Can the ADC Value Be Used as an Imaging “Biopsy” in Endometrial Cancer?
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Selection
2.2. MR Protocol
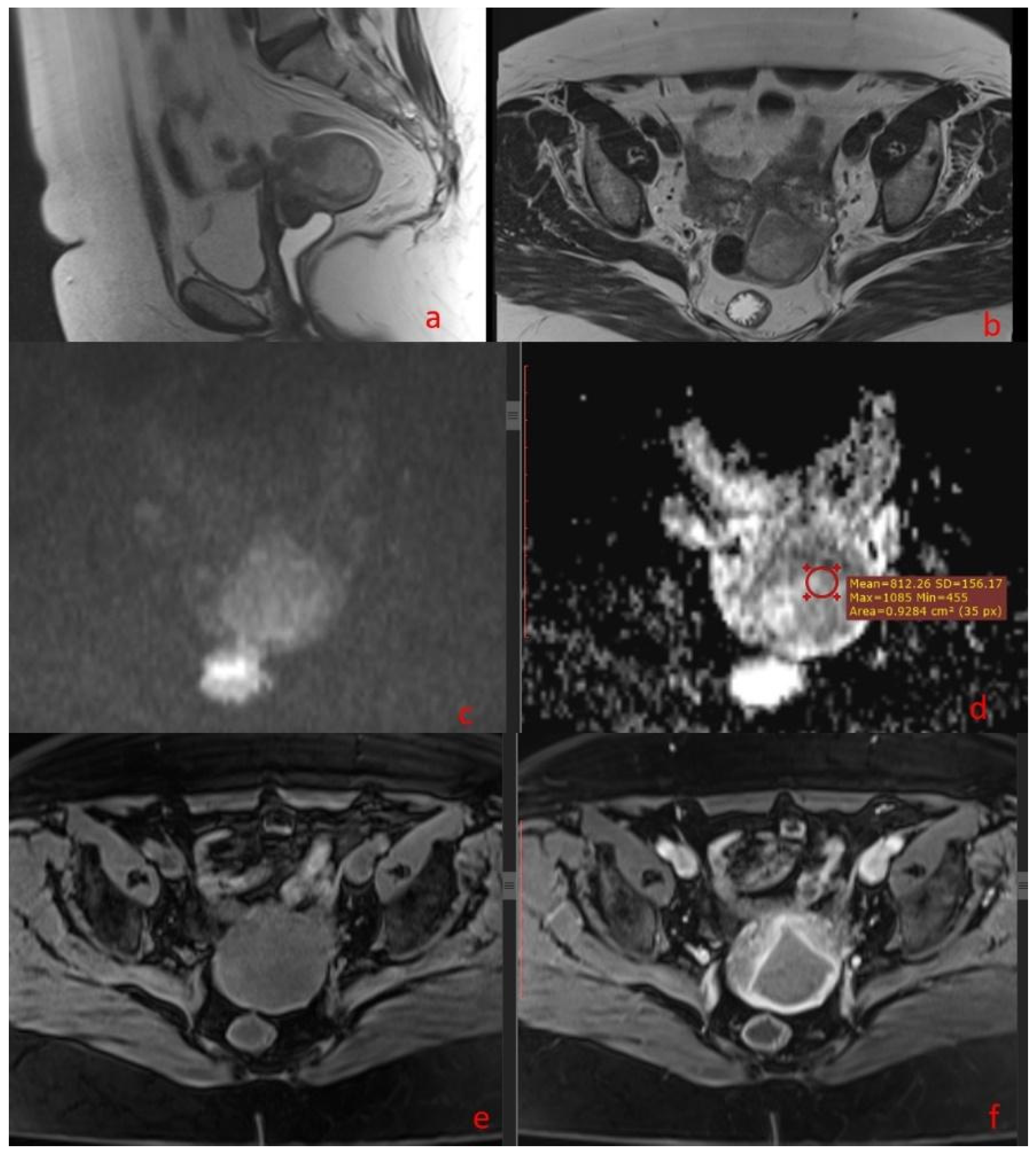
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. MRI Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Çavusoğlu, M.; Ciliz, D.S.; Ozsoy, A.; Duran, S.; Elverici, E.; Atalay, C.R.; Ozdemir, O.; Sakman, B. Diffusion-weighted MRI of postmenopausal women with vaginal bleeding and endometrial thickening: Differentiation of benign and malignant lesions. J. Belg. Soc. Radiol. 2016, 100, 70. [Google Scholar] [CrossRef]
- Bonatti, M.; Pedrinolla, B.; Cybulski, A.J.; Lombardo, F.; Negri, G.; Messini, S.; Tagliaferri, T.; Manfredi, R.; Bonatti, G. Prediction of histological grade of endometrial cancer by means of MRI. Eur. J. Radiol. 2018, 103, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.C.; Xiao, M.L.; Li, Y.; Wei Qiang, J. The diagnostic performance of ADC value for tumor grade, deep myometrial invasion and lymphovascular space invasion in endometrial cancer: A meta-analysis. Acta Radiol. 2019, 60, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, V.; Meydanli, M.M.; Yalçın, I.; Sarı, M.E.; Sahin, H.; Kocaman, E.; Haberal, A.; Dursun, P.; Güngör, T.; Ayhan, A. Comparison of three different risk-stratification models for predicting lymph node involvement in endometrioid endometrial cancer clinically confined to the uterus. J. Gynecol. Oncol. 2017, 28, e78. [Google Scholar] [CrossRef]
- Kishimoto, K.; Tajima, S.; Maeda, I.; Takagi, M.; Ueno, T.; Suzuki, N.; Nakajima, Y. Endometrial cancer: Correlation of apparent diffusion coefficient (ADC) with tumor cellularity and tumor grade. Acta Radiol. 2016, 57, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Rechichi, G.; Galimberti, S.; Signorelli, M.; Franzesi, C.T.; Perego, P.; Valsecchi, M.G.; Sironi, S. Endometrial cancer: Correlation of apparent diffusion coefficient with tumor grade, depth of myometrial invasion, and presence of lymph node metastases. Am. J. Roentgenol. 2011, 197, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhao, T.; Liang, X.; Niu, C.; Ding, C. Can the apparent diffusion coefficient differentiate the grade of endometrioid adenocarcinoma and the histological subtype of endometrial cancer? Acta Radiol. 2018, 59, 363–370. [Google Scholar] [CrossRef]
- Bharwani, N.; Miquel, M.E.; Sahdev, A.; Narayanan, P.; Malietzis, G.; Reznek, R.H.; Rockall, A.G. Diffusion-weighted imaging in the assessment of tumour grade in endometrial cancer. Br. J. Radiol. 2011, 84, 997–1004. [Google Scholar] [CrossRef]
- Tamai, K.; Koyama, T.; Saga, T.; Umeoka, S.; Mikami, Y.; Fujii, S.; Togashi, K. Diffusion-weighted MR imaging of uterine endometrial cancer. J. Magn. Reson. Imaging 2007, 26, 682–687. [Google Scholar] [CrossRef]
- Woo, S.; Cho, J.Y.; Kim, S.Y.; Kim, S.H. Histogram analysis of apparent diffusion coefficient map of diffusion-weighted MRI in endometrial cancer: A preliminary correlation study with histological grade. Acta Radiol. 2014, 55, 1270–1277. [Google Scholar] [CrossRef]
- Abnormal Vaginal Bleeding in Pre- and Peri-Menopausal Women. A Diagnostic Guide for General Practitioners and Gynaecologists. Available online: http://canceraustralia.gov.au/sites/default/files/publications/ncgc-vaginal-bleeding-flowchartsmarch-20111_504af02038614.pdf (accessed on 22 February 2020).
- Yan, B.; Liang, X.; Zhao, T.; Ding, C.; Zhang, M. Is the standard deviation of the apparent diffusion coefficient a potential tool for the preoperative prediction of tumor grade in endometrial cancer? Acta Radiol. 2020, 61, 1724–1732. [Google Scholar] [CrossRef]
- Ma, X.; Shen, M.; He, Y.; Ma, F.; Liu, J.; Zhang, G.; Qiang, J. The role of volumetric ADC histogram analysis in preoperatively evaluating the tumor subtype and grade of endometrial cancer. Eur. J. Radiol. 2021, 140, 109745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, X.; Zhang, X.; Chen, S.; Song, Y.; Xie, L.; Chen, Y.; Ouyang, H. Whole-lesion apparent diffusion coefficient (ADC) histogram as a quantitative biomarker to preoperatively differentiate stage IA endometrial carcinoma from benign endometrial lesions. BMC Med. Imaging 2022, 22, 139. [Google Scholar] [CrossRef] [PubMed]
- Batista, T.P.; Cavalcanti, C.L.C.; Tejo, A.A.G.; Bezerra, A.L.R. Accuracy of preoperative endometrial sampling diagnosis for predicting the final pathology grading in uterine endometrioid carcinoma. Eur. J. Surg. Oncol. 2016, 42, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, I.; Pavelka, J.; Cohn, D.E.; Copeland, L.J.; Ramirez, N.; Manolitsas, T.; Fowler, J.M. Surgical staging for patients presenting with grade 1 endometrial carcinoma. Obstet. Gynecol. 2005, 105, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pertovaara, H.; Dastidar, P.; Vornanen, M.; Paavolainen, L.; Marjomäki, V.; Järvenpää, R.; Eskola, H.; Kellokumpu-Lehtinen, P.L. ADC measurements in diffuse large B-cell lymphoma and follicular lymphoma: A DWI and cellularity study. Eur. J. Radiol. 2013, 82, e158–e164. [Google Scholar] [CrossRef]
- Sugahara, T.; Korogi, Y.; Kochi, M.; Ikushima, I.; Shigematu, Y.; Hirai, T.; Okuda, T.; Liang, L.; Ge, Y.; Komohara, Y.; et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J. Magn. Reson. Imaging 1999, 9, 53–60. [Google Scholar] [CrossRef]
- Nakai, G.; Matsuki, M.; Inada, Y.; Tatsugami, F.; Tanikake, M.; Narabayashi, I.; Yamada, T. Detection and evaluation of pelvic lymph nodes in patients with gynecologic malignancies using body diffusion-weighted magnetic resonance imaging. J. Comput. Assist. Tomogr. 2008, 32, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.M.; Kim, C.K.; Choi, D.; Kwan Park, B. Endometrial cancer: Utility of diffusion-weighted magnetic resonance imaging with background body signal suppression at 3T. J. Magn. Reson. Imaging 2013, 37, 1151–1159. [Google Scholar] [CrossRef]
- Shen, S.H.; Chiou, Y.Y.; Wang, J.H.; Yen, M.S.; Lee, R.C.; Lai, C.R.; Chang, C.Y. Diffusion-weighted single-shot echo-planar imaging with parallel technique in assessment of endometrial cancer. Am. J. Roentgenol. 2008, 190, 481–488. [Google Scholar] [CrossRef]
- Lin, G.; Ng, K.K.; Chang, C.j.; Wang, J.J.; Ho, K.C.; Yen, T.C.; Wu, T.I.; Wang, C.C.; Chen, Y.R.; Huang, Y.T.; et al. Myometrial invasion in endometrial cancer: Diagnostic accuracy of diffusion-weighted 3.0-T MR imaging-initial experience. Radiology 2009, 250, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Matsusue, E.; Kigawa, J.; Sato, S.; Kanasaki, Y.; Nakanishi, J.; Sugihara, S.; Kaminou, T.; Terakawa, N.; Ogawa, T. Diagnostic accuracy of the apparent diffusion coefficient in differentiating benign from malignant uterine endometrial cavity lesions: Initial results. Eur. Radiol. 2008, 18, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Matsuzaki, K.; Nishitani, H. Diffusion-weighted magnetic resonance imaging of endometrial cancer: Differentiation from benign endometrial lesions and preoperative assessment of myometrial invasion. Acta Radiol. 2009, 50, 947–953. [Google Scholar] [CrossRef]
- Husby, J.A.; Salvesen, O.; Magnussen, I.J.; Trovik, J.; Bjørge, L.; Salvesen, H.B.; Haldorsen, I.S. Tumour apparent diffusion coefficient is associated with depth of myometrial invasion and is negatively correlated to tumour volume in endometrial carcinomas. Clin. Radiol. 2015, 70, 487–494. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Patients (n: 92) | ||
|---|---|---|---|
| Age | 63 years old (43–84 years old) | ||
| Symptoms | |||
| Bleeding | 98.9% | ||
| Pelvic pain | 8% | ||
| Fatigue | 9% | ||
| Weight loss | 2% | ||
| Histology | Biopsy | Post-surgical histology | |
| Endometrioid adenocarcinoma | 73 | 64 | |
| Non-endometrioid adenocarcinoma | 10 | 28 | |
| Mixed types | 7 | n/a | |
| Atypic hyperplasia | 2 | n/a | |
| Tumor grade | Biopsy | Post-surgical histology | ADC |
| G1 | 26 | 37 | 35 |
| G2 | 14 | 31 | 33 |
| G3 | 12 | 24 | 24 |
| Missing | 40 | ||
| Tumor characteristics | MRI | Post-surgical histology | |
| Tumor maximum dimension | <2 cm | 15 | n/a |
| >2 cm | 77 | n/a | |
| Depth of myometrial invasion | <50% | 50 | 43 |
| >50% | 42 | 49 | |
| Cervical stroma involvement | Negative | 66 | 68 |
| Positive | 26 | 24 | |
| Lymph node involvement | Negative | 70 | 66 |
| Positive | 22 | 13 | |
| Lymphadenectomy not performed | 13 | ||
| ADC Value | Histologically Proven Myometrial Invasion | ||
|---|---|---|---|
| ADC value | Pearson correlation | 1 | −0.360 ** |
| Sig. (2-tailed) | 0.000 | ||
| N | 92 | 92 | |
| Histologically proven myometrial invasion | Pearson correlation | −0.360 ** | 1 |
| Sig. (2-tailed) | 0.000 | ||
| N | 92 | 92 | |
| ADC Value | Lymphovascular Space Invasion | ||
|---|---|---|---|
| ADC value | Pearson correlation | 1 | −0.522 ** |
| Sig. (2-tailed) | 0.000 | ||
| N | 92 | 92 | |
| Lymphovascular space invasion | Pearson correlation | −0.522 ** | 1 |
| Sig. (2-tailed) | 0.000 | ||
| N | 92 | 92 | |
| Total Number | <50% Myometrial Invasion | >50% Myometrial Invasion | Lymphovascular Space Invasion | Patients with <50% Myometrial Invasion, but with Lymphovascular Space Invasion | |
|---|---|---|---|---|---|
| G1 | 37 | 22 | 15 | 7 | 3 |
| G2 | 31 | 10 | 21 | 17 | 2 |
| G3 | 24 | 10 | 14 | 19 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrila, O.; Nistor, I.; Romedea, N.S.; Negru, D.; Scripcariu, V. Can the ADC Value Be Used as an Imaging “Biopsy” in Endometrial Cancer? Diagnostics 2024, 14, 325. https://doi.org/10.3390/diagnostics14030325
Petrila O, Nistor I, Romedea NS, Negru D, Scripcariu V. Can the ADC Value Be Used as an Imaging “Biopsy” in Endometrial Cancer? Diagnostics. 2024; 14(3):325. https://doi.org/10.3390/diagnostics14030325
Chicago/Turabian StylePetrila, Octavia, Ionut Nistor, Narcis Sandy Romedea, Dragos Negru, and Viorel Scripcariu. 2024. "Can the ADC Value Be Used as an Imaging “Biopsy” in Endometrial Cancer?" Diagnostics 14, no. 3: 325. https://doi.org/10.3390/diagnostics14030325
APA StylePetrila, O., Nistor, I., Romedea, N. S., Negru, D., & Scripcariu, V. (2024). Can the ADC Value Be Used as an Imaging “Biopsy” in Endometrial Cancer? Diagnostics, 14(3), 325. https://doi.org/10.3390/diagnostics14030325





