Ultrasound-Guided Interventions in the Biliary System
Abstract
1. Introduction
2. Biliary Drainage
2.1. Data Background
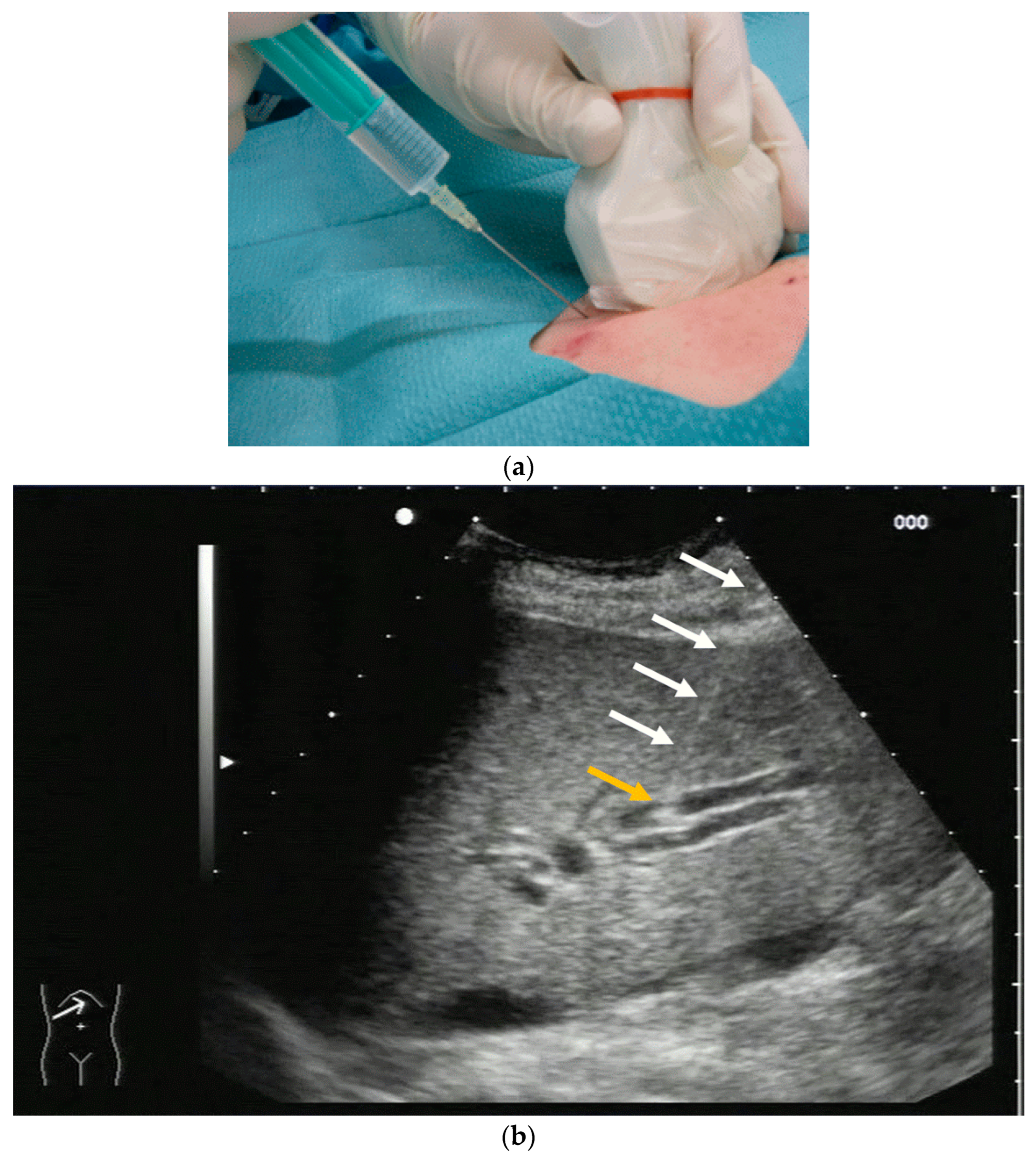
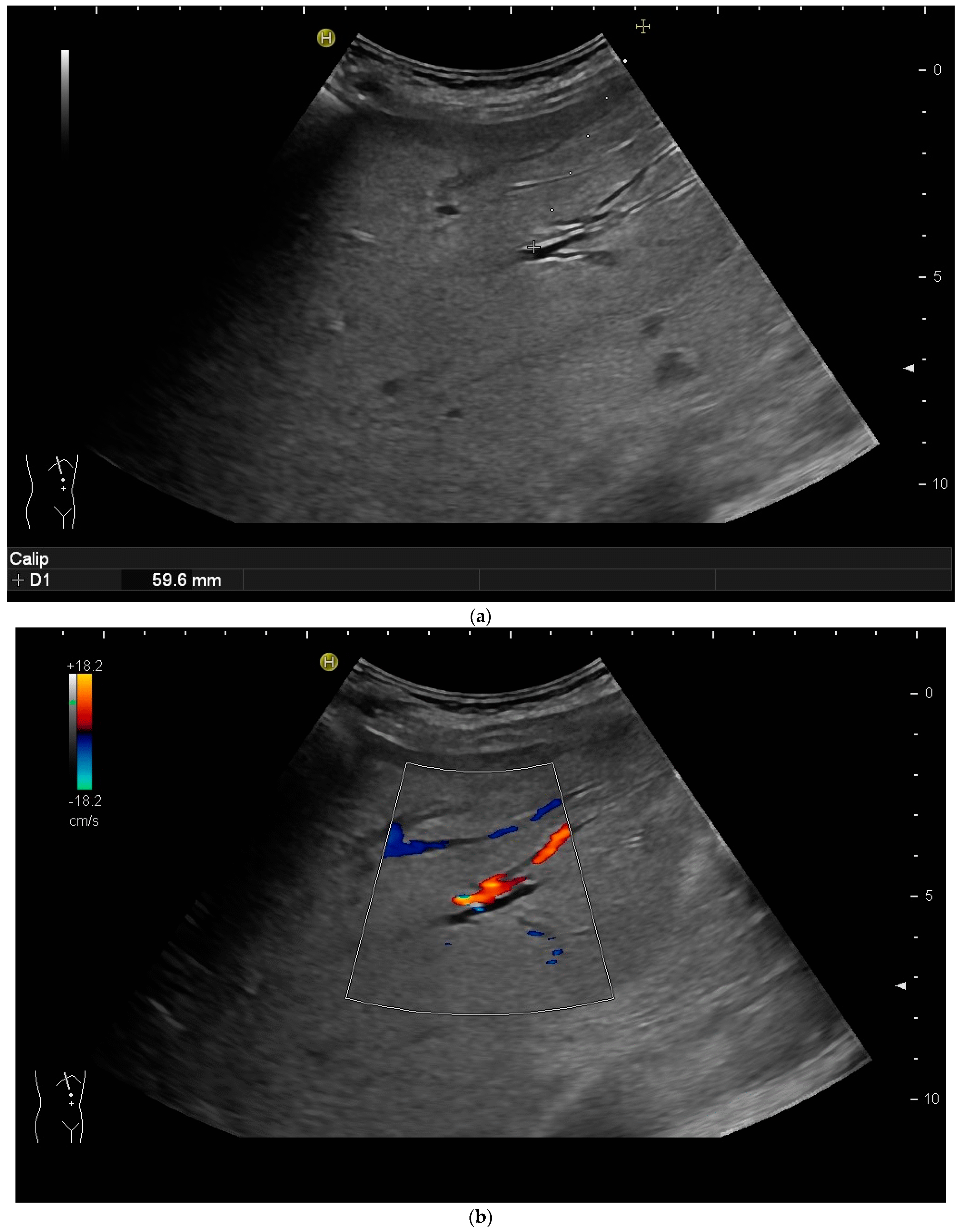
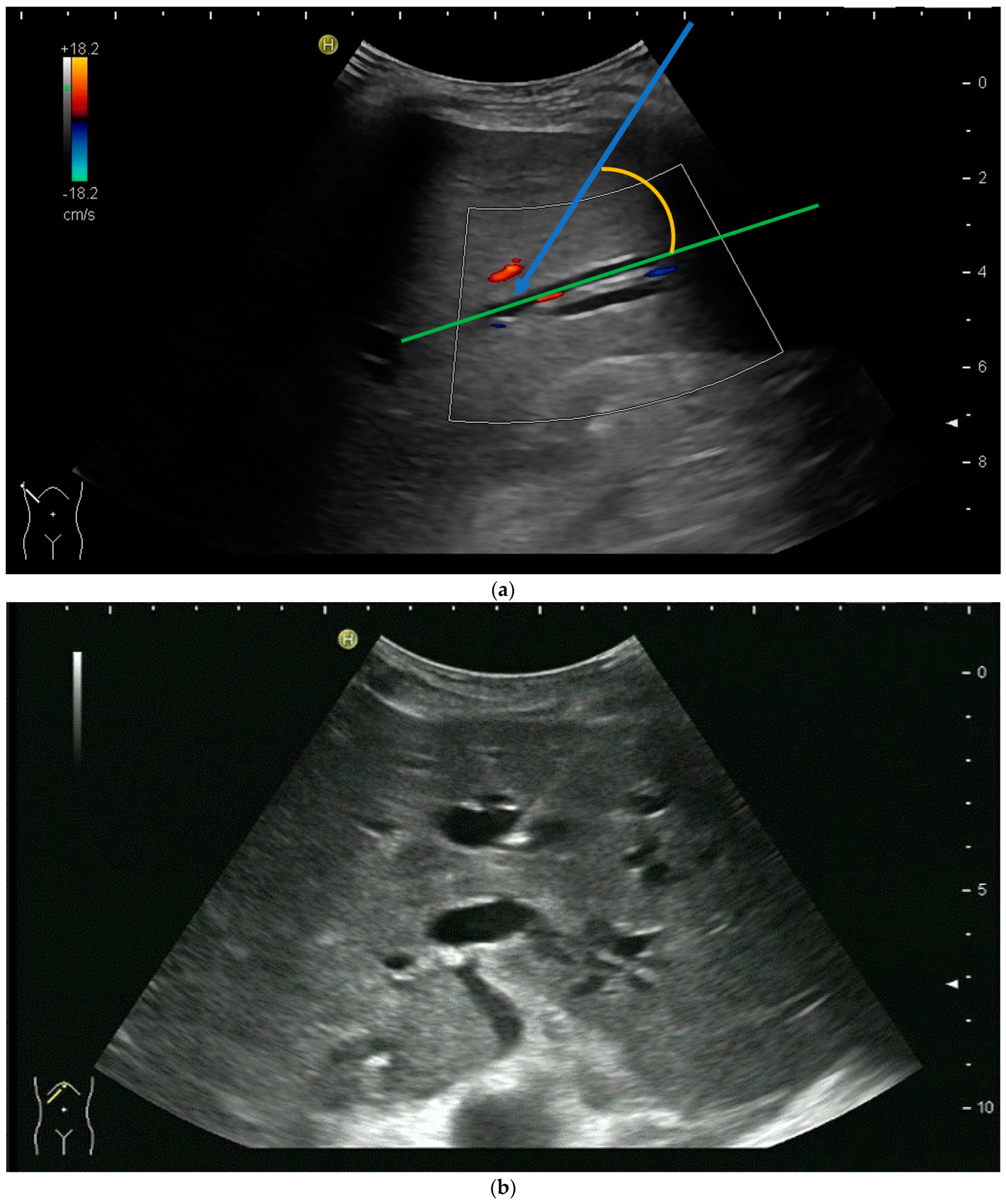
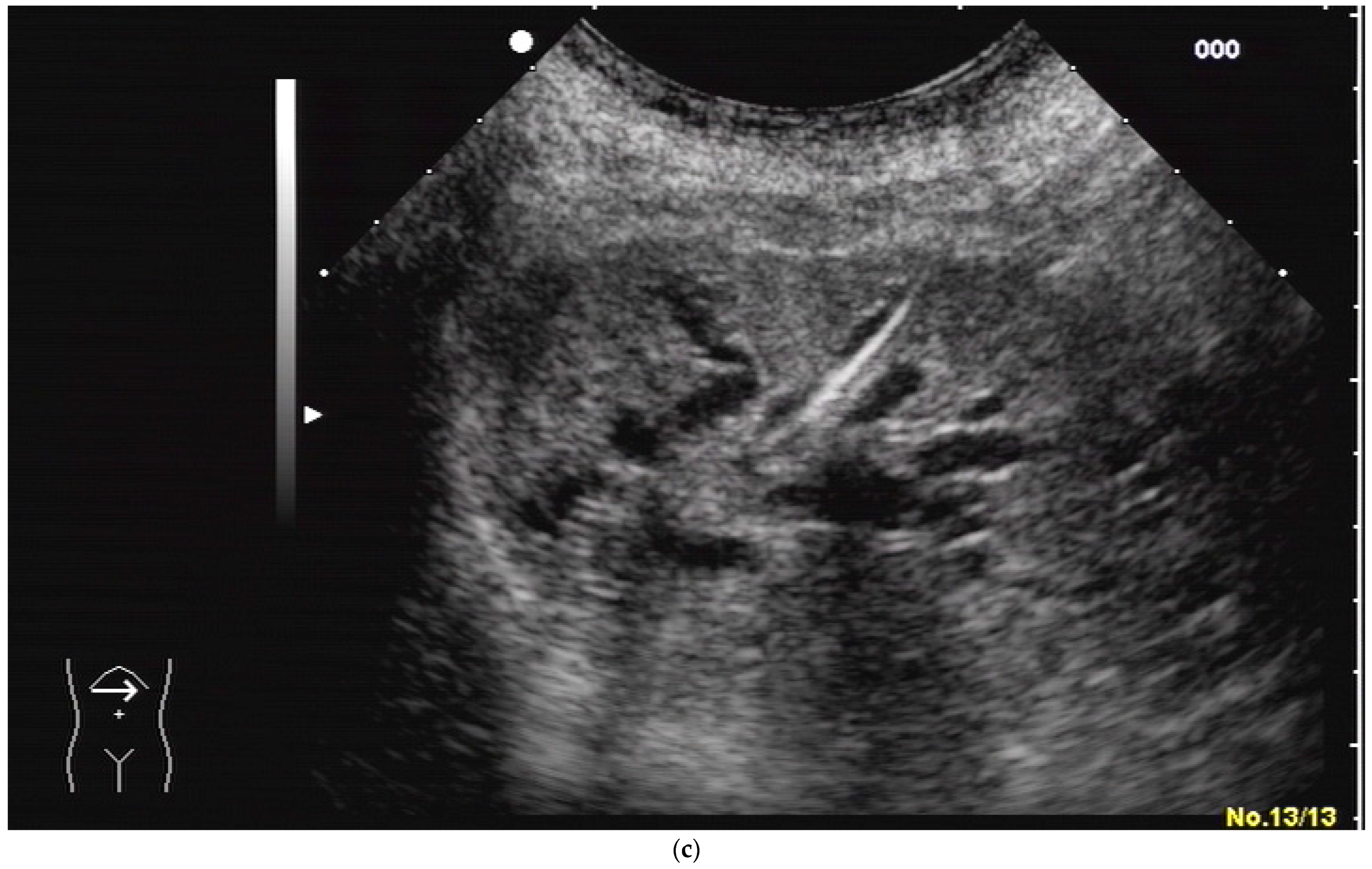
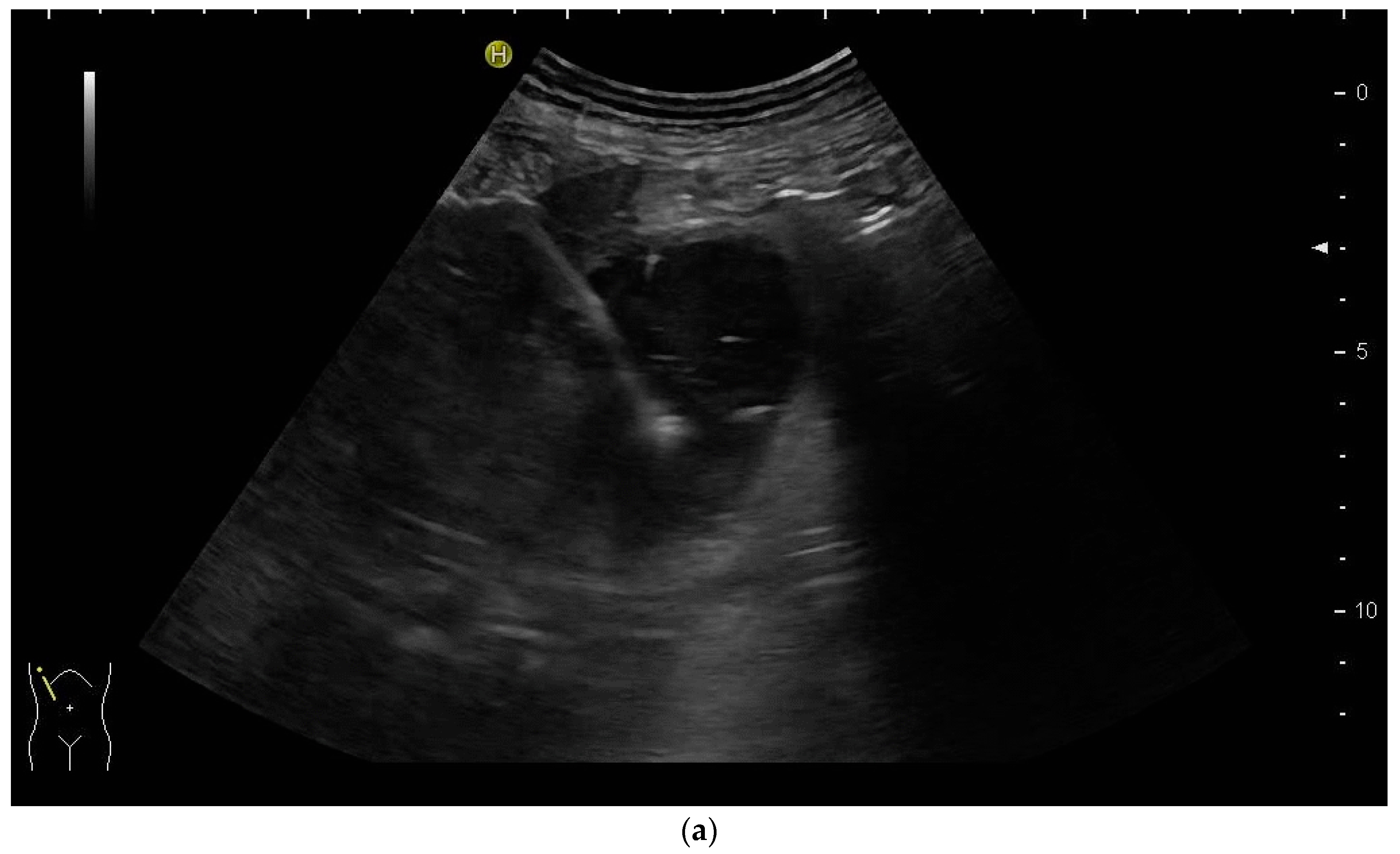
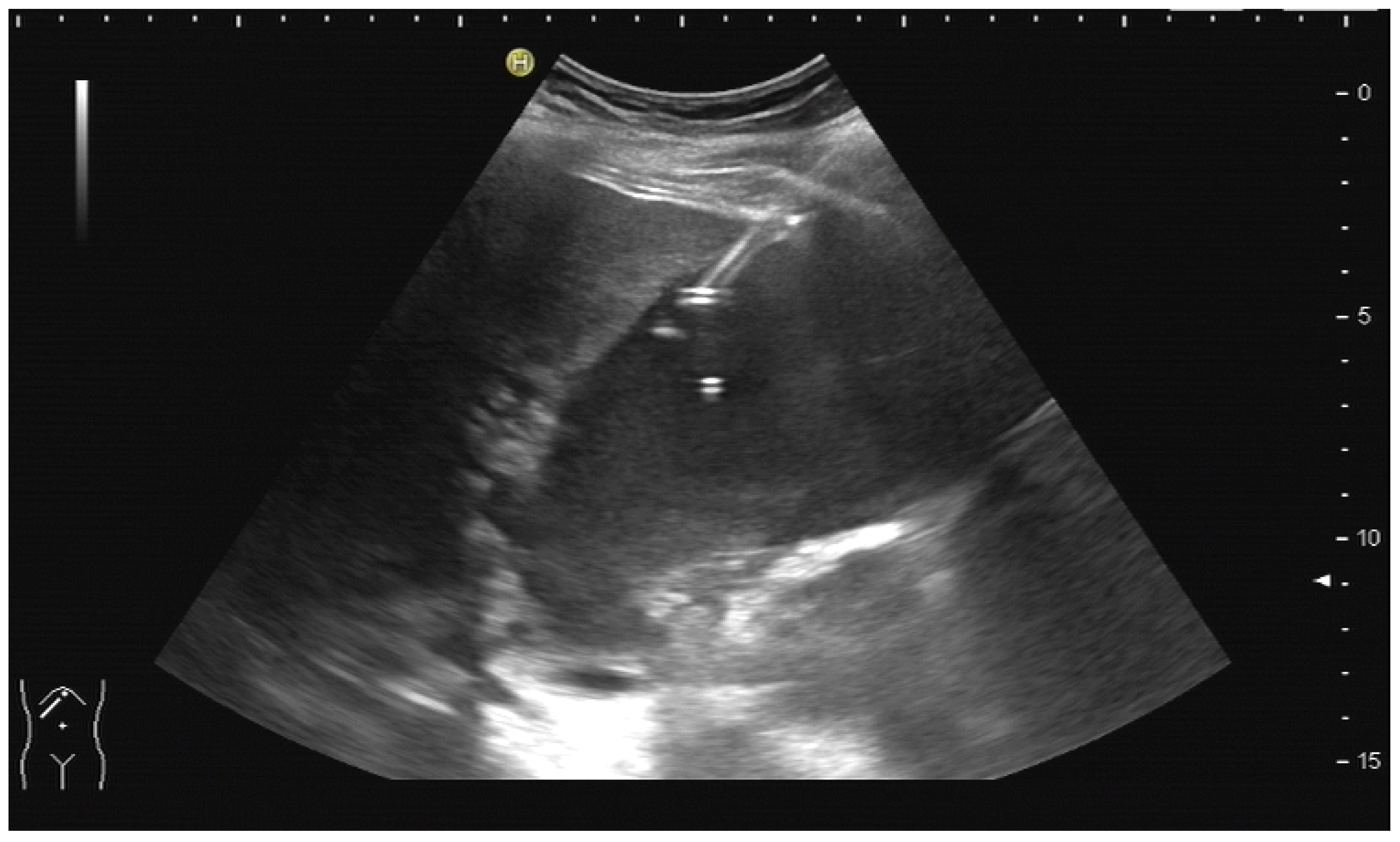
2.2. How to Perform US-Guided PTBD
3. Percutaneous Gall Bladder Drainage (PGBD or Percutaneous Cholecystostomy)
3.1. Data Background
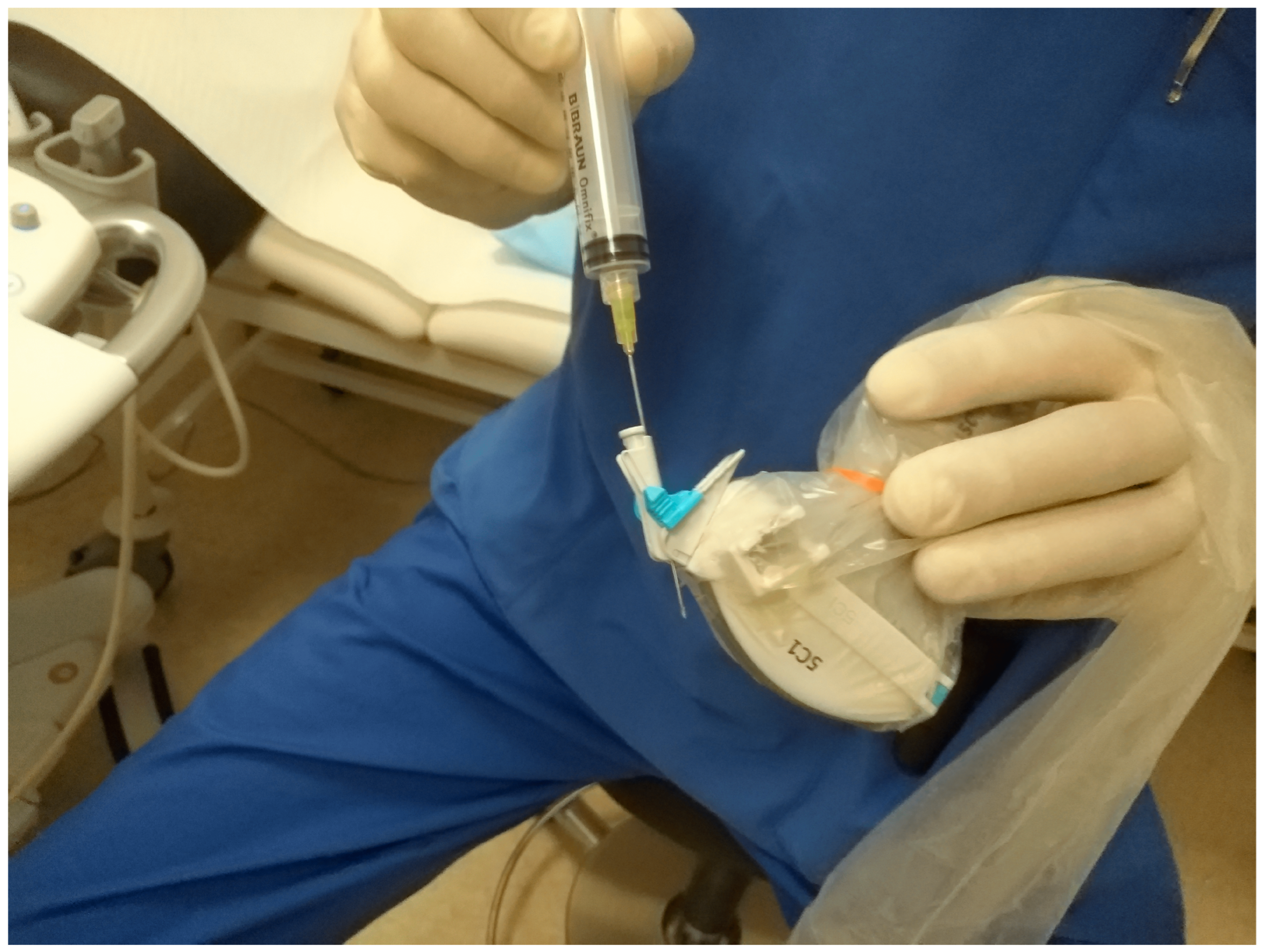
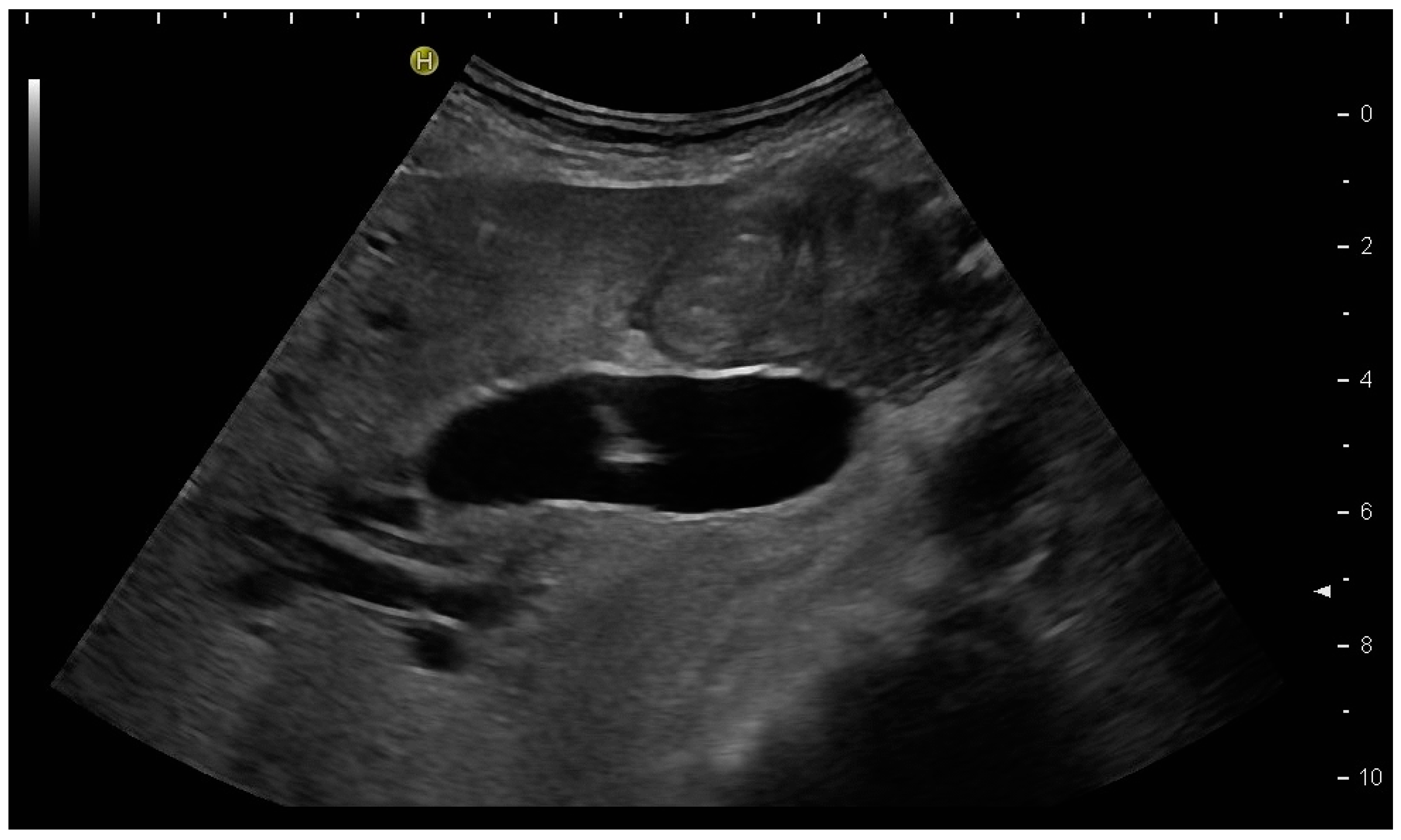
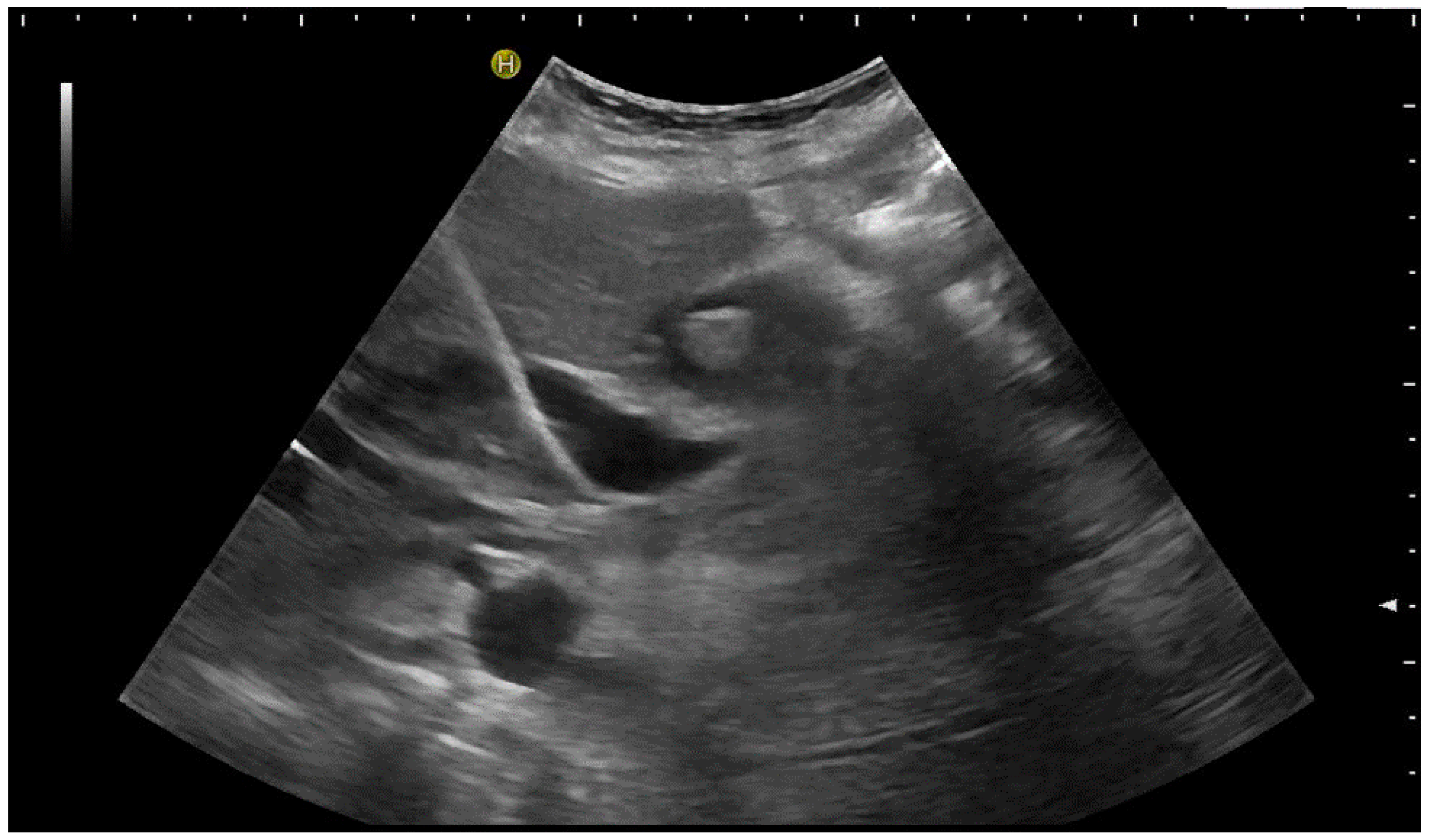
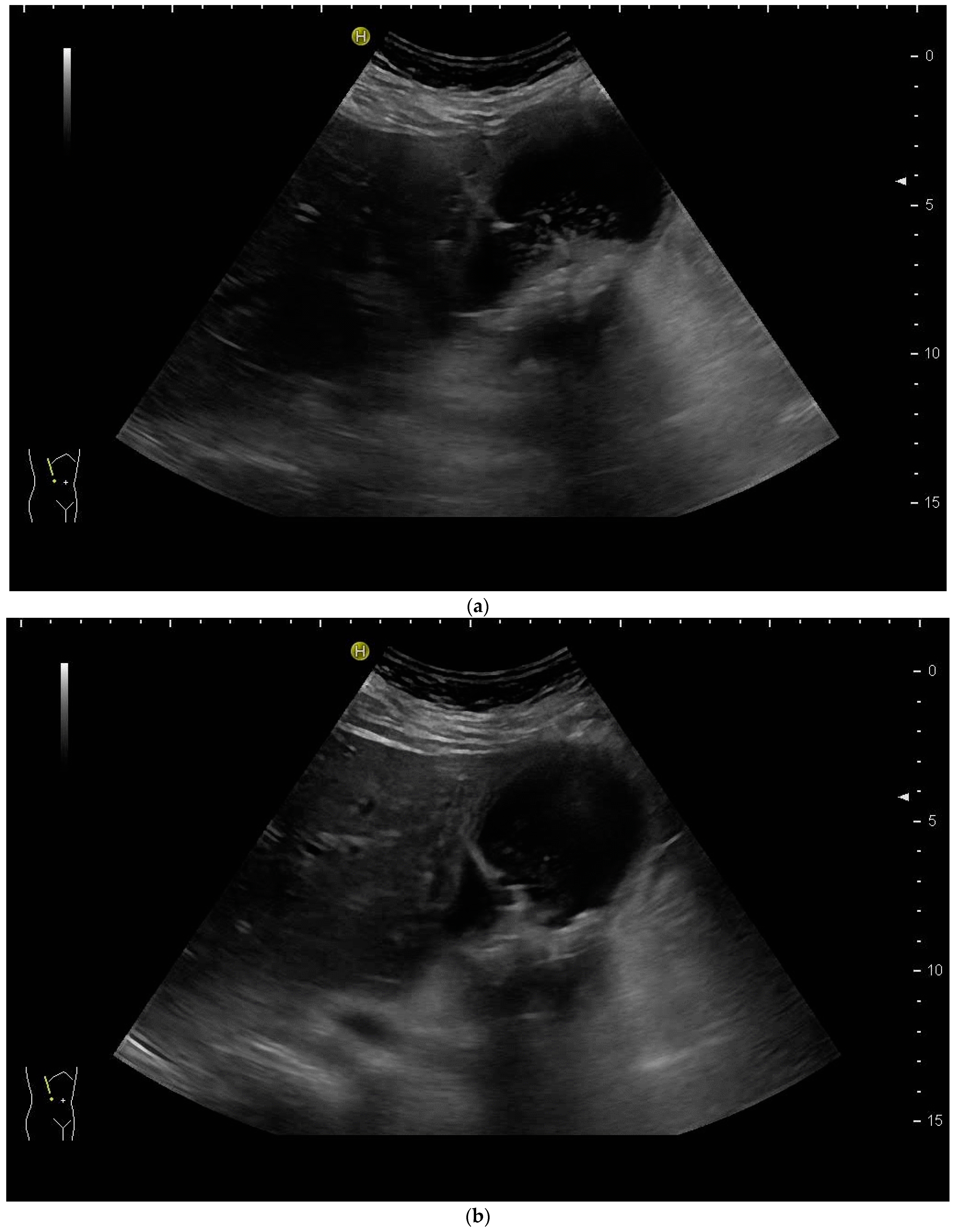
3.2. How to Perform US-Guided PGBD
3.3. Duration of Drainage
4. Percutaneous Gallbladder Aspiration (PGBA)
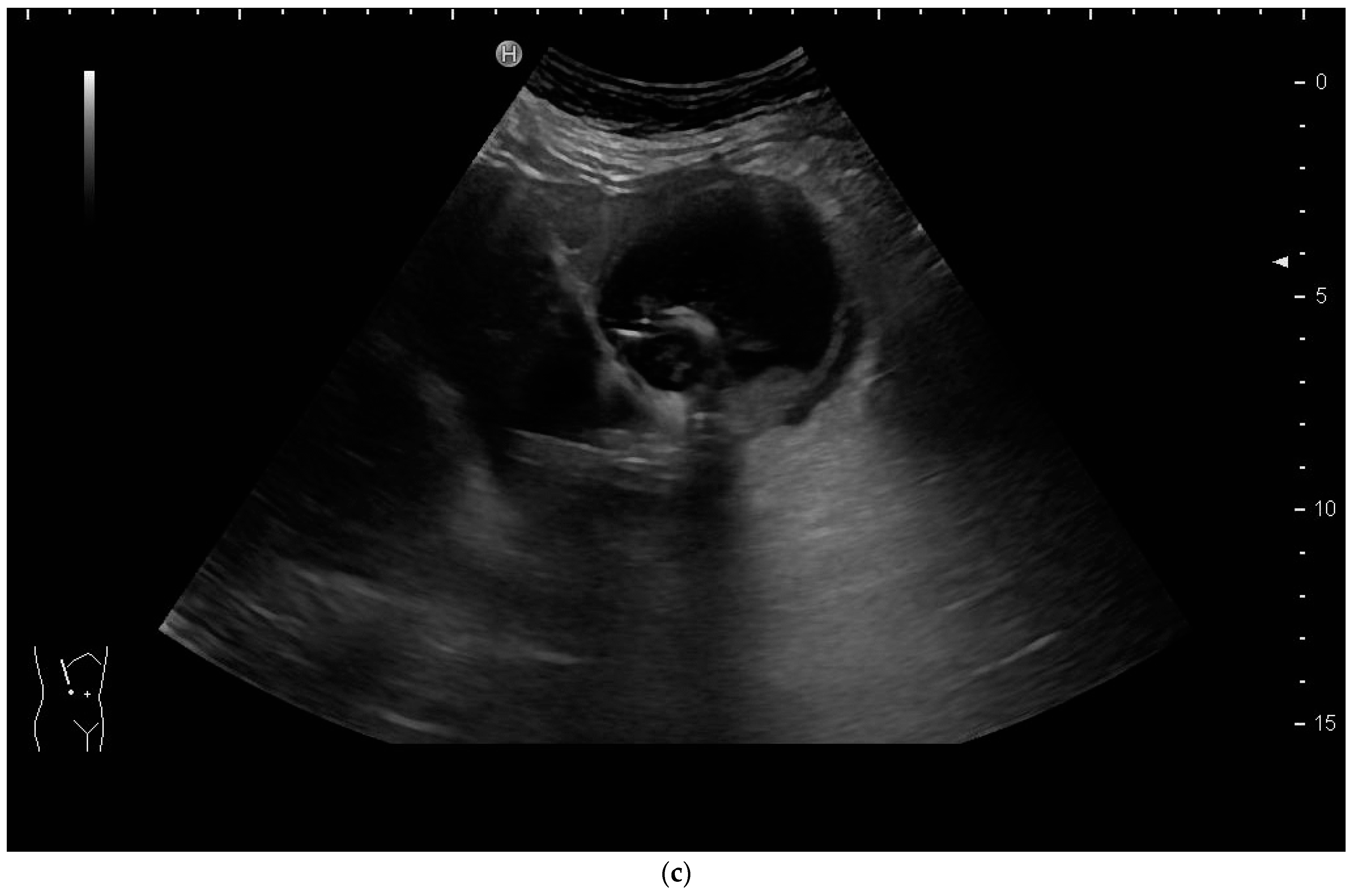
How to Perform US-Guided PGBA
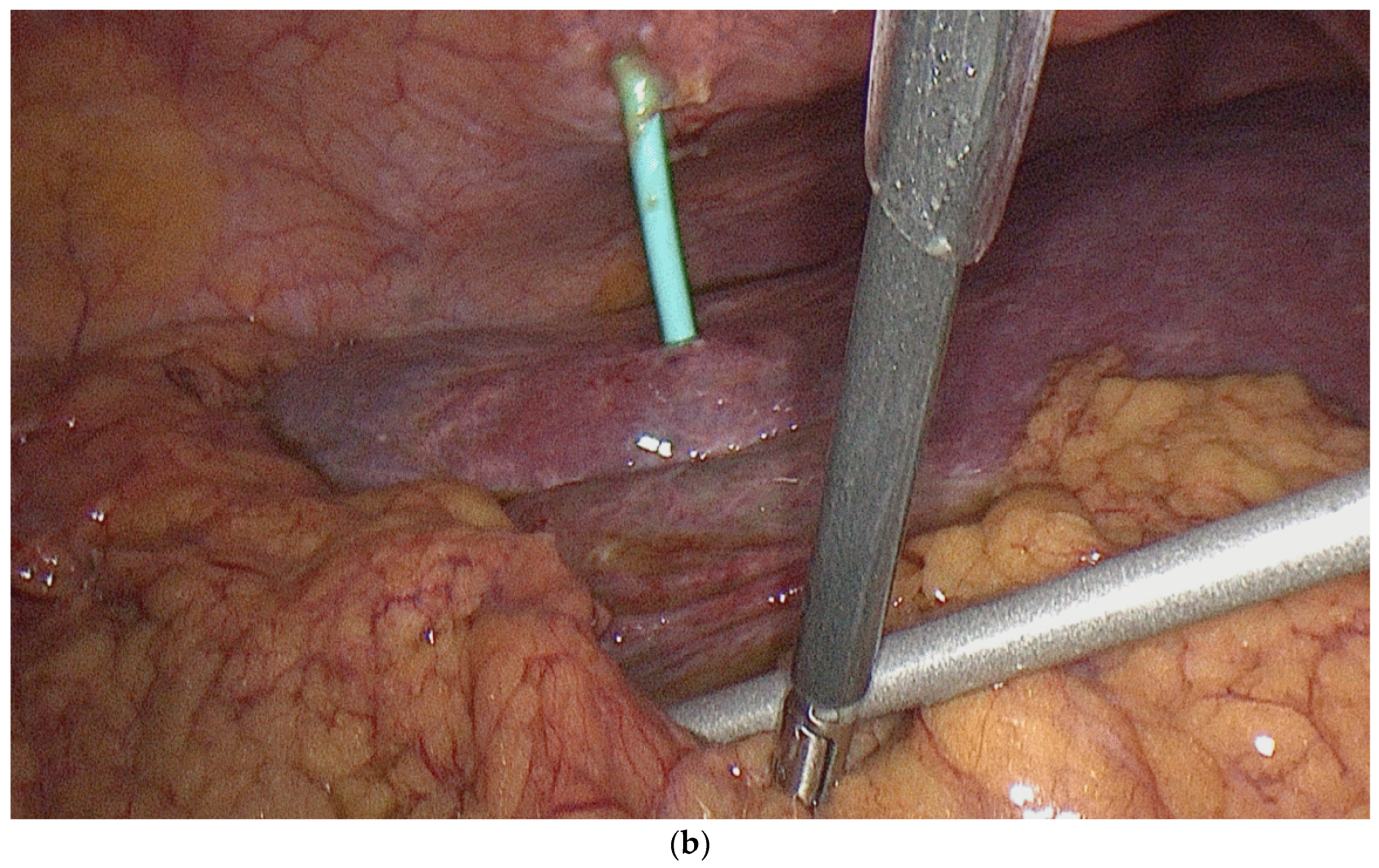
5. Percutaneous Cholecystoenteric Anastomosis
6. Discussion
6.1. Percutaneous Biliary Drainage
6.2. Percutaneous Gallbladder Drainage
6.3. Percutaneous Gallbladder Aspiration
6.4. Percutaneous Cholecystoenteric Anastomosis
7. Conclusions
8. Future Directions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Devane, A.M.; Annam, A.; Brody, L.; Gunn, A.J.; Himes, E.A.; Patel, S.; Tam, A.L.; Dariushnia, S.R. Society of Interventional Radiology Quality Improvement Standards for Percutaneous Cholecystostomy and Percutaneous Transhepatic Biliary Interventions. J. Vasc. Interv. Radiol. 2020, 31, 1849–1856. [Google Scholar] [CrossRef]
- Moll, C.F.; Moura, D.T.H.D.; Ribeiro, I.B.; Proença, I.M.; Monte Junior, E.S.D.; Sánchez-Luna, S.A.; Merchán, M.F.S.; Intriago, J.M.V.; Bernardo, W.M.; Moura, E.G.H.D. Endoscopic Biliary Darinage (EBD) versus Percutaneous Transhepatic Biliary Drainage (PTBD) for biliary drainage in patients with Perihilar Cholangiocarcinoma (PCCA): A systematic review and meta-analysis. Clinics 2023, 78, 100163. [Google Scholar] [CrossRef]
- Cazejust, J.; Pommier, R.; Tavolaro, S. Comment je fais un drainage biliaire percutané? J. D’imagerie Diagn. Interv. 2019, 2, 88–93. [Google Scholar] [CrossRef]
- Nagino, M.; Takada, T.; Kawarada, Y.; Nimura, Y.; Yamashita, Y.; Tsuyuguchi, T.; Wada, K.; Mayumi, T.; Yoshida, M.; Miura, F.; et al. Methods and timing of biliary drainage for acute cholangitis: Tokyo Guidelines. J. Hepato-Biliary-Pancreat. Surg. 2007, 14, 68–77. [Google Scholar] [CrossRef]
- Duan, F.; Cui, L.; Bai, Y.; Li, X.; Yan, J.; Liu, X. Comparison of efficacy and complications of endoscopic and percutaneous biliary drainage in malignant obstructive jaundice: A systematic review and meta-analysis. Cancer Imaging 2017, 17, 27. [Google Scholar] [CrossRef]
- Liu, J.-G.; Wu, J.; Wang, J.; Shu, G.-M.; Wang, Y.-J.; Lou, C.; Zhang, J.; Du, Z. Endoscopic Biliary Drainage Versus Percutaneous Transhepatic Biliary Drainage in Patients with Resectable Hilar Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Dumonceau, J.M.; Tringali, A.; Papanikolaou, I.S.; Blero, D.; Mangiavillano, B.; Schmidt, A.; Vanbiervliet, G.; Costamagna, G.; Devière, J.; García-Cano, J.; et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline—Updated October 2017. Endoscopy 2018, 50, 910–930. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.; Dhindsa, B.; Mashiana, H.; Dhaliwal, A.; Mohan, B.; Jayaraj, M.; Sayles, H.; Singh, S.; Ohning, G.; Bhat, I. EUS-guided biliary drainage: A systematic review and meta-analysis. Endosc. Ultrasound 2020, 9, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Sharaiha, R.Z.; Khan, M.A.; Kamal, F.; Tyberg, A.; Tombazzi, C.R.; Ali, B.; Kahaleh, M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Elmunzer, B.J.; Maranki, J.L.; Gómez, V.; Tavakkoli, A.; Sauer, B.G.; Limketkai, B.N.; Brennan, E.A.; Attridge, E.M.; Brigham, T.J.; Wang, A.Y. ACG Clinical Guideline: Diagnosis and Management of Biliary Strictures. Am. J. Gastroenterol. 2023, 118, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Yee, A.C.; Ho, C.S. Percutaneous transhepatic biliary drainage: A review. Crit. Rev. Diagn. Imaging 1990, 30, 247–279. [Google Scholar]
- Weber, A.; Rosca, B.; Neu, B.; Rösch, T.; Frimberger, E.; Born, P.; Schmid, R.; Prinz, C. Long-term follow-up of percutaneous transhepatic biliary drainage (PTBD) in patients with benign bilioenterostomy stricture. Endoscopy 2009, 41, 323–328. [Google Scholar] [CrossRef]
- Fairchild, A.H.; Hohenwalter, E.J.; Gipson, M.G.; Al-Refaie, W.B.; Braun, A.R.; Cash, B.D.; Kim, C.Y.; Pinchot, J.W.; Scheidt, M.J.; Schramm, K.; et al. ACR Appropriateness Criteria® Radiologic Management of Biliary Obstruction. J. Am. Coll. Radiol. 2019, 16, S196–S213. [Google Scholar] [CrossRef][Green Version]
- Hafezi-Nejad, N.; Liddell, R.P. Percutaneous Biliary Interventions: Clinical Indications, Comparative Effectiveness, Technical Considerations, Complications, and Outcomes. Gastrointest. Endosc. Clin. 2022, 32, 493–505. [Google Scholar] [CrossRef]
- Morettin, L.B.; Dodd, G.D. Percutaneous transhepatic cholangiography. Am. J. Dig. Dis. 1972, 17, 831–845. [Google Scholar] [CrossRef]
- Wimmer, B.; Hauenstein, K.H.; Kauffmann, G.; Friedburg, H. Sonographische perkutane Gallengangsdrainage. RoFo 1981, 135, 466–470. [Google Scholar] [CrossRef]
- Makuuchi, M.; Bandai, Y.; Ito, T.; Watanabe, G.; Wada, T.; Abe, H.; Muroi, T. Ultrasonically guided percutaneous transhepatic bile drainage: A single-step procedure without cholangiography. Radiology 1980, 136, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Makuuchi, M.; Yamazaki, S.; Hasegawa, H.; Bandai, Y.; Ito, T.; Watanabe, G. Ultrasonically guided cholangiography and bile drainage. Ultrasound Med. Biol. 1984, 10, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Kochhar, R.; Mehta, S.K.; Suri, S. A modified technique of ultrasonically guided percutaneous transhepatic biliary drainage. Surg. Endosc. 1989, 3, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, G.C.; Kim, J.Y.; Baik, S.K.; Lee, H.J.; Kim, H.J.; Ryeom, H.K. Ultrasound and fluoroscopy guided percutaneous transhepatic biliary drainage in patients with nondilated bile ducts. Abdom. Imaging 2008, 33, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Ilikan, G.; AKMANGİT, İ. Percutaneous Transhepatic Cholangiography, Percutaneous Biliary Drainage and Metallic Endoprotesis Applications in Malign Biliary Obstructions. J. Contemp. Med. 2020, 11, 97–103. [Google Scholar] [CrossRef]
- Schmitz, D.; Grosse, A.; Hallscheidt, P.; Roseneck, A.; Niemeyer, J.; Rudi, J. Color Doppler ultrasound-guided PTBD with and without metal stent implantation by endoscopic control: Prospective success and early adverse event rates. Z. Gastroenterol. 2015, 53, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Giurazza, F.; Italian College of Interventional Radiology (ICIR) Rising Stars Group; Corvino, F.; Contegiacomo, A.; Marra, P.; Lucarelli, N.M.; Calandri, M.; Silvestre, M.; Corvino, A.; Lucatelli, P.; et al. Safety and effectiveness of ultrasound-guided percutaneous transhepatic biliary drainage: A multicenter experience. J. Ultrasound 2019, 22, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Prasad, U.; Kumar, D.; Kumar, A.; Singh, R.K.; Suman, S.K.; Kumari, P. Ultrasound-Guided Percutaneous Transhepatic Biliary Drainage Outcome at Tertiary Care Centre of North India. Int. J. Sci. Study 2021, 9, 93–99. [Google Scholar]
- Chandel, K.; Singh, J.; Tripathy, T.P.; Patel, R. Is Ultrasound-guided Bedside Percutaneous Transhepatic Biliary Drainage Safe and Feasible in Critically Ill Patients with Severe Cholangitis? A Preliminary Single-center Experience. Indian J. Crit. Care Med. 2023, 27, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ignee, A.; Cui, X.; Schuessler, G.; Dietrich, C.F. Percutaneous transhepatic cholangiography and drainage using extravascular contrast enhanced ultrasound. Z. Gastroenterol. 2015, 53, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Blank, W.; Leitlein, J.; Kubicka, S.; Heinzmann, A. Endocavitary contrast-enhanced ultrasound: A technique whose time has come? J. Clin. Ultrasound 2015, 43, 71–80. [Google Scholar] [CrossRef]
- Xu, E.-J.; Zheng, R.-Q.; Su, Z.-Z.; Li, K.; Ren, J.; Guo, H.-Y. Intra-biliary contrast-enhanced ultrasound for evaluating biliary obstruction during percutaneous transhepatic biliary drainage: A preliminary study. Eur. J. Radiol. 2012, 81, 3846–3850. [Google Scholar] [CrossRef]
- Miyazaki, M.; Shibuya, K.; Tokue, H.; Tsushima, Y. Percutaneous transhepatic biliary drainage assisted by real-time virtual sonography: A retrospective study. BMC Gastroenterol. 2013, 13, 127. [Google Scholar] [CrossRef]
- Wagner, A.; Mayr, C.; Kiesslich, T.; Berr, F.; Friesenbichler, P.; Wolkersdörfer, G.W. Reduced complication rates of percutaneous transhepatic biliary drainage with ultrasound guidance. J. Clin. Ultrasound 2017, 45, 400–407. [Google Scholar] [CrossRef]
- Park, S.E.; Nam, I.C.; Baek, H.J.; Ryu, K.H.; Lim, S.G.; Won, J.H.; Kim, D.R. Effectiveness of ultrasound-guided percutaneous transhepatic biliary drainage to reduce radiation exposure: A single-center experience. PLoS ONE 2023, 17, e0277272. [Google Scholar] [CrossRef]
- Kozlov, A.V.; A Polikarpov, A.; Oleshchuk, N.V.; Tarazov, P.G. Comparative assessment of percutaneous transhepatic cholangiodrainage under roentgenoscopy and ultrasound guidance. Vestn. Rentgenol. Radiol. 2002, 4, 30–33. [Google Scholar]
- Nennstiel, S.; Treiber, M.; Faber, A.; Haller, B.; von Delius, S.; Schmid, R.M.; Neu, B. Comparison of Ultrasound and Fluoroscopically Guided Percutaneous Transhepatic Biliary Drainage. Dig. Dis. 2018, 37, 77–86. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Yen, K.-C.; Liang, P.-C.; Wu, C.-H. Procedure-related risk factors for bleeding after percutaneous transhepatic biliary drainage: A systematic review and meta-analysis. J. Formos. Med. Assoc. 2022, 121, 1680–1688. [Google Scholar] [CrossRef]
- Müller, T.; Martiny, H.; Merz, E.; Döffert, J.; Wüstner, M.; Lessel, W.; Heynemann, H.; Enzmann, T.; Dudwiesus, H.; Nuernberg, D.; et al. DEGUM Recommendations on Infection Prevention in Ultrasound and Endoscopic Ultrasound. Ultraschall Med. 2018, 39, 284–303. [Google Scholar] [CrossRef] [PubMed]
- Nyhsen, C.M.; Humphreys, H.; Koerner, R.J.; Grenier, N.; Brady, A.; Sidhu, P.; Nicolau, C.; Mostbeck, G.; D’onofrio, M.; Gangi, A.; et al. Infection prevention and control in ultrasound—Best practice recommendations from the European Society of Radiology Ultrasound Working Group. Insights Imaging 2017, 8, 523–535. [Google Scholar] [CrossRef]
- Hayashi, N.; Sakai, T.; Kitagawa, M.; Kimoto, T.; Inagaki, R.; Ishii, Y. US-guided left-sided biliary drainage: Nine-year experience. Radiology 1997, 204, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Maralakunte, M.; Rathee, S.; Samanta, J.; Sharma, V.; Mandavdhare, H.; Sinha, S.K.; Dutta, U.; Kochhar, R. Percutaneous transhepatic biliary drainage in patients at higher risk for adverse events: Experience from a tertiary care referral center. Abdom. Imaging 2020, 45, 2547–2553. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cha, S.J. US-guided Percutaneous Transhepatic Biliary Drainage: Comparative Study of Right-sided and Left-sided Approach. J. Korean Radiol. Soc. 2002, 46, 115–118. [Google Scholar] [CrossRef]
- Filipović, A.N.; Mašulović, D.; Zakošek, M.; Filipović, T.; Galun, D. Total Fluoroscopy Time Reduction During Ultrasound- and Fluoroscopy-Guided Percutaneous Transhepatic Biliary Drainage Procedure: Importance of Adjusting the Puncture Angle. J. Pharmacol. Exp. Ther. 2021, 27, e933889-1–e933889-7. [Google Scholar] [CrossRef]
- Zorger, N.; Feuerbach, S. Technik der perkutanen transhepatischen Cholangio-Drainage (PTCD). J. Gastroenterol. Hepatol. Erkrank. 2010, 8, 21–26. [Google Scholar]
- Covey, A.M.; Brown, K.T. Percutaneous Transhepatic Biliary Drainage. Tech. Vasc. Interv. Radiol. 2008, 11, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Götzberger, M.; Nüssler, N.; Braden, B.; Dietrich, C.F.; Müller, T. Acute Cholecystitis in high-risk surgical patients: Sonographic and endoscopic treatment options. Z. Gastroenterol. 2021, 59, 983–990. [Google Scholar] [PubMed]
- Bozic, D.; Ardalic, Z.; Mestrovic, A.; Ivisic, J.B.; Alicic, D.; Zaja, I.; Ivanovic, T.; Bozic, I.; Puljiz, Z.; Bratanic, A. Assessment of Gallbladder Drainage Methods in the Treatment of Acute Cholecystitis: A Literature Review. Medicina 2024, 60, 5. [Google Scholar] [CrossRef]
- Mori, Y.; Itoi, T.; Baron, T.H.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Ukai, T.; Shikata, S.; Noguchi, Y.; Teoh, A.Y.B.; et al. Tokyo Guidelines 2018: Management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 87–95. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Lorentzen, T.; Appelbaum, L.; Buscarini, E.; Cantisani, V.; Correas, J.M.; Cui, X.W.; D’Onofrio, M.; Gilja, O.H.; Hocke, M.; et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part III—Abdominal Treatment Procedures (Long Version). Ultraschall Med—Eur. J. Ultrasound 2016, 37, E1–E32. [Google Scholar] [CrossRef]
- Pisano, M.; Allievi, N.; Gurusamy, K.; Borzellino, G.; Cimbanassi, S.; Boerna, D.; Coccolini, F.; Tufo, A.; Di Martino, M.; Leung, J.; et al. 2020 World Society of Emergency Surgery updated guidelines for the diagnosis and treatment of acute calculus cholecystitis. World J. Emerg. Surg. 2020, 15, 61. [Google Scholar] [CrossRef]
- Gutt, C.; Jenssen, C.; Barreiros, A.P.; Götze, T.O.; Stokes, C.S.; Jansen, P.L.; Neubrand, M.; Lammert, F. Updated S3-Guideline for Prophylaxis, Diagnosis and Treatment of Gallstones. German Society for Digestive and Metabolic Diseases (DGVS) and German Society for Surgery of the Alimentary Tract (DGAV)—AWMF Registry 021/008. Z. Gastroenterol. 2018, 56, 912–966. [Google Scholar]
- Coccolini, F.; Cucinotta, E.; Mingoli, A.; Zago, M.; Altieri, G.; Biloslavo, A.; Caronna, R.; Cengeli, I.; Cicuttin, E.; Cirocchi, R.; et al. Acute cholecystitis management in high-risk, critically ill, and unfit-for-surgery patients: The Italian Society of Emergency Surgery and Trauma (SICUT) guidelines. Updates Surg. 2023, 75, 1–13. [Google Scholar] [CrossRef]
- Coelho, J.C.U.; Costa MAR da Enne, M.; Torres, O.J.M.; Andraus, W.; Campos, A.C.L. Acute cholecystitis in high-risk patients. surgical, radiological, or endoscopic treatment? Brazilian college of digestive surgery position paper. ABCD Arq. Bras. Cir. Dig. 2023, 36, e1749. [Google Scholar] [CrossRef]
- Elyaderani, M.; Gabriele, O.F. Percutaneous Cholecystostomy and Cholangiography in Patients with Obstructive Jaundice. Radiology 1979, 130, 601–602. [Google Scholar] [CrossRef]
- Loozen, C.S.; van Santvoort, H.C.; van Duijvendijk, P.; Besselink, M.G.; Gouma, D.J.; Nieuwenhuijzen, G.A.; Kelder, J.C.; Donkervoort, S.C.; van Geloven, A.A.; Kruyt, P.M.; et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ 2018, 363, k3965. [Google Scholar] [CrossRef]
- Cirocchi, R.; Amato, L.; Ungania, S.; Buononato, M.; Tebala, G.D.; Cirillo, B.; Avenia, S.; Cozza, V.; Costa, G.; Davies, R.J.; et al. Management of Acute Cholecystitis in High-Risk Patients: Percutaneous Gallbladder Drainage as a Definitive Treatment vs. Emergency Cholecystectomy—Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 4903. [Google Scholar] [CrossRef]
- Stanek, A.; Dohan, A.; Barkun, J.; Barkun, A.; Reinhold, C.; Valenti, D.; Cassinotto, C.; Gallix, B. Percutaneous cholecystostomy: A simple bridge to surgery or an alternative option for the management of acute cholecystitis? Am. J. Surg. 2018, 216, 595–603. [Google Scholar] [CrossRef]
- Zarour, S.; Imam, A.; Kouniavsky, G.; Lin, G.; Zbar, A.; Mavor, E. Percutaneous cholecystostomy in the management of high-risk patients presenting with acute cholecystitis: Timing and outcome at a single institution. Am. J. Surg. 2017, 214, 456–461. [Google Scholar] [CrossRef]
- Fleming, C.A.; Ismail, M.; Kavanagh, R.G.; Heneghan, H.M.; Prichard, R.S.; Geoghegan, J.; Brophy, D.P.; McDermott, E.W. Clinical and Survival Outcomes Using Percutaneous Cholecystostomy Tube Alone or Subsequent Interval Cholecystectomy to Treat Acute Cholecystitis. J. Gastrointest. Surg. 2020, 24, 627–632. [Google Scholar] [CrossRef]
- Jeon, H.W.; Jung, K.U.; Lee, M.Y.; Hong, H.P.; Shin, J.H.; Lee, S.R. Surgical outcomes of percutaneous transhepatic gallbladder drainage in acute cholecystitis grade II patients according to time of surgery. Asian J. Surg. 2021, 44, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.; Christensen, S.D.; Kirkegård, J.; Larsen, L.P.; Knudsen, A.R.; Mortensen, F.V. Percutaneous cholecystostomy is an effective treatment option for acute calculous cholecystitis: A 10-year experience. HPB 2015, 17, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Tyberg, A.; Duarte-Chavez, R.; Shahid, H.M.; Sarkar, A.; Simon, A.; Shah-Khan, S.M.; Gaidhane, M.; Mohammad, T.F.; Nosher, J.; Wise, S.S.; et al. Endoscopic Ultrasound-Guided Gallbladder Drainage Versus Percutaneous Drainage in Patients With Acute Cholecystitis Undergoing Elective Cholecystectomy. Clin. Transl. Gastroenterol. 2023, 14, e00593. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Suzuki, K.; Yahagi, M.; Murata, T.; Sako, H.; Ishii, Y. The Efficacy of PTGBD for Acute Cholecystitis Based on the Tokyo Guidelines 2018. World J. Surg. 2019, 43, 2789–2796. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-L.; Sung, C.-M.; Fu, C.-Y.; Liao, C.-H.; Wang, S.-Y.; Hsu, J.-T.; Yeh, T.-S.; Yeh, C.-N.; Jan, Y.-Y. Management of Patients With Acute Cholecystitis After Percutaneous Cholecystostomy: From the Acute Stage to Definitive Surgical Treatment. Front. Surg. 2021, 8, 616320. [Google Scholar] [CrossRef] [PubMed]
- Turhan, N.; Duran, C.; Ertorul, D.; Bulut Batur, Ü. The role of frailty score in early surgical treatment of elderly cholecystitis patients. Eur. Res. J. 2023, 9, 108–115. [Google Scholar] [CrossRef]
- Cook, M.D.; Karim, S.A.; Jensen, H.K.; Bennett, J.L.; Burdine, L.J.; Bhavaraju, A.; Sexton, K.W.; Kalkwarf, K.J. Percutaneous Cholecystostomy Tubes versus Medical Management for Acute Cholecystitis. Am. Surg. 2022, 88, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.Y.B.; Kitano, M.; Itoi, T.; Pérez-Miranda, M.; Ogura, T.; Chan, S.M.; Serna-Higuera, C.; Omoto, S.; Torres-Yuste, R.; Tsuichiya, T.; et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: An international randomised multicentre controlled superiority trial (DRAC 1). Gut 2020, 69, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Turiño, S.Y.; Shabanzadeh, D.M.; Eichen, N.M.; Jørgensen, S.L.; Sørensen, L.T.; Jørgensen, L.N. Percutaneous Cholecystostomy Versus Conservative Treatment for Acute Cholecystitis: A Cohort Study. J. Gastrointest. Surg. 2019, 23, 297–303. [Google Scholar] [CrossRef]
- Gurusamy, K.S.; Rossi, M.; Davidson, B.R. Percutaneous cholecystostomy for high-risk surgical patients with acute calculous cholecystitis. Cochrane Database Syst. Rev. 2013, CD007088. [Google Scholar] [CrossRef]
- Ambe, P.C.; Kaptanis, S.; Papadakis, M.; Weber, S.A.; Jansen, S.; Zirngibl, H. The Treatment of Critically Ill Patients with Acute Cholecystitis. Dtsch. Ärztebl. Int. 2016, 113, 545–551. [Google Scholar] [CrossRef]
- Rassameehiran, S.; Tantrachoti, P.; Nugent, K. Percutaneous Gallbladder Aspiration for Acute Cholecystitis. Bayl. Univ. Med. Cent. Proc. 2016, 29, 381–384. [Google Scholar] [CrossRef][Green Version]
- Van Dijk, A.H.; De Reuver, P.R.; Tasma, T.N.; Van Dieren, S.; Hugh, T.J.; Boermeester, M.A. Systematic review of antibiotic treatment for acute calculous cholecystitis. Br. J. Surg. 2016, 103, 797–811. [Google Scholar] [CrossRef]
- Haas, I.; Lahat, E.; Griton, Y.; Shmulevsky, P.; Shichman, S.; Elad, G.; Kammar, C.; Yaslovich, O.; Kendror, S.; Ben-Ari, A.; et al. Percutaneous aspiration of the gall bladder for the treatment of acute cholecystitis: A prospective study. Surg. Endosc. 2016, 30, 1948–1951. [Google Scholar] [CrossRef]
- Janssen, E.R.; Hendriks, T.; Natroshvili, T.; Bremers, A.J. Retrospective Analysis of Non-Surgical Treatment of Acute Cholecystitis. Surg. Infect. 2020, 21, 428–432. [Google Scholar] [CrossRef]
- Popowicz, A. Management of Acute Cholecystits. Surgery, Drainage and Gallbladder Aspiration. Available online: https://openarchive.ki.se/xmlui/bitstream/handle/10616/48469/Thesis_Agnieszka_Popowicz.pdf?sequence=3&isAllowed=y (accessed on 7 January 2024).
- Mohan, B.P.; Khan, S.R.; Trakroo, S.; Ponnada, S.; Jayaraj, M.; Asokkumar, R.; Adler, D.G. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: A systematic review and comparative meta-analysis. Endoscopy 2020, 52, 96–106. [Google Scholar] [CrossRef]
- Hemerly, M.C.; de Moura, D.T.H.; Junior, E.S.D.M.; Proença, I.M.; Ribeiro, I.B.; Yvamoto, E.Y.; Ribas, P.H.B.V.; Sánchez-Luna, S.A.; Bernardo, W.M.; de Moura, E.G.H. Endoscopic ultrasound (EUS)-guided cholecystostomy versus percutaneous cholecystostomy (PTC) in the management of acute cholecystitis in patients unfit for surgery: A systematic review and meta-analysis. Surg. Endosc. 2022, 37, 2421–2438. [Google Scholar] [CrossRef]
- Boregowda, U.; Chen, M.; Saligram, S. Endoscopic Ultrasound-Guided Gallbladder Drainage versus Percutaneous Gallbladder Drainage for Acute Cholecystitis: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 657. [Google Scholar] [CrossRef]
- Kamezaki, H.; Tsuyuguchi, T.; Shimura, K.; Sakamoto, D.; Senoo, J.; Mizumoto, H.; Kubota, M.; Yoshida, Y.; Azemoto, R.; Sugiyama, H.; et al. Safety and Efficacy of Early Tube Removal following Percutaneous Transhepatic Gallbladder Drainage: An Observational Study. Surg. Laparosc. Endosc. Percutaneous Tech. 2020, 30, 164–168. [Google Scholar] [CrossRef]
- Atar, E.; Bachar, G.; Berlin, S.; Neiman, C.; Bleich-Belenky, E.; Litvin, S.; Knihznik, M.; Belenky, A.; Ram, E. Percutaneous cholecystostomy in critically ill patients with acute cholecystitis: Complications and late outcome. Clin. Radiol. 2014, 69, e247–e252. [Google Scholar] [CrossRef]
- Beland, M.D.; Patel, L.; Ahn, S.H.; Grand, D.J. Image-Guided Cholecystostomy Tube Placement: Short- and Long-Term Outcomes of Transhepatic Versus Transperitoneal Placement. Am. J. Roentgenol. 2019, 212, 201–204. [Google Scholar] [CrossRef]
- Loberant, N.; Notes, Y.; Eitan, A.; Yakir, O.; Bickel, A. Comparison of early outcome from transperitoneal versus transhepatic percutaneous cholecystostomy. Hepatogastroenterology 2010, 57, 12–17. [Google Scholar] [PubMed]
- Heinzmann, A.; Müller, T.; Leitlein, J.; Braun, B.; Kubicka, S.; Blank, W. Endocavitary Contrast Enhanced Ultrasound (CEUS)—Work in Progress. Ultraschall Med.—Eur. J. Ultrasound 2012, 33, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.N.; Ghio, M.; Sadri, L.; Sarkar, S.; Kasotakis, G.; Narsule, C.; Sarkar, B. Percutaneous Cholecystostomy in Acute Cholecystitis—Predictors of Recurrence and Interval Cholecystectomy. J. Surg. Res. 2018, 232, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Aydiner, Ö.; Raşa, H.K. Percutaneous Cholecystostomy is a Feasible and Safe Option for High-Risk Acute Cholecystitis Patients. Clin. Exp. Health Sci. 2023, 13, 782–785. [Google Scholar] [CrossRef]
- Bundy, J.; Srinivasa, R.N.; Gemmete, J.J.; Shields, J.J.; Chick, J.F.B. Percutaneous Cholecystostomy: Long-Term Outcomes in 324 Patients. Cardiovasc. Interv. Radiol. 2018, 41, 928–934. [Google Scholar] [CrossRef]
- Pang, K.W.; Tan, C.H.N.; Loh, S.; Chang, K.Y.S.; Iyer, S.G.; Madhavan, K.; Kow, W.C.A. Outcomes of Percutaneous Cholecystostomy for Acute Cholecystitis. Mol. Med. 2016, 40, 2735–2744. [Google Scholar] [CrossRef]
- Macchini, D.; Degrate, L.; Oldani, M.; Leni, D.; Padalino, P.; Romano, F.; Gianotti, L. Timing of percutaneous cholecystostomy tube removal: Systematic review. Minerva Chir. 2016, 71, 415–426. [Google Scholar]
- Berenson, A.; Doran, M.; Strollo, B.; Burton, J.; Townsend, M.; Babin, J.; Millien, J.; Brown, R.; Fuhrman, G. An Analysis of Outcomes and Management Strategies for Patients with Cholecystostomy Tubes. Am. Surg. 2023, 89, 4424–4430. [Google Scholar] [CrossRef]
- Cha, B.H.; Song, H.H.; Kim, Y.N.; Jeon, W.J.; Kim, J.D.; Lee, H.H.; Lee, B.S.; Lee, S.H. Percutaneous Cholecystostomy Is Appropriate as Definitive Treatment for Acute Cholecystitis in Critically Ill Patients: A Single Center, Cross-sectional Study. Korean J. Gastroenterol. 2014, 63, 32–38. [Google Scholar] [CrossRef]
- Loftus, T.J.; Brakenridge, S.C.; Moore, F.A.; Dessaigne, C.G.; Sarosi, G.A., Jr.; Zingarelli, W.J.; Jordan, J.R.; Croft, C.A.; Smith, R.S.; Efron, P.A.; et al. Routine surveillance cholangiography following percutaneous cholecystostomy delays drain removal and cholecystectomy. J. Trauma. Acute Care Surg. 2017, 82, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Fujita, N.; Noda, Y.; Kobayashi, G.; Kimura, K.; Sugawara, T.; Horaguchi, J. Percutaneous Cholecystostomy Versus Gallbladder Aspiration for Acute Cholecystitis:A Prospective Randomized Controlled Trial. Am. J. Roentgenol. 2004, 183, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Barragan, C.; Alshehri, H.; Marom, G.; Glazer, Y.; Swanstrom, L.; Shlomovitz, E. A Pilot Study of Percutaneous Cholecystoenteric Anastomosis: A New Option for High-Risk Patients with Symptomatic Gallstones. J. Vasc. Interv. Radiol. 2024, 35, 74–79. [Google Scholar] [CrossRef] [PubMed]
- van Loon, F.H.J.; Scholten, H.J.; Korsten, H.H.M.; Dierick-van Daele, A.T.M.; Bouwman, A.R.A. The learning curve for ultrasound-guided peripheral intravenous cannulation in adults: A multicenter study. Med. Ultrason. 2022, 24, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Jayanti, S.; Juergens, C.; Makris, A.; Hennessy, A.; Nguyen, P. The Learning Curves for Transradial and Ultrasound-Guided Arterial Access: An Analysis of the SURF Trial. Heart Lung Circ. 2021, 30, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, G.R.; Helayel, P.E.; da Conceição, D.B.; Garzel, I.S.; Pavei, P.; Ceccon, M.S. Learning Curves and Mathematical Models for Interventional Ultrasound Basic Skills. Anesth. Analg. 2008, 106, 568. [Google Scholar] [CrossRef]
- Hoskins, M.J.M.; Nolan, B.C.M.; Evans, K.L.P.; Phillips, B.P. Educating health professionals in ultrasound guided peripheral intravenous cannulation: A systematic review of teaching methods, competence assessment, and patient outcomes. Medicine 2023, 102, e33624. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; van der Leij, C.; Katoh, M.; Benten, D.; Hendriks, B.M.F.; Hatzidakis, A. CIRSE Standards of Practice on Percutaneous Transhepatic Cholangiography, Biliary Drainage and Stenting. Cardiovasc. Interv. Radiol. 2021, 44, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, K.S.; Jineesh, V.; Keshava, S.N. Indian College of Radiology and Imaging Evidence-Based Guidelines for Percutaneous Image-Guided Biliary Procedures. Indian J. Radiol. Imaging 2021, 31, 421–440. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Itoi, T.; Baron, T.H.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Ukai, T.; Shikata, S.; Teoh, A.Y.B.; Kim, M.; et al. Indications and techniques of biliary drainage for acute cholangitis in updated Tokyo Guidelines 2018. J. Hepato-Biliary-Pancreatic Sci. 2017, 24, 537–549. [Google Scholar] [CrossRef]
- Ahmed, O.; Rogers, A.C.; Bolger, J.C.; Mastrosimone, A.; Lee, M.J.; Keeling, A.N.; Cheriyan, D.; Robb, W.B. Meta-analysis of outcomes of endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for the management of acute cholecystitis. Surg. Endosc. 2018, 32, 1627–1635. [Google Scholar] [CrossRef]
- Podboy, A.; Yuan, J.; Stave, C.D.; Chan, S.M.; Hwang, J.H.; Teoh, A.Y.B. Comparison of EUS-guided endoscopic transpapillary and percutaneous gallbladder drainage for acute cholecystitis: A systematic review with network meta-analysis. Gastrointest. Endosc. 2021, 93, 797–804.e1. [Google Scholar] [CrossRef]
- Bang, J.Y.; Arnoletti, J.P.; Wagner, A.; Varadarajulu, S. EUS-guided gallbladder drainage in acute cholecystitis: Long-term problems with surgical approach. Gut 2023. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, T.; Braden, B. Ultrasound-Guided Interventions in the Biliary System. Diagnostics 2024, 14, 403. https://doi.org/10.3390/diagnostics14040403
Müller T, Braden B. Ultrasound-Guided Interventions in the Biliary System. Diagnostics. 2024; 14(4):403. https://doi.org/10.3390/diagnostics14040403
Chicago/Turabian StyleMüller, Thomas, and Barbara Braden. 2024. "Ultrasound-Guided Interventions in the Biliary System" Diagnostics 14, no. 4: 403. https://doi.org/10.3390/diagnostics14040403
APA StyleMüller, T., & Braden, B. (2024). Ultrasound-Guided Interventions in the Biliary System. Diagnostics, 14(4), 403. https://doi.org/10.3390/diagnostics14040403





