Hepatic Artery Delineation on Ultrasound Volumes Comparing B-Flow and Color Doppler for Postoperative Monitoring of Pediatric Liver Transplants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
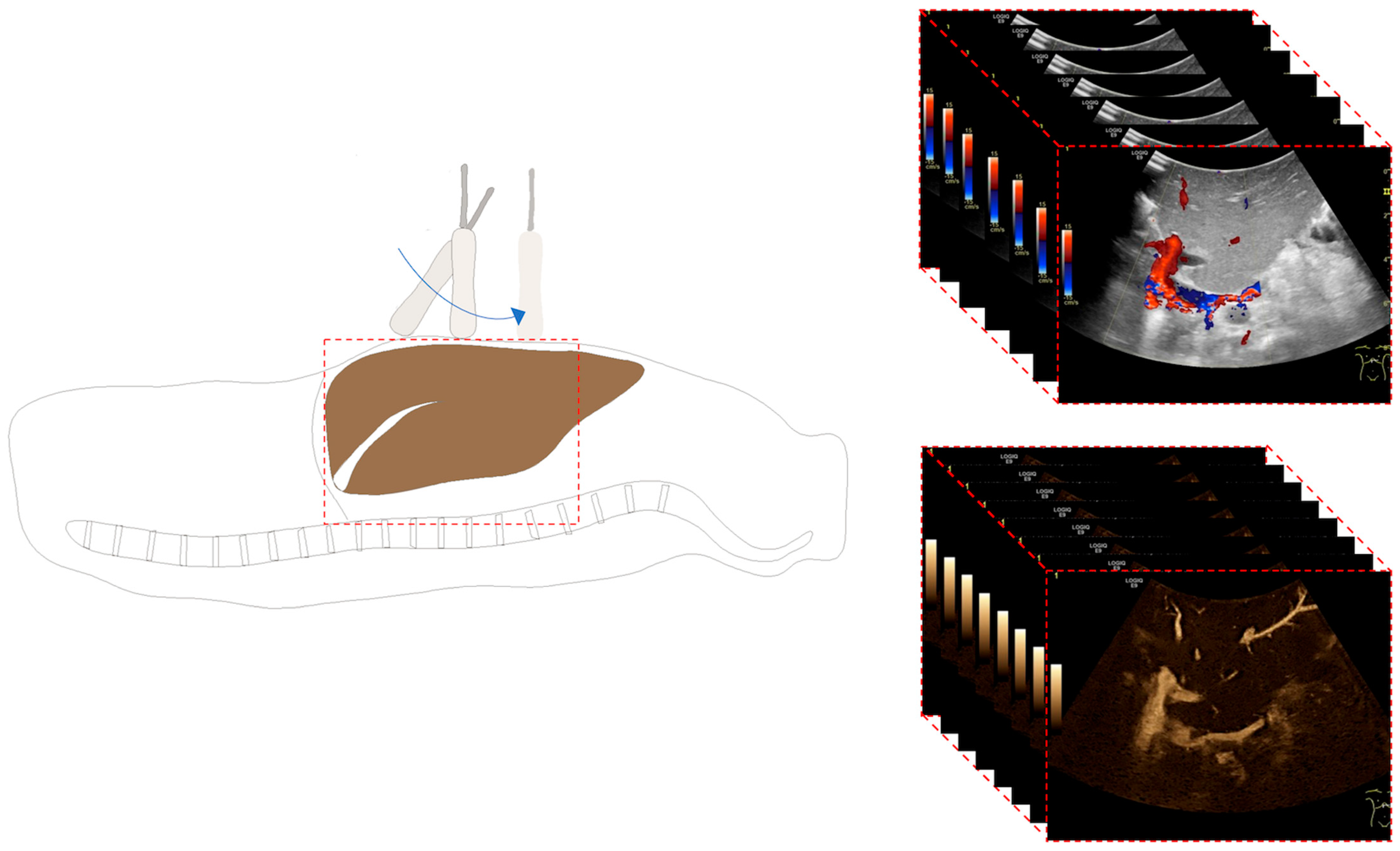
2.2. Ultrasound Examination
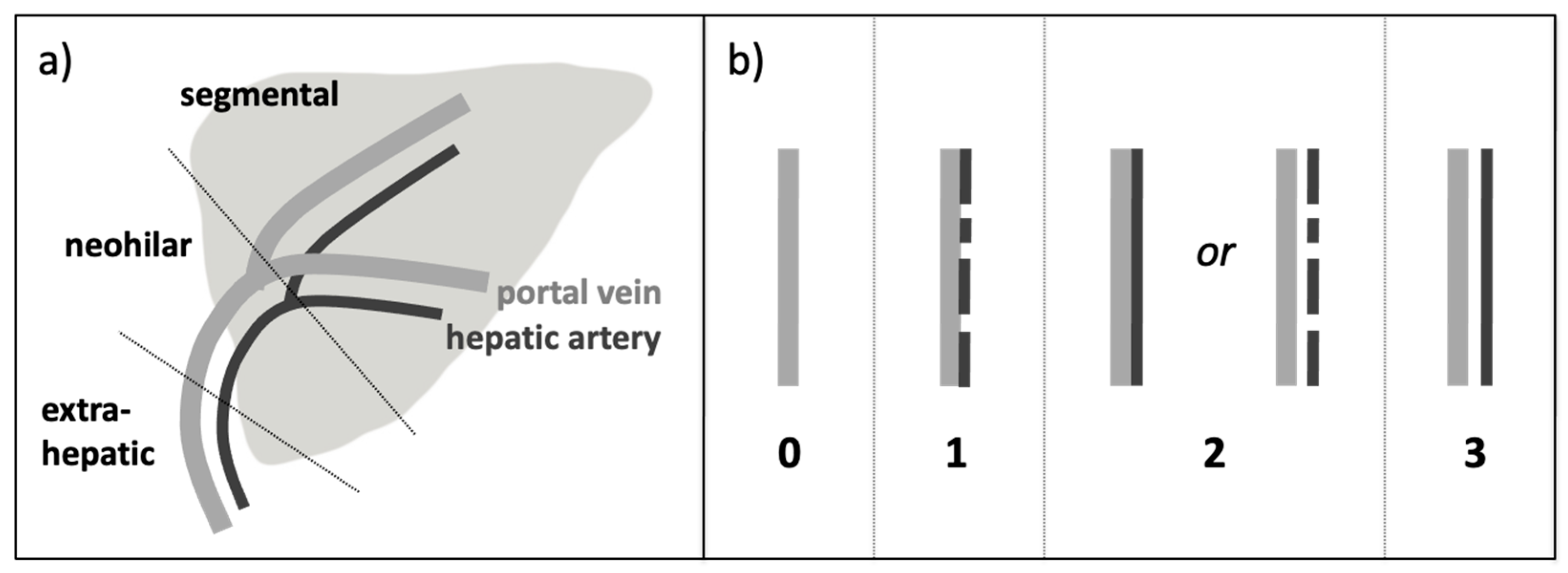
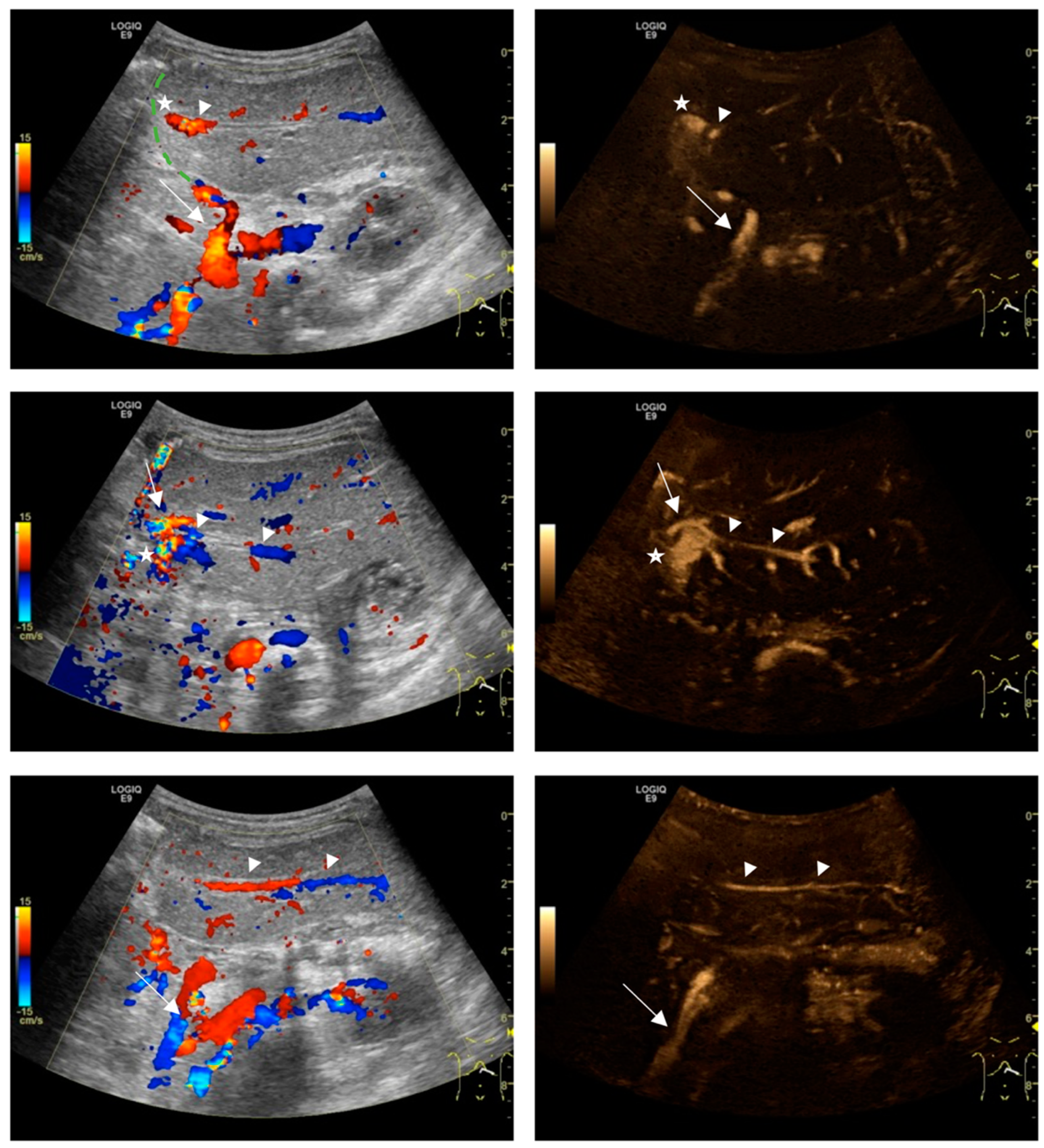
2.3. Image Analysis
- -
- S 0 = HA not detectable, only PV detectable
- -
- S 1 = HA discontinuously detectable but not separable by a gap from the PV
- -
- S 2 = HA continuously detectable but not separable by a gap from the PVor HA discontinuously detectable but separable by a gap from the PV
- -
- S 3 = HA continuously detectable and completely separable by a gap from the PV
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumann, U.; Karam, V.; Adam, R.; Fondevila, C.; Dhawan, A.; Sokal, E.; Jacquemin, E.; Kelly, D.A.; Grabhorn, E.; Pawlowska, J.; et al. Prognosis of Children Undergoing Liver Transplantation: A 30-Year European Study. Pediatrics 2022, 150, e2022057424. [Google Scholar] [CrossRef]
- Neto, J.S.; Fonseca, E.A.; Vincenzi, R.; Pugliese, R.; Benavides, M.R.; Roda, K.; Porta, G.; Miura, I.K.; Porta, A.; Borges, C.; et al. Technical Choices in Pediatric Living Donor Liver Transplantation: The Path to Reduce Vascular Complications and Improve Survival. Liver Transplant. 2020, 26, 1644–1651. [Google Scholar] [CrossRef]
- Seda-Neto, J.; da Fonseca, E.A.; Pugliese, R.; Candido, H.L.; Benavides, M.R.; Afonso, R.C.; Neiva, R.; Porta, G.; Miura, I.K.; Teng, H.W.; et al. Twenty Years of Experience in Pediatric Living Donor Liver Transplantation. Transplantation 2016, 100, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Astarcıoglu, I.; Egeli, T.; Gulcu, A.; Ozbilgin, M.; Agalar, C.; Cesmeli, E.B.; Kaya, E.; Karademir, S.; Unek, T. Vascular Complications After Liver Transplantation. Transplantation 2023, 21, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Sieders, E.; Peeters, P.M.; TenVergert, E.M.; de Jong, K.P.; Porte, R.J.; Zwaveling, J.H.; Bijleveld, C.M.; Slooff, M.J. Early vascular complications after pediatric liver transplantation. Liver Transplant. 2000, 6, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.P.; Hong, J.C.; Farmer, D.G.; Ghobrial, R.M.; Yersiz, H.; Hiatt, J.R.; Busuttil, R.W. Vascular Complications of Orthotopic Liver Transplantation: Experience in More than 4,200 Patients. J. Am. Coll. Surg. 2009, 208, 896–903. [Google Scholar] [CrossRef]
- Bekker, J.; Ploem, S.; De Jong, K.P. Early Hepatic Artery Thrombosis after Liver Transplantation: A Systematic Review of the Incidence, Outcome and Risk Factors. Am. J. Transplant. 2009, 9, 746–757. [Google Scholar] [CrossRef]
- Horvat, N.; Marcelino, A.S.Z.; Horvat, J.V.; Yamanari, T.R.; Araújo-Filho, J.d.A.B.; Panizza, P.; Seda-Neto, J.; da Fonseca, E.A.; Carnevale, F.C.; Cerri, L.M.d.O.; et al. Pediatric Liver Transplant: Techniques and Complications. Radiographics 2017, 37, 1612–1631. [Google Scholar] [CrossRef]
- Pozniak, M.A.; Zagzebski, J.A.; Scanlan, K.A. Spectral and color Doppler artifacts. Radiographics 1992, 12, 35–44. [Google Scholar] [CrossRef]
- Terslev, L.; Diamantopoulos, A.P.; Døhn, U.M.; Schmidt, W.A.; Torp-Pedersen, S. Settings and artefacts relevant for Doppler ultrasound in large vessel vasculitis. Arthritis Res. Ther. 2017, 19, 167. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.M.; Kim, Y.J.; Lee, J.Y.; Han, J.K.; Choi, B.I. High-Definition Flow Doppler Ultrasonographic Technique to Assess Hepatic Vasculature Compared with Color or Power Doppler Ultrasonography. J. Ultrasound Med. 2008, 27, 1491–1501. [Google Scholar] [CrossRef]
- Wachsberg, R.H. B-Flow, a Non-Doppler Technology for Flow Mapping: Early Experience in the Abdomen. Ultrasound Q. 2003, 19, 114–122. [Google Scholar] [CrossRef]
- Fujimoto, T.; Shibata, Y.; Ohno, Y. A New Era in Diagnostic Ultrasound, Superb Microvascular Imaging: Preliminary Results in Pediatric Hepato-Gastrointestinal Disorders. Eur. J. Pediatr. Surg. 2016, 27, 020–025. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Fang, H.; Liu, X.; Xia, Q.; Li, F. Additional value of superb microvascular imaging for assessing hepatic arterial blood flow after pediatric liver transplantation. Pediatr. Transplant. 2020, 24, e13785. [Google Scholar] [CrossRef]
- Collaku, E.; Simonini, R.; Balbi, M.; Bonaffini, P.A.; Valle, C.; Morzenti, C.; Faseli, R.F.; Ferrari, A.; Ippolito, D.; Marra, P.; et al. Superb Microvascular Imaging (SMI) Compared with Color Doppler Ultrasound for the Assessment of Hepatic Artery in Pediatric Liver Transplants: A Feasibility Study. Diagnostics 2022, 12, 1476. [Google Scholar] [CrossRef] [PubMed]
- Wachsberg, R.H. B-flow imaging of the hepatic vasculature: Correlation with color Doppler sonography. AJR Am. J. Roentgenol. 2007, 188, W522–W533. [Google Scholar] [CrossRef]
- Hofmann, A.G.; Mlekusch, I.; Wickenhauser, G.; Assadian, A.; Taher, F. Clinical Applications of B-Flow Ultrasound: A Scoping Review of the Literature. Diagnostics 2023, 13, 397. [Google Scholar] [CrossRef]
- Dammann, E.; Ording-Müller, L.-S.; Franchi-Abella, S.; Verhagen, M.V.; McGuirk, S.P.; Bokkers, R.P.H.; Clapuyt, P.R.M.; Deganello, A.; Tandoi, F.; Goyet, J.d.V.d.; et al. European Society of Pediatric Radiology survey of perioperative imaging in pediatric liver transplantation: (3) postoperative imaging. Pediatr. Radiol. 2024, 54, 276–284. [Google Scholar] [CrossRef]
- Parsai, A.; Zerizer, I.; Hohmann, J.; Bongartz, G.; Beglinger, C.; Sperandeo, G. Remote sonographic interpretation: Comparison of standardized video clips to still images. J. Clin. Ultrasound 2012, 40, 495–501. [Google Scholar] [CrossRef]
- Dormagen, J.B.; Gaarder, M.; Drolsum, A. Standardized cine-loop documentation in abdominal ultrasound facilitates offline image interpretation. Acta Radiol. 2013, 56, 3–9. [Google Scholar] [CrossRef]
- Groth, M.; Ernst, M.; Deindl, P.; Herrmann, J. B-Flow Sonography for Evaluation of Basal Cerebral Arteries in Newborns. Clin. Neuroradiol. 2019, 29, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Groth, M.; Dammann, E.; Arndt, F.; Ernst, M.; Herrmann, J. Comparison of B-Mode with B-Flow Sonography for the Evaluation of Femoral Arteries in Infants. RoFo—Fortschritte Auf Dem Geb. Der Rontgenstrahlen Und Der Nukl. 2017, 189, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Eurotransplant Manual—Version 6.7. January 2024. Available online: https://www.eurotransplant.org/allocation/eurotransplant-manual/ (accessed on 8 February 2024).
- Johnson, A.W.; Stoneman, P.; McClung, M.S.; Van Wagoner, N.; Corey, T.E.; Bruening, D.A.; Hunter, T.D.; Myrer, J.W.; Ridge, S.T. Use of Cine Loops and Structural Landmarks in Ultrasound Image Processing Improves Reliability and Reduces Error in the Assessment of Foot and Leg Muscles. J. Ultrasound Med. 2020, 39, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Jandzinski, D.; van Wijngaarden, E.; Dogra, V.; Fisher, S.G.; Conde, A.; Rubens, D. Renal Sonography With 2-Dimensional Versus Cine Organ Imaging. J. Ultrasound Med. 2007, 26, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Fernández, I.; Ruiz-González, E.; Lubian-Gutiérrez, M.; Lubián-Fernández, S.P.; Fontela, Y.C.; Roca-Cornejo, C.; Olmo-Duran, P.; Lubián-López, S.P. Ultrasonographic Estimation of Total Brain Volume: 3D Reliability and 2D Estimation. Enabling Routine Estimation During NICU Admission in the Preterm Infant. Front. Pediatr. 2021, 9, 708396. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Säring, D.; der Mauer, M.A.; Groth, M.; Well, E.J.-V. Forensic age assessment of the knee: Proposal of a new classification system using two-dimensional ultrasound volumes and comparison to MRI. Eur. Radiol. 2021, 31, 3237–3247. [Google Scholar] [CrossRef]
- Stenman, C.; Thorelius, L.; Knutsson, A.; Smedby, Ö. Radiographer-acquired and radiologist-reviewed ultrasound examination—Agreement with radiologist’s bedside evaluation. Acta Radiol. 2010, 52, 70–74. [Google Scholar] [CrossRef]
- Herrmann, J.; Petit, P.; Franchi-Abella, S.; Verhagen, M.V.; McGuirk, S.P.; Dammann, E.; Bokkers, R.P.H.; Clapuyt, P.R.M.; Deganello, A.; Tandoi, F.; et al. European Society of Pediatric Radiology survey of perioperative imaging in pediatric liver transplantation: (2) intraoperative imaging. Pediatr. Radiol. 2024, 54, 269–275. [Google Scholar] [CrossRef]
- Jung, E.; Kammerer, S.; Brandenstein, M.; Putz, F.; Stroszczynski, C.; Jung, F. High resolution flow (HR Flow) and Glazing Flow in cases of hepatic flow changes: Comparison to color-coded Doppler sonography (CCDS). Clin. Hemorheol. Microcirc. 2021, 79, 3–17. [Google Scholar] [CrossRef]
- Franke, D.; Daugherty, R.J.; Ključevšek, D.; Ntoulia, A.; Rafailidis, V.; Takahashi, M.S.; Torres, A.; Viteri, B.; Volberg, F.M. Contrast-enhanced ultrasound of transplant organs—liver and kidney—In children. Pediatr. Radiol. 2021, 51, 2284–2302. [Google Scholar] [CrossRef]
- Craig, E.V.; Heller, M.T. Complications of liver transplant. Abdom. Radiol. 2021, 46, 43–67. [Google Scholar] [CrossRef] [PubMed]
- Clevert, D.-A.; Johnson, T.; Jung, E.M.; Flach, P.M.; Strautz, T.I.; Ritter, G.; Gallegos, M.T.; Kubale, R.; Becker, C.; Reiser, M. Color Doppler, power Doppler and B-flow ultrasound in the assessment of ICA stenosis: Comparison with 64-MD-CT angiography. Eur. Radiol. 2007, 17, 2149–2159. [Google Scholar] [CrossRef]
- Matsumoto, N.; Ogawa, M.; Miura, T.; Shiozawa, K.; Abe, M.; Nakagawara, H.; Moriyama, M. B-flow imaging of vascular structure for the diagnosis of liver tumor. J. Med. Ultrason. 2013, 40, 409–415. [Google Scholar] [CrossRef]
- Furuse, J.; Maru, Y.; Mera, K.; Sumi, H.; Yoshino, M.; Yokoyama, Y.; Hashimoto, H.; Ejiri, A. Visualization of blood flow in hepatic vessels and hepatocellular carcinoma using B-flow sonography. J. Clin. Ultrasound 2001, 29, 1–6. [Google Scholar] [CrossRef]
- Dammann, E.; Groth, M.; Schild, R.-S.; Lemke, A.; Oh, J.; Adam, G.; Herrmann, J. B-Flow Sonography vs. Color Doppler Sonography for the Assessment of Vascularity in Pediatric Kidney Transplantation. RöFo Fortschritte Auf Dem Geb. Der Röntgenstrahlen Und Der Nukl. 2020, 193, 49–60. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Zhang, B.; Liu, B.; Pan, H. Meta-analysis of the diagnostic value of contrast-enhanced ultrasound for the detection of vascular complications after liver transplantation. Rev. Española Enfermedades Dig. 2018, 111, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S.; Cantisani, V.; Deganello, A.; Dietrich, C.; Duran, C.; Franke, D.; Harkanyi, Z.; Kosiak, W.; Miele, V.; Ntoulia, A.; et al. Role of Contrast-Enhanced Ultrasound (CEUS) in Paediatric Practice: An EFSUMB Position Statement. Ultraschall Med.—Eur. J. Ultrasound 2016, 38, 33–43. [Google Scholar] [CrossRef] [PubMed]

| Parameter | |
|---|---|
| Age at liver transplantation (years) | 6.9 ± 6.9 |
| Sex | |
| Number (no.) of males | 17 |
| No. of females | 18 |
| Weight at liver transplantation (kg) | 25.2 ± 22.2 |
| Preoperative patient status | |
| No. with high urgency | 20 |
| No. with chronic disease | 17 |
| Type of liver transplantation | |
| No. with orthotopic, full graft | 8 |
| No. with reduced graft | 1 |
| No. with split graft, deceased donor | 21 |
| No. with split graft, living donor | 7 |
| Liver transplantations | |
| No. with first | 32 |
| No. with second | 5 |
| Ischemic time (min) | |
| Cold | 621.1 ± 176.6 |
| Warm | 36.1 ± 16.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dammann, E.; Steinmeister, L.; Groth, M.; Herden, U.; Fischer, L.; Brinkert, F.; Beime, J.; Tozakidou, M.; Bannas, P.; Herrmann, J. Hepatic Artery Delineation on Ultrasound Volumes Comparing B-Flow and Color Doppler for Postoperative Monitoring of Pediatric Liver Transplants. Diagnostics 2024, 14, 617. https://doi.org/10.3390/diagnostics14060617
Dammann E, Steinmeister L, Groth M, Herden U, Fischer L, Brinkert F, Beime J, Tozakidou M, Bannas P, Herrmann J. Hepatic Artery Delineation on Ultrasound Volumes Comparing B-Flow and Color Doppler for Postoperative Monitoring of Pediatric Liver Transplants. Diagnostics. 2024; 14(6):617. https://doi.org/10.3390/diagnostics14060617
Chicago/Turabian StyleDammann, Elena, Leonhard Steinmeister, Michael Groth, Uta Herden, Lutz Fischer, Florian Brinkert, Jan Beime, Magdalini Tozakidou, Peter Bannas, and Jochen Herrmann. 2024. "Hepatic Artery Delineation on Ultrasound Volumes Comparing B-Flow and Color Doppler for Postoperative Monitoring of Pediatric Liver Transplants" Diagnostics 14, no. 6: 617. https://doi.org/10.3390/diagnostics14060617
APA StyleDammann, E., Steinmeister, L., Groth, M., Herden, U., Fischer, L., Brinkert, F., Beime, J., Tozakidou, M., Bannas, P., & Herrmann, J. (2024). Hepatic Artery Delineation on Ultrasound Volumes Comparing B-Flow and Color Doppler for Postoperative Monitoring of Pediatric Liver Transplants. Diagnostics, 14(6), 617. https://doi.org/10.3390/diagnostics14060617







