Relationship between Bilateral Landmarks of Facial Asymmetry in Skeletal Class II and Class III in Vertical Dimension: 3D Facial Scan and Cone-Beam Computed Tomography
Abstract
1. Introduction
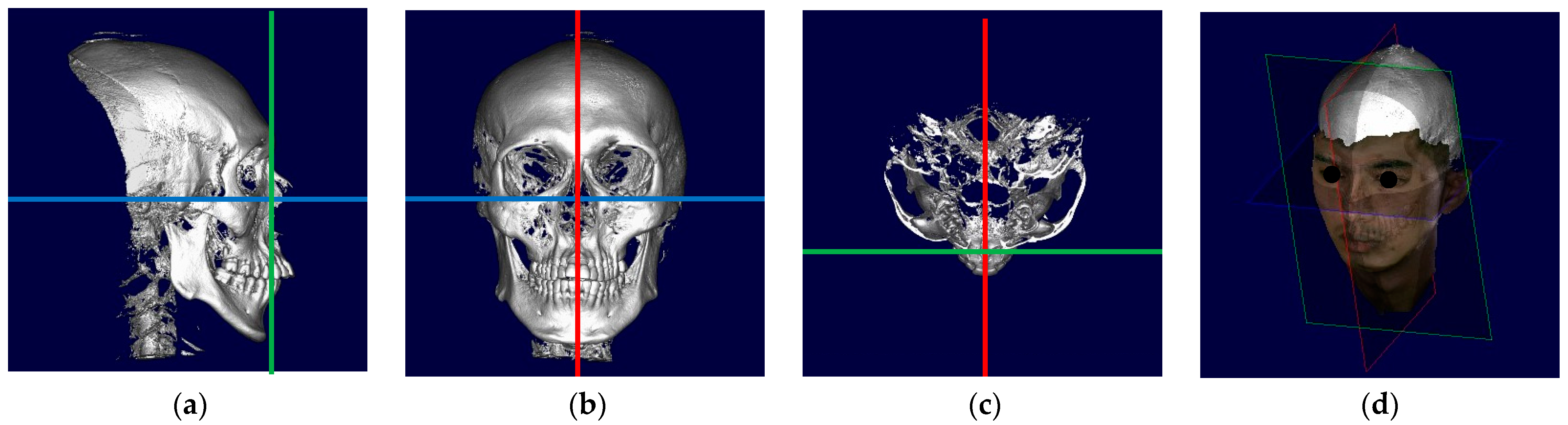
2. Materials and Methods
2.1. Patient Inclusion and Exclusion
2.2. Data Acquisition and Assessment
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peck, S.; Peck, L.; Kataja, M. Skeletal asymmetry in esthetically pleasing faces. Angle Orthod. 1991, 61, 43–48. [Google Scholar] [PubMed]
- Ko, E.W.; Huang, C.S.; Chen, Y.R. Characteristics and corrective outcome of face asymmetry by orthognathic surgery. J. Oral Maxillofac. Surg. 2009, 67, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Lindauer, S.J. Asymmetries: Diagnosis and treatment. Semin. Orthod. 1998, 4, 133. [Google Scholar] [CrossRef] [PubMed]
- Manosudprasit, M.; Manosudprasit, A.; Manosudprasit, A.; Traisrisin, K. Dentofacial Asymmetry: A Literature Review. J. Med. Assoc. Thai. 2017, 100, 50. [Google Scholar]
- Cheong, Y.W.; Lo, L.J. Facial asymmetry: Etiology, evaluation, and management. Chang. Gung Med. J. 2011, 34, 341–351. [Google Scholar]
- Enquist, M.; Arak, A. Symmetry, beauty and evolution. Nature 1994, 372, 169–172. [Google Scholar] [CrossRef]
- Choi, J.W.; Park, H.; Kwon, S.M.; Lee, J.Y. Surgery-first orthognathic approach for the correction of facial asymmetry. J. Craniomaxillofac. Surg. 2021, 49, 435–442. [Google Scholar] [CrossRef]
- Haraguchi, S.; Takada, K.; Yasuda, Y. Facial asymmetry in subjects with skeletal Class III deformity. Angle Orthod. 2002, 72, 28–35. [Google Scholar]
- Severt, T.R.; Proffit, W.R. The prevalence of facial asymmetry in the dentofacial deformities population at the University of North Carolina. Int. J. Adult Orthodon. Orthognath. Surg. 1997, 12, 171–176. [Google Scholar]
- Thiesen, G.; Gribel, B.F.; Freitas, M.P.M.; Oliver, D.R.; Kim, K.B. Craniofacial features affecting mandibular asymmetries in skeletal Class II patients. J. Orofac. Orthop. 2017, 78, 437–445. [Google Scholar] [CrossRef]
- Kim, H.; Lee, W.; Kook, Y.-A.; Kim, Y. Comparison of the condyle-fossa relationship between skeletal class III malocclusion patients with and without asymmetry: A retrospective three-dimensional cone-beam computed tomograpy study. Korean J. Orthod. 2013, 43, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Do Egito Vasconcelos, B.C.; Gonçalves, F.; Andrade, A.; Guillen, M.; Landim, F. Mandibular asymmetry: Literature review and case report. Braz. J. Otorhinolaryngol. (Engl. Ed.) 2012, 78, 137. [Google Scholar] [CrossRef] [PubMed]
- Bishara, S.E.; Burkey, P.S.; Kharouf, J.G. Dental and facial asymmetries: A review. Angle Orthod. 1994, 64, 89–98. [Google Scholar]
- Moshkelgosha, V.; Fathinejad, S.; Pakizeh, Z.; Shamsa, M.; Golkari, A. Photographic Facial Soft Tissue Analysis by Means of Linear and Angular Measurements in an Adolescent Persian Population. Open Dent. J. 2015, 9, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Plooij, J.M.; Maal, T.J.; Haers, P.; Borstlap, W.A.; Kuijpers-Jagtman, A.M.; Bergé, S.J. Digital three-dimensional image fusion processes for planning and evaluating orthodontics and orthognathic surgery. A systematic review. Int. J. Oral Maxillofac. Surg. 2011, 40, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Singh, H.; Mishra, S.; Sharma, P.; Kapoor, P.; Chandra, L. Facial asymmetry revisited: Part I- diagnosis and treatment planning. J. Oral Biol. Craniofac. Res. 2018, 8, 7–14. [Google Scholar] [CrossRef] [PubMed]
- De Groeve, P.; Schutyser, F.; Van Cleynenbreugel, J.; Suetens, P. Registration of 3D Photographs with Spiral CT Images for Soft Tissue Simulation in Maxillofacial Surgery. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2001, Berlin/Heidelberg, Germany, 14–17 October 2001. [Google Scholar]
- Swennen, G.R.; Schutyser, F.; Barth, E.L.; De Groeve, P.; De Mey, A. A new method of 3-D cephalometry Part I: The anatomic Cartesian 3-D reference system. J. Craniofac. Surg. 2006, 17, 314–325. [Google Scholar] [CrossRef]
- Khambay, B.S.; Nebel, J.C.; Bowman, J.; Ayoub, A.F.; Walker, F.; Hadley, D. 3D stereophotogrammetric image superimposition onto 3D CT scan images: The future of orthognathic surgery. A pilot study. Int. J. Adult Orthodon. Orthognath. Surg. 2002, 17, 331–341. [Google Scholar]
- Vale, F.; Scherzberg, J.; Cavaleiro, J.; Sanz, D.; Caramelo, F.; Maló, L.; Marcelino, J.P. 3D virtual planning in orthognathic surgery and CAD/CAM surgical splints generation in one patient with craniofacial microsomia: A case report. Dent. Press J. Orthod. 2016, 21, 89–100. [Google Scholar] [CrossRef]
- Marradi, F.; Staderini, E.; Zimbalatti, M.A.; Rossi, A.; Grippaudo, C.; Gallenzi, P. How to Obtain an Orthodontic Virtual Patient through Superimposition of Three-Dimensional Data: A Systematic Review. Appl. Sci. 2020, 10, 5354. [Google Scholar] [CrossRef]
- Nur, R.B.; Çakan, D.G.; Arun, T. Evaluation of facial hard and soft tissue asymmetry using cone-beam computed tomography. Am. J. Orthod. Dentofacial. Orthop. 2016, 149, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, D.; Dominguez-Mompell, R.; Mallya, S.; Moschik, C.; Pan, H.C.; Miller, J.; Moon, W. Changes in the midpalatal and pterygopalatine sutures induced by micro-implant-supported skeletal expander, analyzed with a novel 3D method based on CBCT imaging. Prog. Orthod. 2017, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Elkenawy, I.; Fijany, L.; Colak, O.; Paredes, N.A.; Gargoum, A.; Abedini, S.; Cantarella, D.; Dominguez-Mompell, R.; Sfogliano, L.; Moon, W. An assessment of the magnitude, parallelism, and asymmetry of micro-implant-assisted rapid maxillary expansion in non-growing patients. Prog. Orthod. 2020, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.M. Hemispheric and facial asymmetry: Gender differences. Laterality 2000, 5, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, V.F.; Sforza, C.; Poggio, C.E.; Tartaglia, G. Distance from symmetry: A three-dimensional evaluation of facial asymmetry. J. Oral Maxillofac. Surg. 1994, 52, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Woo, T.L. On the Asymmetry of the Human Skull. Biometrika 1931, 22, 324–352. [Google Scholar] [CrossRef]
- Lu, K.H. Harmonic analysis of the human face. Biometrics 1965, 21, 491–505. [Google Scholar] [CrossRef]
- Kawamoto, H.K.; Kim, S.S.; Jarrahy, R.; Bradley, J.P. Differential diagnosis of the idiopathic laterally deviated mandible. Plast. Reconstr. Surg. 2009, 124, 1599–1609. [Google Scholar] [CrossRef]
- Lee, M.S.; Chung, D.H.; Lee, J.W.; Cha, K.S. Assessing soft-tissue characteristics of facial asymmetry with photographs. Am. J. Orthod. Dentofacial. Orthop. 2010, 138, 23–31. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Youn, I.-S.; Lee, K.-H.; Lim, H.-J. Classification of facial asymmetry by cluster analysis. Am. J. Orthod. Dentofacial Orthop. 2007, 132, 279. [Google Scholar] [CrossRef]
- Kim, J.A.-O.; Park, H.K.; Shin, S.W.; Park, J.H.; Jung, H.D.; Jung, Y.A.-O. Three-dimensional evaluation of the correlation between lip canting and craniofacial planes. Korean J. Orthod. 2020, 25, 258–267. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, E.J.; Kim, B.C.; Cho, K.H.; Lee, K.H.; Hwang, H.S. Correlations of frontal lip-line canting with craniofacial morphology and muscular activity. Am. J. Orthod. Dentofacial Orthop. 2007, 132, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Gazit-Rappaport, T.; Weinreb, M.; Gazit, E. Quantitative evaluation of lip symmetry in functional asymmetry. Eur. J. Orthod. 2003, 25, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-T.; Wang, J.-L.; Chen, S.-C.; Pan, C.-Y.; Chen, C.-M.; Tseng, Y.-C. Correlation between facial asymmetry of skeletal class III jaw relationship and morphology of the temporomandibular joint: A cone beam computed tomography study. J. Dent. Sci. 2023, 18, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.; Joshi, M.R. An assessment of asymmetry in the normal craniofacial complex. Angle Orthod. 1978, 48, 141–148. [Google Scholar] [PubMed]
- Burstone, C.J. Diagnosis and treatment planning of patients with asymmetries. Semin. Orthod. 1998, 4, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, N.; Momoi, Y.; Ariji, Y.; Nawa, H.; Muramatsu, A.; Goto, S.; Ariji, E. Can Cephalometric Indices and Subjective Evaluation Be Consistent for Facial Asymmetry? Angle Orthod. 2005, 75, 651–655. [Google Scholar]
- Ayoub, A.F.; Xiao, Y.; Khambay, B.; Siebert, J.P.; Hadley, D. Towards building a photo-realistic virtual human face for craniomaxillofacial diagnosis and treatment planning. Int. J. Oral Maxillofac. Surg. 2007, 36, 423–428. [Google Scholar] [CrossRef]
- Grauer, D.; Cevidanes, L.S.; Proffit, W.R. Working with DICOM craniofacial images. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 460–470. [Google Scholar] [CrossRef]
- Swennen, G.R.; Mollemans, W.; De Clercq, C.; Abeloos, J.; Lamoral, P.; Lippens, F.; Neyt, N.; Casselman, J.; Schutyser, F. A cone-beam computed tomography triple scan procedure to obtain a three-dimensional augmented virtual skull model appropriate for orthognathic surgery planning. J. Craniofac. Surg. 2009, 20, 297–307. [Google Scholar] [CrossRef]
- Reyneke, J.P.; Tsakiris, P.; Kienle, F. A simple classification for surgical treatment planning of maxillomandibular asymmetry. Br. J. Oral Maxillofac. Surg. 1997, 35, 349–351. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jeon, J.; Rhee, J.T.; Hong, J. Change of lip cant after bimaxillary orthognathic surgery. J. Oral. Maxillofac. Surg. 2010, 68, 1106–1111. [Google Scholar] [CrossRef]
- Terajima, M.; Nakasima, A.; Aoki, Y.; Goto, T.K.; Tokumori, K.; Mori, N.; Hoshino, Y. A 3-dimensional method for analyzing the morphology of patients with maxillofacial deformities. Am. J. Orthod. Dentofacial. Orthop. 2009, 136, 857–867. [Google Scholar] [CrossRef]
- Ras, F.; Habets, L.L.; van Ginkel, F.C.; Prahl-Andersen, B. Method for quantifying facial asymmetry in three dimensions using stereophotogrammetry. Angle Orthod. 1995, 65, 233–239. [Google Scholar]

| Total, n = 52 | Skeletal Class II, n = 26 | Skeletal Class III, n = 26 | |
|---|---|---|---|
| Age (year, mean ± SD) | 24.40 ± 3.79 | 24.43 ± 4.30 | 24.37 ± 3.29 |
| Menton deviation (mm, mean ± SD) | 3.76 ± 0.99 | 3.95 ± 1.09 | 3.58 ± 0.87 |
| Side of menton deviation | |||
| Right | 28 | 18 | 10 |
| Left | 24 | 8 | 16 |
| Gender | |||
| Female | 27 | 16 | 11 |
| Menton deviation to the right | 18 | 13 | 5 |
| Menton deviation to the left | 9 | 3 | 6 |
| Male | 25 | 10 | 15 |
| Menton deviation to the right | 10 | 5 | 5 |
| Menton deviation to the left | 15 | 5 | 10 |
| Bilateral Landmarks | Same Side | Other Side | p-Value |
|---|---|---|---|
| Condylar head | 73.1% | 26.9% | 0.001 * |
| Jugal process | 67.3% | 32.7% | 0.008 * |
| Occlusal plane | 86.5% | 13.5% | <0.001 * |
| Exocanthion | 80.8% | 19.2% | <0.001 * |
| Lip commissure | 75.0% | 25.0% | <0.001 * |
| Bilateral Landmarks | Skeletal Class II, n = 26 | Skeletal Class III, n = 26 | p-Value |
|---|---|---|---|
| Condylar head | 76.9% | 69.2% | 0.092 |
| Jugal process | 69.2% | 65.4% | 0.777 |
| Occlusal plane | 84.6% | 88.5% | 0.165 |
| Exocanthion | 88.5% | 73.1% | 0.262 |
| Lip commissure | 76.9% | 73.1% | 0.402 |
| Vertical Difference in Bilateral Landmark (Millimeter) | Skeletal Class II | Skeletal Class III | p-Value between Class II and III |
|---|---|---|---|
| Hard tissue landmarks | |||
| Condylar head (Cor-Col) | 0.91 ± 2.00 | 1.45 ± 1.57 | 0.510 |
| Jugal process (Jpr-Jpl) | 0.40 ± 2.98 | 1.08 ± 1.96 | 0.707 |
| Occlusal plane (Opr-Opl) | 0.98 ± 1.08 | 1.34 ± 0.69 | 0.472 |
| Soft tissue landmarks | |||
| Exocanthion (Exo’r-Exo’l) | 1.20 ± 1.58 | 1.34 ± 1.62 | 0.706 |
| Lip commissure (Lc’r-Lc’l) | 0.94 ± 1.33 | 1.32 ± 1.08 | 0.487 |
| Menton Deviation | Condylar Difference | Jugal process Difference | Occlusal Difference | Exocanthion Difference | Commissure Difference | |
|---|---|---|---|---|---|---|
| Menton deviation | 1 | |||||
| Condylar difference | −0.031 | 1 | ||||
| Jugal process difference | −0.493 ** | 0.223 | 1 | |||
| Occlusal difference | 0.337 * | 0.161 | −0.069 | 1 | ||
| Exocanthion difference | 0.176 | 0.179 | 0.096 | 0.064 | 1 | |
| Commissure difference | 0.056 | 0.316 * | 0.193 | −0.017 | 0.182 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jearanai, T.; Samruajbenjakun, B.; Chanmanee, P. Relationship between Bilateral Landmarks of Facial Asymmetry in Skeletal Class II and Class III in Vertical Dimension: 3D Facial Scan and Cone-Beam Computed Tomography. Diagnostics 2024, 14, 590. https://doi.org/10.3390/diagnostics14060590
Jearanai T, Samruajbenjakun B, Chanmanee P. Relationship between Bilateral Landmarks of Facial Asymmetry in Skeletal Class II and Class III in Vertical Dimension: 3D Facial Scan and Cone-Beam Computed Tomography. Diagnostics. 2024; 14(6):590. https://doi.org/10.3390/diagnostics14060590
Chicago/Turabian StyleJearanai, Tanapat, Bancha Samruajbenjakun, and Pannapat Chanmanee. 2024. "Relationship between Bilateral Landmarks of Facial Asymmetry in Skeletal Class II and Class III in Vertical Dimension: 3D Facial Scan and Cone-Beam Computed Tomography" Diagnostics 14, no. 6: 590. https://doi.org/10.3390/diagnostics14060590
APA StyleJearanai, T., Samruajbenjakun, B., & Chanmanee, P. (2024). Relationship between Bilateral Landmarks of Facial Asymmetry in Skeletal Class II and Class III in Vertical Dimension: 3D Facial Scan and Cone-Beam Computed Tomography. Diagnostics, 14(6), 590. https://doi.org/10.3390/diagnostics14060590






