Pre-Analytical Factors Affecting Extracellular DNA in Saliva
Abstract
1. Introduction
2. Methods
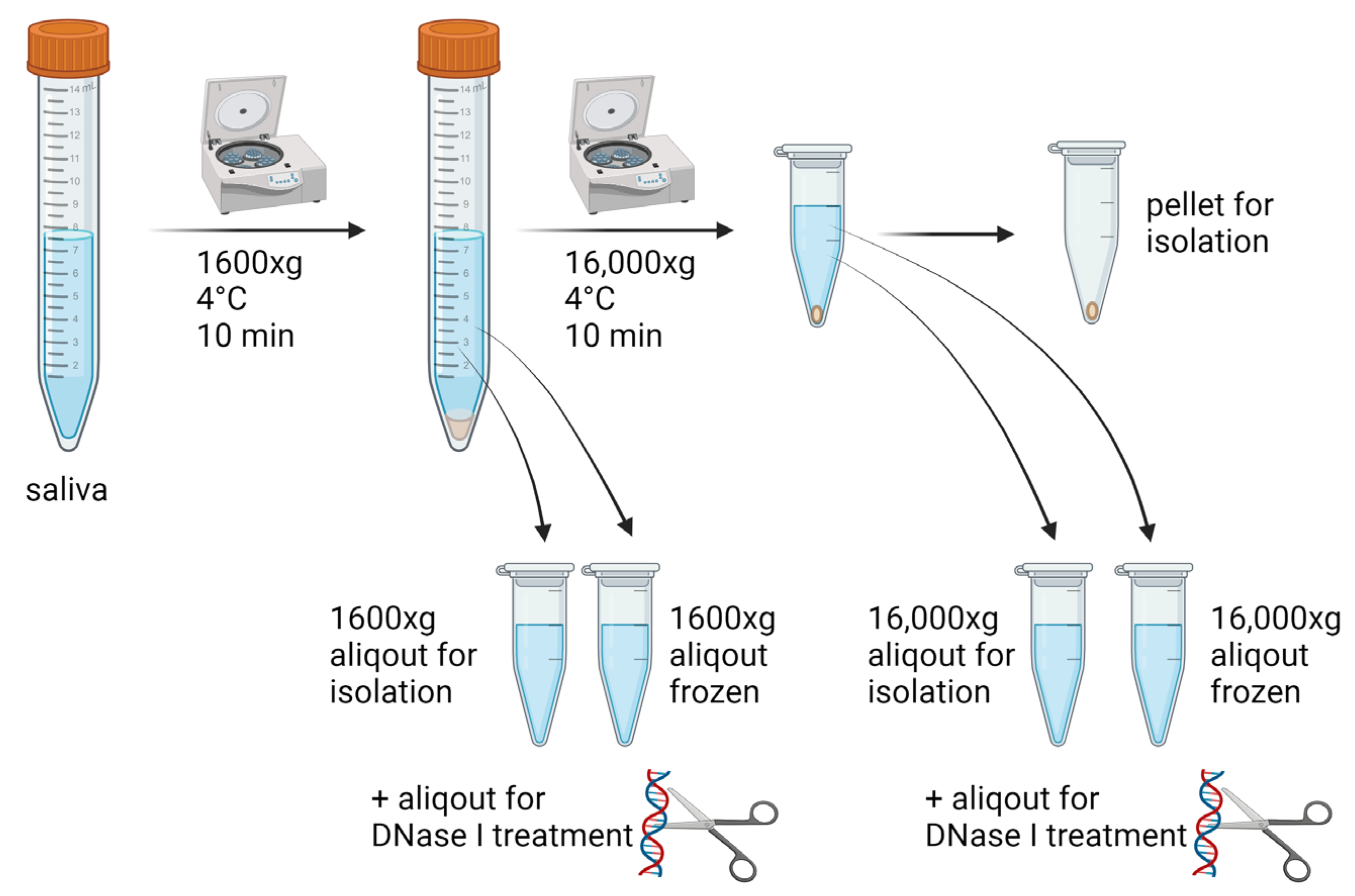
2.1. Saliva Collection and Sample Processing
2.2. Extracellular DNA Isolation and Quantification
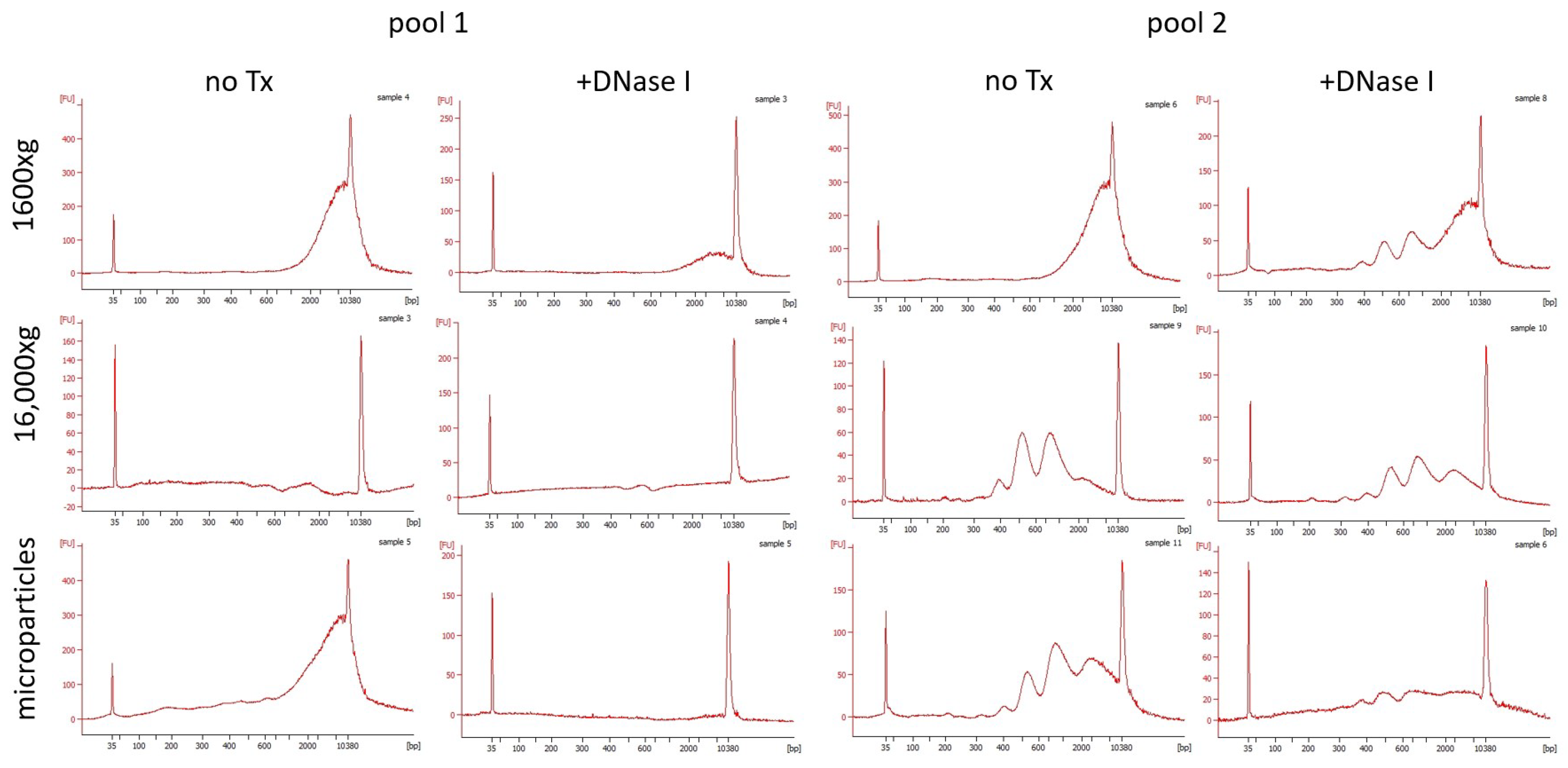
2.3. DNA Fragmentation
2.4. DNase Activity
2.5. Statistical Analysis
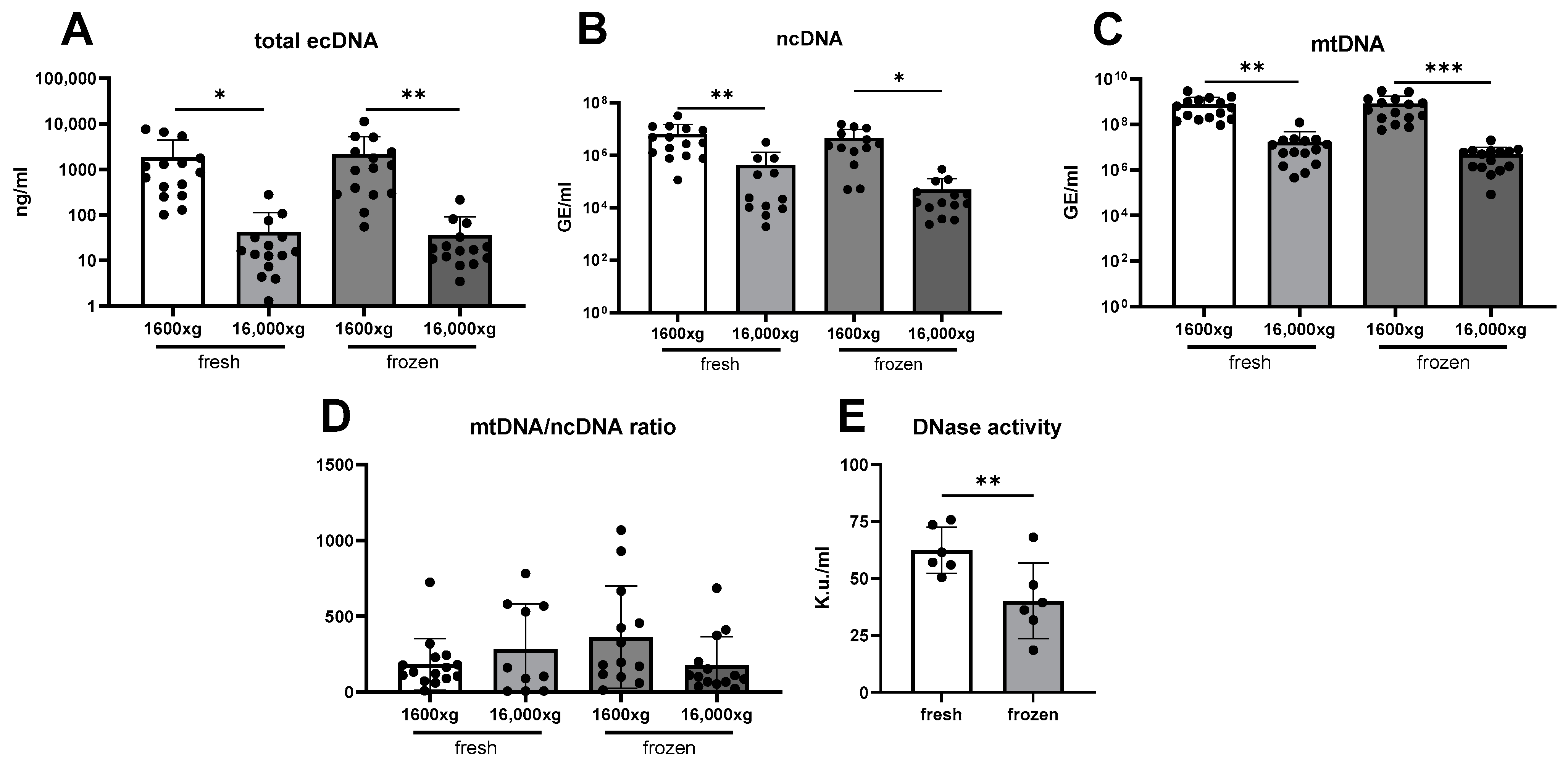
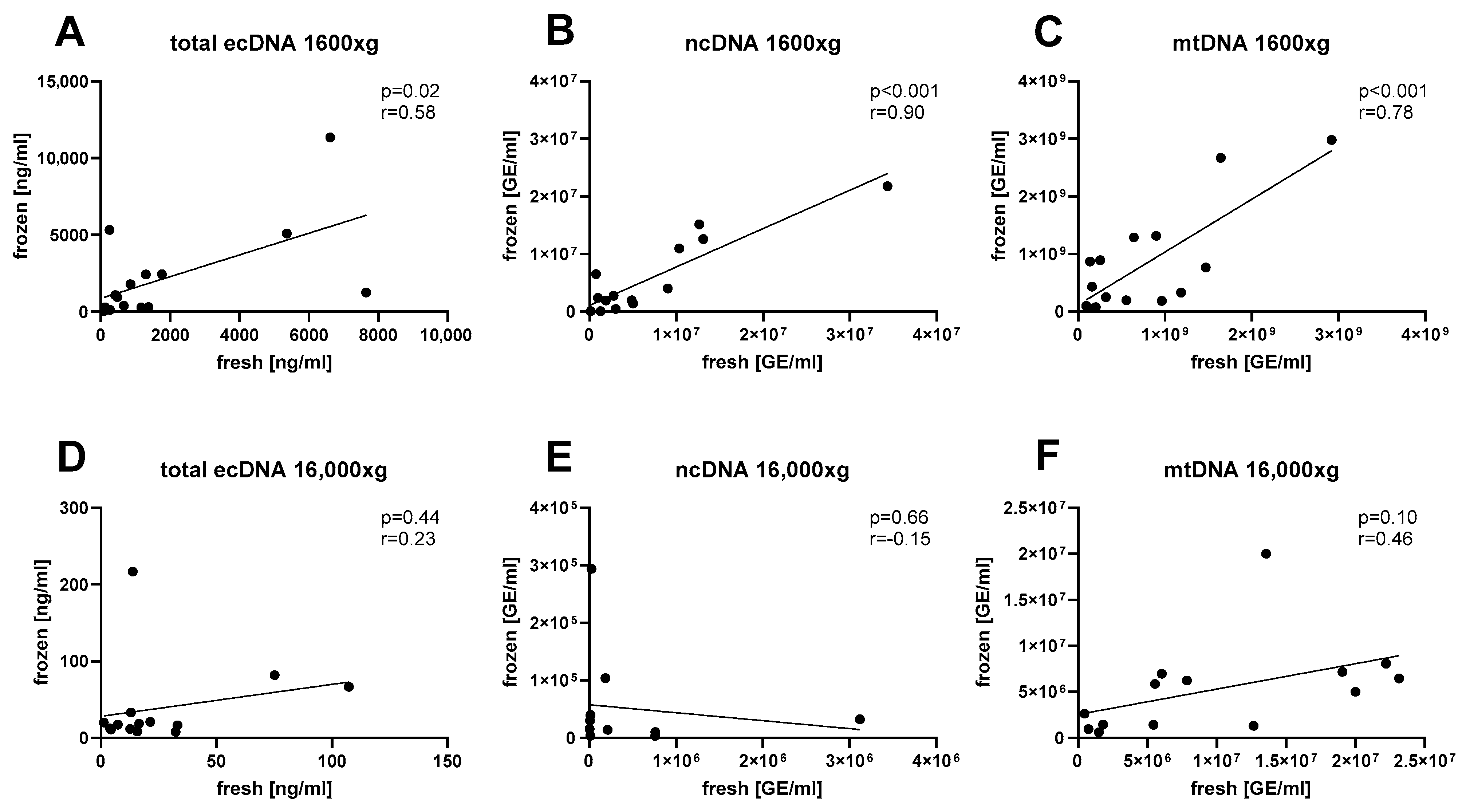
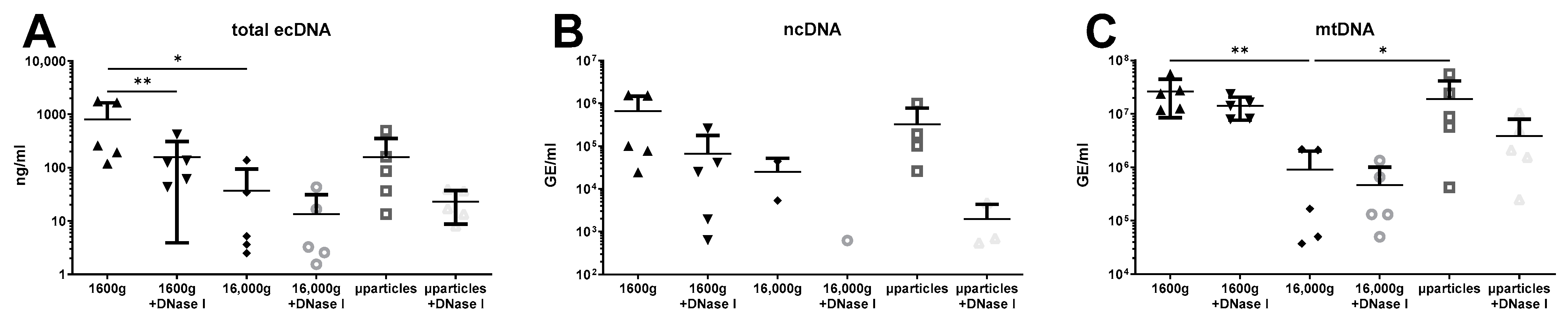
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Celec, P.; Vlková, B.; Lauková, L.; Bábíčková, J.; Boor, P. Cell-free DNA: The role in pathophysiology and as a biomarker in kidney diseases. Expert Rev. Mol. Med. 2018, 20, e1. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.D.; Han, D.S.C.; Jiang, P.; Chiu, R.W.K. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science 2021, 372, eaaw3616. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.C.; Lo, Y.M.D. Cell-free DNA and RNA in plasma as new tools for molecular diagnostics. Expert Rev. Mol. Diagn. 2003, 3, 785–797. [Google Scholar] [CrossRef]
- Lo, Y.M.D.; Chan, K.C.A.; Sun, H.; Chen, E.Z.; Jiang, P.; Lun, F.M.F.; Zheng, Y.W.; Leung, T.Y.; Lau, T.K.; Cantor, C.R.; et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci. Transl. Med. 2010, 2, 61ra91. [Google Scholar] [CrossRef]
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and death of circulating cell-free DNA. Cancer Biol. Ther. 2019, 20, 1057–1067. [Google Scholar] [CrossRef]
- Hung, E.C.; Shing, T.K.; Chim, S.S.; Yeung, P.C.; Chan, R.W.; Chik, K.W.; Lee, V.; Tsui, N.B.; Li, C.K.; Wong, C.S.; et al. Presence of donor-derived DNA and cells in the urine of sex-mismatched hematopoietic stem cell transplant recipients: Implication for the transrenal hypothesis. Clin. Chem. 2009, 55, 715–722. [Google Scholar] [CrossRef]
- Tsui, N.B.Y.; Jiang, P.; Chow, K.C.K.; Su, X.; Leung, T.Y.; Sun, H.; Chan, K.C.A.; Chiu, R.W.K.; Lo, Y.M.D. High resolution size analysis of fetal DNA in the urine of pregnant women by paired-end massively parallel sequencing. PLoS ONE 2012, 7, e48319. [Google Scholar] [CrossRef]
- McEwen, A.E.; Leary, S.E.S.; Lockwood, C.M. Beyond the Blood: CSF-Derived cfDNA for Diagnosis and Characterization of CNS Tumors. Front. Cell Dev. Biol. 2020, 8, 45. [Google Scholar] [CrossRef]
- Mair, R.; Mouliere, F. Cell-free DNA technologies for the analysis of brain cancer. Br. J. Cancer 2022, 126, 371–378. [Google Scholar] [CrossRef]
- Meddeb, R.; Pisareva, E.; Thierry, A.R. Guidelines for the Preanalytical Conditions for Analyzing Circulating Cell-Free DNA. Clin. Chem. 2019, 65, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, A.J.; Ungerer, V.; Diehl, F.; Anker, P.; Dor, Y.; Fleischhacker, M.; Gahan, P.B.; Hui, L.; Holdenrieder, S.; Thierry, A.R. Towards systematic nomenclature for cell-free DNA. Hum. Genet. 2021, 140, 565–578. [Google Scholar] [CrossRef]
- Bronkhorst, A.J.; Aucamp, J.; Pretorius, P.J. Methodological Variables in the Analysis of Cell-Free DNA. Adv. Exp. Med. Biol. 2016, 924, 157–163. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Carreras-Presas, C.M.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva diagnostics—Current views and directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef]
- Nonaka, T.; Wong, D.T. Saliva Diagnostics. Annu. Rev. Anal. Chem. 2022, 15, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Gudiseva, H.V.; Hansen, M.; Gutierrez, L.; Collins, D.W.; He, J.; Verkuil, L.D.; Danford, I.D.; Sagaser, A.; Bowman, A.S.; Salowe, R.; et al. Saliva DNA quality and genotyping efficiency in a predominantly elderly population. BMC Med. Genom. 2016, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Koni, A.C.; Scott, R.A.; Wang, G.; Bailey, M.E.; Peplies, J.; Bammann, K.; Pitsiladis, Y.P. DNA yield and quality of saliva samples and suitability for large-scale epidemiological studies in children. Int. J. Obes. 2011, 35 (Suppl. 1), S113–S118. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.R.; Chapleau, R.R. Comparing DNA quantity and quality using saliva collection following food and beverage consumption. BMC Res. Notes 2019, 12, 165. [Google Scholar] [CrossRef]
- Swarup, N.; Cheng, J.; Choi, I.; Heo, Y.J.; Kordi, M.; Aziz, M.; Arora, A.; Li, F.; Chia, D.; Wei, F.; et al. Multi-faceted attributes of salivary cell-free DNA as liquid biopsy biomarkers for gastric cancer detection. Biomark. Res. 2023, 11, 90. [Google Scholar] [CrossRef]
- Brooks, P.J.; Malkin, E.Z.; De Michino, S.; Bratman, S.V. Isolation of salivary cell-free DNA for cancer detection. PLoS ONE 2023, 18, e0285214. [Google Scholar] [CrossRef]
- Yao, W.; Mei, C.; Nan, X.; Hui, L. Evaluation and comparison of in vitro degradation kinetics of DNA in serum, urine and saliva: A qualitative study. Gene 2016, 590, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Rapado-González, Ó.; López-Cedrún, J.L.; Lago-Lestón, R.M.; Abalo, A.; Rubin-Roger, G.; Salgado-Barreira, Á.; López-López, R.; Muinelo-Romay, L.; Suárez-Cunqueiro, M.M. Integrity and quantity of salivary cell-free DNA as a potential molecular biomarker in oral cancer: A preliminary study. J. Oral Pathol. Med. 2022, 51, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Sayal, L.; Hamadah, O.; Almasri, A.; Idrees, M.; Thomson, P.; Kujan, O. Saliva-based cell-free DNA and cell-free mitochondrial DNA in head and neck cancers have promising screening and early detection role. J. Oral Pathol. Med. 2023, 52, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sayal, L.; Hamadah, O.; AlMasri, A.; Idrees, M.; Kassem, I.; Habbal, W.; Alsalamah, B.; Kujan, O. Salivary-Based Cell-Free Mitochondrial DNA Level Is an Independent Prognostic Biomarker for Patients with Head and Neck Squamous Cell Carcinoma. J. Pers. Med. 2023, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, V.; Sharma, S.; Deshpande, A.; Zhou, H.; Gimzewski, J.; Wong, D.T. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS ONE 2010, 5, e8577. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S.; Gauley, J.; Ullal, A.J. Microparticles as a source of extracellular DNA. Immunol. Res. 2011, 49, 227–234. [Google Scholar] [CrossRef]
- Sorber, L.; Zwaenepoel, K.; Jacobs, J.; De Winne, K.; Goethals, S.; Reclusa, P.; Van Casteren, K.; Augustus, E.; Lardon, F.; Roeyen, G.; et al. Circulating Cell-Free DNA and RNA Analysis as Liquid Biopsy: Optimal Centrifugation Protocol. Cancers 2019, 11, 458. [Google Scholar] [CrossRef]
- Shin, K.H.; Lee, S.M.; Park, K.; Choi, H.; Kim, I.S.; Yoon, S.H.; Oh, S.H. Effects of Different Centrifugation Protocols on the Detection of EGFR Mutations in Plasma Cell-Free DNA. Am. J. Clin. Pathol. 2022, 158, 206–211. [Google Scholar] [CrossRef]
- Cox, D.R.A.; Wong, B.K.L.; Yang, L.; Yoshino, O.; Testro, A.; Muralidharan, V.; Dobrovic, A. High Speed Centrifugation Before Frozen Storage of Plasma Is Critical for Quantitative Analysis of Mitochondrial-Derived Cell-Free DNA. Clin. Chem. 2020, 66, 1111–1114. [Google Scholar] [CrossRef]
- Trumpff, C.; Rausser, S.; Haahr, R.; Karan, K.R.; Gouspillou, G.; Puterman, E.; Kirschbaum, C.; Picard, M. Dynamic behavior of cell-free mitochondrial DNA in human saliva. Psychoneuroendocrinology 2022, 143, 105852. [Google Scholar] [CrossRef]
- Schneyer, L.H. Coagulation of salivary mucoid by freezing and thawing of saliva. Proc. Soc. Exp. Biol. Med. 1956, 91, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, H.; Takayasu, L.; Isshi, K.; Hara, Y.; Ono, S.; Kato, M.; Sumiyama, K.; Suda, W. Effect of storage temperature and flash-freezing on salivary microbiota profiles based on 16S rRNA-targeted sequencing. Eur. J. Oral Sci. 2022, 130, e12852. [Google Scholar] [CrossRef] [PubMed]
- Garbieri, T.F.; Brozoski, D.T.; Dionísio, T.J.; Santos, C.F.; Neves, L.T. Human DNA extraction from whole saliva that was fresh or stored for 3, 6 or 12 months using five different protocols. J. Appl. Oral Sci. 2017, 25, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Meddeb, R.; Dache, Z.A.A.; Thezenas, S.; Otandault, A.; Tanos, R.; Pastor, B.; Sanchez, C.; Azzi, J.; Tousch, G.; Azan, S.; et al. Quantifying circulating cell-free DNA in humans. Sci. Rep. 2019, 9, 5220. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Wolkowitz, O.M.; Picard, M.; Ohlsson, L.; Bersani, F.S.; Fernström, J.; Westrin, Å.; Hough, C.M.; Lin, J.; Reus, V.I.; et al. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology 2018, 43, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Srivastava, S.; Singh, S.A.; Das, A.K.; Das, G.C.; Dhar, B.; Ghosh, S.K.; Mondal, R. Cell-free mitochondrial DNA copy number variation in head and neck squamous cell carcinoma: A study of non-invasive biomarker from Northeast India. Tumour Biol. 2017, 39, 1010428317736643. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ma, M.L.; Chan, R.W.Y.; Lam, W.K.J.; Peng, W.; Gai, W.; Hu, X.; Ding, S.C.; Ji, L.; Zhou, Q.; et al. Fragmentation landscape of cell-free DNA revealed by deconvolutional analysis of end motifs. Proc. Natl. Acad. Sci. USA 2023, 120, e2220982120. [Google Scholar] [CrossRef]
- Bhati, A.; Varghese, A.; Rajan, G.; Sridhar, V.; Mohan, Y.; Pradeep, S.; Babu, S.; Kaikkolante, N.; Sarma, M.; Arun, S.; et al. An effective method for saliva stabilization and magnetic nanoparticles based DNA extraction for genomic applications. Anal. Biochem. 2021, 624, 114182. [Google Scholar] [CrossRef]
- Han, D.S.C.; Ni, M.; Chan, R.W.Y.; Chan, V.W.H.; Lui, K.O.; Chiu, R.W.K.; Lo, Y.M.D. The Biology of Cell-free DNA Fragmentation and the Roles of DNASE1, DNASE1L3, and DFFB. Am. J. Hum. Genet. 2020, 106, 202–214. [Google Scholar] [CrossRef]
- Vlková, B.; Szemes, T.; Minárik, G.; Turna, J.; Celec, P. Does maternal saliva contain fetal DNA usable for prenatal diagnostics? Med. Hypotheses 2010, 74, 258–260. [Google Scholar] [CrossRef]
- Kamodyová, N.; Durdiaková, J.; Celec, P.; Sedláčková, T.; Repiská, G.; Sviežená, B.; Minárik, G. Prevalence and persistence of male DNA identified in mixed saliva samples after intense kissing. Forensic Sci. Int. Genet. 2013, 7, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Silva, L.M.; Theofilou, V.I.; Greenwell-Wild, T.; Li, L.; Williams, D.W.; Ikeuchi, T.; Brenchley, L.; Bugge, T.H.; Diaz, P.I.; et al. Neutrophil extracellular traps and extracellular histones potentiate IL-17 inflammation in periodontitis. J. Exp. Med. 2023, 220, e20221751. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chu, C.J.; Pan, W.; Li, Y.; Huang, H.; Zhao, L. The Correlation between Periodontal Parameters and Cell-Free DNA in the Gingival Crevicular Fluid, Saliva, and Plasma in Chinese Patients: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 6902. [Google Scholar] [CrossRef]
- Taki, T.; Kibayashi, K. Characterization of cellular and extracellular DNA in saliva. Leg. Med. 2015, 17, 471–474. [Google Scholar] [CrossRef]
| Fresh | Frozen | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1600× g | 16,000× g | 1600× g | 16,000× g | |||||||||||
| Total ecDNA | ncDNA | mtDNA | Total ecDNA | ncDNA | mtDNA | Total ecDNA | ncDNA | mtDNA | Total ecDNA | ncDNA | mtDNA | |||
| fresh | 1600× g | total ecDNA | ||||||||||||
| ncDNA | 0.89 | |||||||||||||
| *** | ||||||||||||||
| mtDNA | 0.68 | 0.61 | ||||||||||||
| ** | * | |||||||||||||
| 16,000× g | total ecDNA | 0.48 | 0.64 | 0.68 | ||||||||||
| ** | ** | |||||||||||||
| ncDNA | −0.10 | −0.07 | 0.16 | 0.06 | ||||||||||
| mtDNA | 0.47 | 0.63 | 0.68 | 1.00 | 0.20 | |||||||||
| ** | ** | *** | ||||||||||||
| frozen | 1600× g | total ecDNA | 0.64 | 0.48 | 0.76 | 0.32 | −0.14 | 0.32 | ||||||
| ** | *** | |||||||||||||
| ncDNA | 0.86 | 0.90 | 0.81 | 0.67 | −0.03 | 0.67 | 0.84 | |||||||
| *** | *** | *** | ** | ** | *** | |||||||||
| mtDNA | 0.45 | 0.40 | 0.78 | 0.35 | −0.16 | 0.35 | 0.79 | 0.62 | ||||||
| *** | *** | * | ||||||||||||
| 16,000× g | total ecDNA | 0.51 | 0.66 | 0.71 | 1.00 | −0.09 | 0.99 | 0.36 | 0.70 | 0.39 | ||||
| * | ** | ** | *** | *** | ** | |||||||||
| ncDNA | 0.50 | 0.65 | 0.70 | 1.00 | −0.15 | 0.99 | 0.34 | 0.69 | 0.37 | 1.00 | ||||
| ** | ** | *** | *** | ** | *** | |||||||||
| mtDNA | 0.49 | 0.65 | 0.69 | 1.00 | −0.09 | 0.99 | 0.34 | 0.69 | 0.37 | 1.00 | 1.00 | |||
| ** | ** | *** | *** | ** | *** | *** | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janovičová, Ľ.; Holániová, D.; Vlková, B.; Celec, P. Pre-Analytical Factors Affecting Extracellular DNA in Saliva. Diagnostics 2024, 14, 249. https://doi.org/10.3390/diagnostics14030249
Janovičová Ľ, Holániová D, Vlková B, Celec P. Pre-Analytical Factors Affecting Extracellular DNA in Saliva. Diagnostics. 2024; 14(3):249. https://doi.org/10.3390/diagnostics14030249
Chicago/Turabian StyleJanovičová, Ľubica, Dominika Holániová, Barbora Vlková, and Peter Celec. 2024. "Pre-Analytical Factors Affecting Extracellular DNA in Saliva" Diagnostics 14, no. 3: 249. https://doi.org/10.3390/diagnostics14030249
APA StyleJanovičová, Ľ., Holániová, D., Vlková, B., & Celec, P. (2024). Pre-Analytical Factors Affecting Extracellular DNA in Saliva. Diagnostics, 14(3), 249. https://doi.org/10.3390/diagnostics14030249






