (Figure 2).
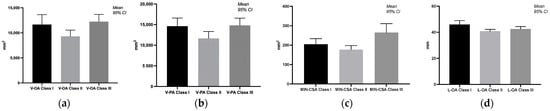
Figure 2.
Graphical representation of the significant differences in the volume of pharyngeal airway spaces in different malocclusion classes. (a) Differences between the volume of the oropharynx in different malocclusions; (b) differences between the volume of the pharynx in different malocclusions; (c) differences between the areas of the narrowest part of the pharynx; (d) differences in the vertical length of the oropharynx in different malocclusions.
The statistical analysis (Mann–Whitney U test) highlights significant gender differences in pharyngeal volumes and morphologies across different malocclusion classes. Notably, males consistently displayed larger volumes and areas in specific measurements (e.g., V-HA, V-PA, and L-PA in class I; MIN-CSA and L-PA in class II; L-HA and MIN-CSA in class III), suggesting a predisposition towards larger pharyngeal dimensions compared to females (Table 3, Figure 3).

Table 3.
Gender differences in pharyngeal volumes and morphologies across different malocclusion classes using the Mann–Whitney U test.
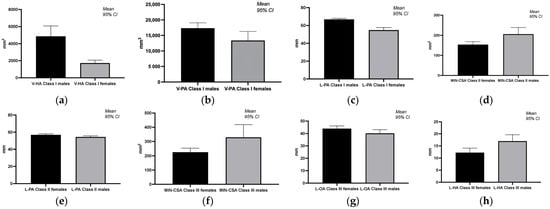
Figure 3.
Graphical representation of significant differences in pharyngeal airway volumes and morphologies across different malocclusion classes and genders. (a) Gender differences in volume of the hypopharynx in class I; (b) gender differences in volume of the pharynx in class I; (c) gender differences in the sum of vertical length of oropharynx and hypopharynx in class I; (d) gender differences in the narrowest part of the pharynx in class II; (e) gender differences in the sum of vertical length of oropharynx and hypopharynx in class II; (f) gender differences in the narrowest part of the pharynx in class III; (g) gender differences in the vertical length of oropharynx in class III; (h) gender differences in the vertical length of hypopharynx in class III.
4. Discussion
In several studies, upper airway volumes were observed to differ by skeletal class [1]. Other studies demonstrate the correlation between class II and obstructive sleep apnea [18]. Regarding the delimitation of the segments, we used the same anatomical landmarks as those used by Zheng et al. [1]. Various studies have explored the relationship between malocclusion type (class I, class II, and class III), gender, and airway volume, revealing intricate interactions among these variables. Nath et al. [8] focused on the skeletal malocclusion’s influence on oropharyngeal airway volume using cone beam computed tomography (CBCT) analysis. This research found significant differences in airway volume among patients with different classes of skeletal malocclusion, underscoring the utility of CBCT in airway assessment [8]. Our results showed significant differences in the volume and area measurements of the oropharynx and pharynx when comparing different classes of anomalies (class II and class III) in orthodontic patients. The total volume of the pharynx was increased in patients with class III anomalies. The area of the narrowest part of the pharynx (MIN-CSA) was significantly decreased in classes I and II when compared to class III (Table 2, Figure 2). Rivlin et al. [18] demonstrated that an anterior mandible position in patients with Angle class III leads to an increased pharyngeal space. In patients with Angle class II, the mandible adopts a retrognathic position, and pharyngeal space proves to be significantly reduced [18]. Other researchers observed a difference in patients with Angle class III, where the hypopharynx volume was higher than in patients with Angle class II [1,28]. Understanding these differences can affect treatment planning and outcomes in orthodontic patients with different malocclusions. A CBCT-based comparison of pharyngeal airway area and volume in patients with Angle class I and class II malocclusions revealed that male patients had a greater area than female patients. However, no association was found between Angle classes I or II malocclusions and oropharyngeal airway volume [29]. A study by Kim et al. [30] highlighted gender-specific differences in airway dimensions among smokers, demonstrating that women have higher wall-area percentages and lower luminal areas, internal diameters, and airway thicknesses than men in anatomically matched airways. These findings highlight the influence of gender on airway dimensions [30]. According to Dominelli et al. [31], healthy women have central airways significantly smaller (~26–35%) than men, with the trachea showing the most significant difference. This difference persists even when subjects are matched for height [31]. Gungor and Turkkahraman reviewed the effects of airway problems on maxillary growth, highlighting the role of specific dental and skeletal malocclusions in airway volume differences [32]. S. Kim et al., in their study about identifying optimal oropharyngeal airway sizes for men and women, found nuanced differences in airway management between genders [33]. These differences affect clinical practices, such as airway management and assessing respiratory health risks. Some studies [34,35] about the association between gender, malocclusion, and the volume of the hypopharynx provide insights into the morphological characteristics of the hypopharynx and its variations by gender. A study by Zhang et al. [34] highlighted morphological characteristics of the male and female hypopharynx through MRI imaging. It was observed that female subjects had a smaller laryngeal cavity and piriform fossa compared to males, indicating gender differences in the morphology of the hypopharynx [34]. According to our study, males have a predisposition towards larger pharyngeal dimensions compared to females across the different malocclusion classes. Male orthodontic patients presented higher volumes and areas in measurements (V-HA, V-PA, and L-PA values for class I; MIN-CSA and L-PA in class II; and MIN-CSA and L-HA in class III). While specific studies directly associating malocclusion with the volume of the hypopharynx were not found, research into the general effects of malocclusion on oral health indicates potential pathways through which malocclusion could impact the hypopharyngeal region. For instance, Rantavuori et al. observed gender differences in the association between malocclusion traits and oral health-related quality of life in Finnish adults, suggesting that malocclusion can have systemic effects that might extend to the hypopharyngeal area in terms of overall health and quality of life [35]. A study focusing on patients with catathrenia, a sleep-related breathing disorder, found that these patients had a statistically smaller sagittal diameter of the hypopharynx than the standard reference [36]. This finding, presented by Yu et al., indicates an association between malocclusion (as an indirect factor through conditions such as catathrenia) and a narrow hypopharynx. However, it does not directly address the role of gender [36]. The interplay between gender, malocclusion, and hypopharynx volume remains under-researched. The available studies primarily focus on gender differences in hypopharynx morphology or the broader impacts of malocclusion. Direct studies exploring all three variables are scarce, highlighting an area for future research. According to our study, malocclusion type and gender significantly influence airway volume. The differences resulting from the present study follow the findings from the literature [8,29,37] and have important implications for the assessment and treatment planning in individuals with malocclusion. They highlight the need for personalized approaches for dental occlusion types and gender-specific airway characteristics.
From a clinical point of view, the findings of our study are relevant when orthodontic treatment is initiated in patients with different classes of anomalies. In the case of class II anomalies, treatment options that might reduce the volume of the pharynx should be avoided. The use of digital dentistry might enhance the outcome of the treatment [19,38,39,40,41,42,43,44].
The limitations of the present study are the following. The evaluation of the airways was performed only in sagittal malocclusion. This study is retrospective. The unregulated respiratory cycle during image acquisition and inadvertent variations in tongue positioning during CBCT scans could affect the accuracy of static 3D images. We lacked control over variables like head position, tongue position, and breathing during CBCT scans. While cone beam computed tomography (CBCT) offers detailed images and has revolutionized dental radiography, it still has limitations. These include the potential for distortion in the imaging of airway structures and the reliance on the proper setting of Hounsfield units for optimal visualization. The interpretation of these images may also be subject to operator error or variability. This study divides participants into three skeletal classes based on ANB angle measurements. However, it does not mention the distribution of other potentially influential factors, such as ethnicity or body mass index. This study provides a snapshot in time and does not track changes throughout orthodontic treatment or as participants age. Therefore, it cannot establish causality or assess the long-term implications of the observed skeletal patterns on airway volume.
Future research could consider replicating the analysis while incorporating sagittal discrepancies, other malocclusions, and functional alterations. By deepening the understanding of airway alterations related to maxillofacial morphology, researchers can more effectively detect patients who are at risk of airway dysfunction early on. Further investigations and analysis after finishing the orthodontic correction of different malocclusions may help elucidate the clinical significance of these anatomical variations and their relationship to orthodontic treatment outcomes. However, future research should focus on prospective studies with larger sample sizes and standardized protocols to further validate these findings and enhance treatment strategies for patients with orthodontic and airway concerns. Also, the analysis can be extended to include the role of surrounding soft tissues, such as tonsil size and tongue posture, in affecting airway volume and orthodontic treatment outcomes. Gender-specific research to delve deeper into the observed gender differences in pharyngeal volumes and morphologies, potentially including hormonal influences, is also necessary.
5. Conclusions
Within the limitations of the present study, the narrowest segment of the pharynx had the highest values in patients with Angle class III. The volume of the oropharynx was found to be greater in patients with Angle class III versus patients with Angle class II.
The findings from this study underscore the importance of considering gender when evaluating pharyngeal volumes and areas in orthodontic patients with malocclusions. Understanding these differences is crucial for the diagnosis and treatment planning of malocclusions, potentially impacting approaches to managing airway-related issues in orthodontic patients.
Author Contributions
Conceptualization, S.I.P. and B.A.; methodology, S.I.P., A.P., M.M. and D.C.; software, B.A. and L.M.; validation, L.M., R.V.P. and S.I.P.; formal analysis, M.M.; investigation, D.C. and B.A.; resources, K.M.J., S.I.P. and A.P.; data curation, M.M. and K.M.J.; writing—original draft preparation, S.I.P., A.P., M.M. and B.A.; writing—review and editing, S.I.P., A.P., D.C. and K.M.J.; visualization, L.M. and R.V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of George Emil Palade University of Medicine, Pharmacy, Science and Technology of Targu Mures (2904/8 March 2024) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zheng, Z.H.; Yamaguchi, T.; Kurihara, A.; Li, H.F.; Maki, K. Three-dimensional evaluation of upper airway in patients with different anteroposterior skeletal patterns. Orthod. Craniofacial Res. 2014, 17, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Brito, F.C.; Brunetto, D.P.; Nojima, M.C.G. Three-dimensional study of the upper airway in different skeletal Class II malocclusion patterns. Angle Orthod. 2019, 89, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Azevêdo, M.S.; Machado, A.W.; Barbosa, I.D.S.; Esteves, L.S.; Rocha, V.Á.C.; Bittencourt, M.A.V. Evaluation of upper airways after bimaxillary orthognathic surgery in patients with skeletal Class III pattern using cone-beam computed tomography. Dent. Press J. Orthod. 2016, 21, 34–41. [Google Scholar] [CrossRef]
- Golchini, E.; Rasoolijazi, H.; Momeni, F.; Shafaat, P.; Ahadi, R.; Jafarabadi, M.A.; Rahimian, S. Investigation of the relationship between mandibular morphology and upper airway dimensions. J. Craniofacial Surg. 2020, 31, 1353–1361. [Google Scholar] [CrossRef]
- Masoud, A.I.; Jackson, G.W.; Carley, D.W. Sleep and airway assessment: A review for dentists. Cranio 2017, 35, 206–222. [Google Scholar] [CrossRef]
- Georgiadis, T.; Angelopoulos, C.; Papadopoulos, M.A.; Kolokitha, O.E. Three-Dimensional Cone-Beam Computed Tomography Evaluation of Changes in Naso-Maxillary Complex Associated with Rapid Palatal Expansion. Diagnostics 2023, 13, 1322. [Google Scholar] [CrossRef] [PubMed]
- Kochar, G.D.; Chakranarayan, A.; Kohli, S.; Kohli, V.S.; Khanna, V.; Jayan, B.; Chopra, S.S.; Verma, M. Effect of surgical mandibular advancement on pharyngeal airway dimensions: A three-dimensional computed tomography study. Int. J. Oral Maxillofac. Surg. 2016, 45, 553–559. [Google Scholar] [CrossRef]
- Nath, M.; Ahmed, J.; Ongole, R.; Denny, C.; Shenoy, N. CBCT analysis of pharyngeal airway volume and comparison of airway volume among patients with skeletal Class I, Class II, and Class III malocclusion: A retrospective study. Cranio 2021, 39, 379–390. [Google Scholar] [CrossRef]
- Jacobs, R.; Salmon, B.; Codari, M.; Hassan, B.; Bornstein, M.M. Cone beam computed tomography in implant dentistry: Recommendations for clinical use. BMC Oral Health 2018, 18, 88. [Google Scholar] [CrossRef]
- Zimmerman, J.N.; Vora, S.R.; Pliska, B.T. Reliability of upper airway assessment using CBCT. Eur. J. Orthod. 2019, 41, 101–108. [Google Scholar] [CrossRef]
- Yamashita, A.L.; Iwaki Filho, L.; Leite, P.C.C.; de Lima Navarro, R.; Ramos, A.L.; Previdelli, I.T.S.; Dal Molin Ribeiro, M.H.; Iwaki, L.C.V. Three-dimensional analysis of the pharyngeal airway space and hyoid bone position after orthognathic surgery. J. Cranio-Maxillofac. Surg. 2017, 45, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R. Cone-beam computed tomography and three-dimensional orthodontics. Where we are and future perspectives. J. Orthod. 2019, 46 (Suppl. 1), 45–48. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, D.C., III. Dentistry’s Great Awakening. Cranio 2018, 36, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Gholinia, F.; Habibi, L.; Amrollahi Boyouki, M. Cephalometric evaluation of the upper airway in different skeletal classifications of jaws. J. Craniofacial Surg. 2019, 30, e469–e474. [Google Scholar] [CrossRef]
- D’Onofrio, L. Oral dysfunction as a cause of malocclusion. Orthod. Craniofacial Res. 2019, 22, 43–48. [Google Scholar] [CrossRef]
- Alhammadi, M.S.; Almashraqi, A.A.; Halboub, E.; Almahdi, S.; Jali, T.; Atafi, A.; Alomar, F. Pharyngeal airway spaces in different skeletal malocclusions: A CBCT 3D assessment. Cranio 2019, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sfondrini, M.F.; Gallo, S.; Pascadopoli, M.; Gandini, P.; Roncoroni, C.; Scribante, A. Upper Airway Dimensions among Different Skeletal Malocclusions: A Retrospective Observational Study by Cephalometric Analysis. Dent. J. 2024, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, J.; Hoffstein, V.; Kalbfleisch, J.; McNichols, W.; Zamel, N.; Bryan, A.C. Upper Airway Morphology in Patients with Idiopathic Obstructive Sleep Apnea. Surv. Anesthesiol. 1985, 29, 42. [Google Scholar] [CrossRef]
- Di Carlo, G.; Polimeni, A.; Melsen, B.; Cattaneo, P.M. The relationship between upper airways and craniofacial morphology studied in 3D. A CBCT study. Orthod. Craniofac. Res. 2015, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alswairki, H.J.; Alam, M.K.; Rahman, S.A.; Alsuwailem, R.; Alanazi, S.H. Upper airway changes in diverse orthodontic looms: A systematic review and meta-analysis. Appl. Sci. 2022, 12, 916. [Google Scholar] [CrossRef]
- Kwong, J.C.; Palomo, J.M.; Landers, M.A.; Figueroa, A.; Hans, M.G. Image quality produced by different cone-beam computed tomography settings. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarim, A.Z.; Khalifa, A.R.; Hassan, M.G.; Abdou, A.; Duman, S.B.; Rezallah, N.N.; Abdraboh, A.E.; Ghoneima, A. Three-Dimensional Assessment of the Pharyngeal Airway in Growing versus Non-Growing Subjects with/without Cleft Lip and Palate. Anatomia 2023, 2, 192–205. [Google Scholar] [CrossRef]
- Fountoulaki, G.; Thurzo, A. Change in the Constricted Airway in Patients after Clear Aligner Treatment: A Retrospective Study. Diagnostics 2022, 12, 2201. [Google Scholar] [CrossRef] [PubMed]
- Osorio, F.; Perilla, M.; Doyle, D.J.; Palomo, J.M. Cone beam computed tomography: An innovative tool for airway assessment. Anesth. Analg. 2008, 106, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, V.; Farronato, M.; Ugolini, A.; Cossellu, G.; Gaffuri, F.; Parisi, F.M.R.; Cavagnetto, D.; Abate, A.; Maspero, C. Volumetric Changes in the Upper Airways after Rapid and Slow Maxillary Expansion in Growing Patients: A Case-Control Study. Materials 2020, 13, 2239. [Google Scholar] [CrossRef] [PubMed]
- Ortu, E.; Giannoni, M.; Ortu, M.; Gatto, R.; Monaco, A. Oropharyngeal airway changes after rapid maxillary expansion: The state of the art. Int. J. Clin. Exp. Med. 2014, 7, 1632–1638. [Google Scholar] [PubMed]
- Tsolakis, I.A.; Kolokitha, O.-E. Comparing Airway Analysis in Two-Time Points after Rapid Palatal Expansion: A CBCT Study. J. Clin. Med. 2023, 12, 4686. [Google Scholar] [CrossRef]
- Kirjavainen, M.; Kirjavainen, T. Upper airway dimensions in Class II malocclusion. Ef-fects of headgear treatment. Angle Orthod. 2007, 77, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, F.; Yosaf, U.; Qayyum, F.; Jamil, A.; Jamil, M. CBCT Based Comparison of Pharyngeal Airway Area and Volume in patients with Angle’s Class I and Class II Malocclusion: A Retrospective Study. Pak. J. Med. Health Sci. 2022, 16, 21–23. [Google Scholar] [CrossRef]
- Kim, Y.I.; Schroeder, J.; Lynch, D.; Newell, J.; Make, B.; Friedlander, A.; Estépar, R.S.; Hanania, N.A.; Washko, G.; Murphy, J.R.; et al. Gender differences of airway dimensions in anatomically matched sites on CT in smokers. COPD 2011, 8, 285–292. [Google Scholar] [CrossRef]
- Dominelli, P.B.; Ripoll, J.G.; Cross, T.J.; Baker, S.E.; Wiggins, C.C.; Welch, B.T.; Joyner, M.J. Sex differences in large conducting airway anatomy. J. Appl. Physiol. 1985, 125, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Gungor, A.Y.; Turkkahraman, H. Effects of Airway Problems on Maxillary Growth: A Review. Eur. J. Dent. 2009, 3, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, J.E.; Kim, Y.H.; Kang, B.C.; Heo, S.B.; Kim, C.K.; Park, W.K. An assessment of oropharyngeal airway position using a fibreoptic bronchoscope. Anaesthesia 2014, 69, 53–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Honda, K.; Wei, J.; Kitamura, T. Morphological characteristics of male and female hypopharynx: A magnetic resonance imaging-based study. J. Acoust. Soc. Am. 2019, 145, 734. [Google Scholar] [CrossRef]
- Rantavuori, K.; Silvola, A.-S.; Suominen, A.; Masood, M.; Suominen, A.L.; Lahti, S. Gender differences in the association between malocclusion traits and oral health-related quality of life in Finnish adults. Eur. J. Oral Sci. 2023, 131, e12927. [Google Scholar] [CrossRef]
- Yu, M.; Hao, Z.L.; Xu, L.Y.; Wen, Y.F.; Han, F.; Gao, X.M. Craniofacial anatomical Characteristics of patients with catathrenia. Zhonghua Kou Qiang Yi Xue Za Zhi 2023, 58, 659–669. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H. Study on Airway Size in Class III Malocclusion by the Gender. Korean J. Phys. Anthropol. 2017, 30, 145. [Google Scholar] [CrossRef]
- Meehan, M.; Teschner, M.; Girod, S. Three-dimensional simulation and prediction of craniofacial surgery. Orthod. Craniofacial. Res. 2003, 6 (Suppl. 1), 102–107. [Google Scholar] [CrossRef]
- Savoldi, F.; Dagassan-Berndt, D.; Patcas, R.; Mak, W.S.; Kanavakis, G.; Verna, C.; Gu, M.; Bornstein, M.M. The use of CBCT in orthodontics with special focus on upper airway analysis in patients with sleep-disordered breathing. Dentomaxillofacial Radiol. 2024, 53, 178–188. [Google Scholar] [CrossRef]
- Christovam, I.O.; Lisboa, C.O.; Ferreira, D.M.; Cury-Saramago, A.A.; Mattos, C.T. Upper airway dimensions in patients undergoing orthognathic surgery: A systematic review and meta-analysis. Int. J. Oral. Maxillofac. Surg. 2016, 45, 460–471. [Google Scholar] [CrossRef]
- Hsu, W.C.; Kang, K.T.; Yao, C.J.; Chou, C.H.; Weng, W.C.; Lee, P.L.; Chen, Y.J. Evaluation of Upper Airway in Children with Obstructive Sleep Apnea Using Cone-Beam Computed Tomography. Laryngoscope 2021, 131, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Masoud, A.I.; Alwadei, F.H. Two-dimensional upper airway normative values in children aged 7 to 17 years. Cranio 2022, 40, 536–543. [Google Scholar] [CrossRef]
- Gurani, S.F.; Cattaneo, P.M.; Rafaelsen, S.R.; Pedersen, M.R.; Thorn, J.J.; Pinholt, E.M. The effect of altered head and tongue posture on upper airway volume based on a validated upper airway analysis-An MRI pilot study. Orthod. Craniofac. Res. 2020, 23, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Masoud, A.I.; Alwadei, A.H.; Gowharji, L.F.; Park, C.G.; Carley, D.W. Relating three-dimensional airway measurements to the apnea-hypopnea index in pediatric sleep apnea patients. Orthod. Craniofac. Res. 2021, 24, 137–146. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).