The Applicability and Limitations of the Spectrofluorometric Method for Determination of ALDH1 Activity in Serum and Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
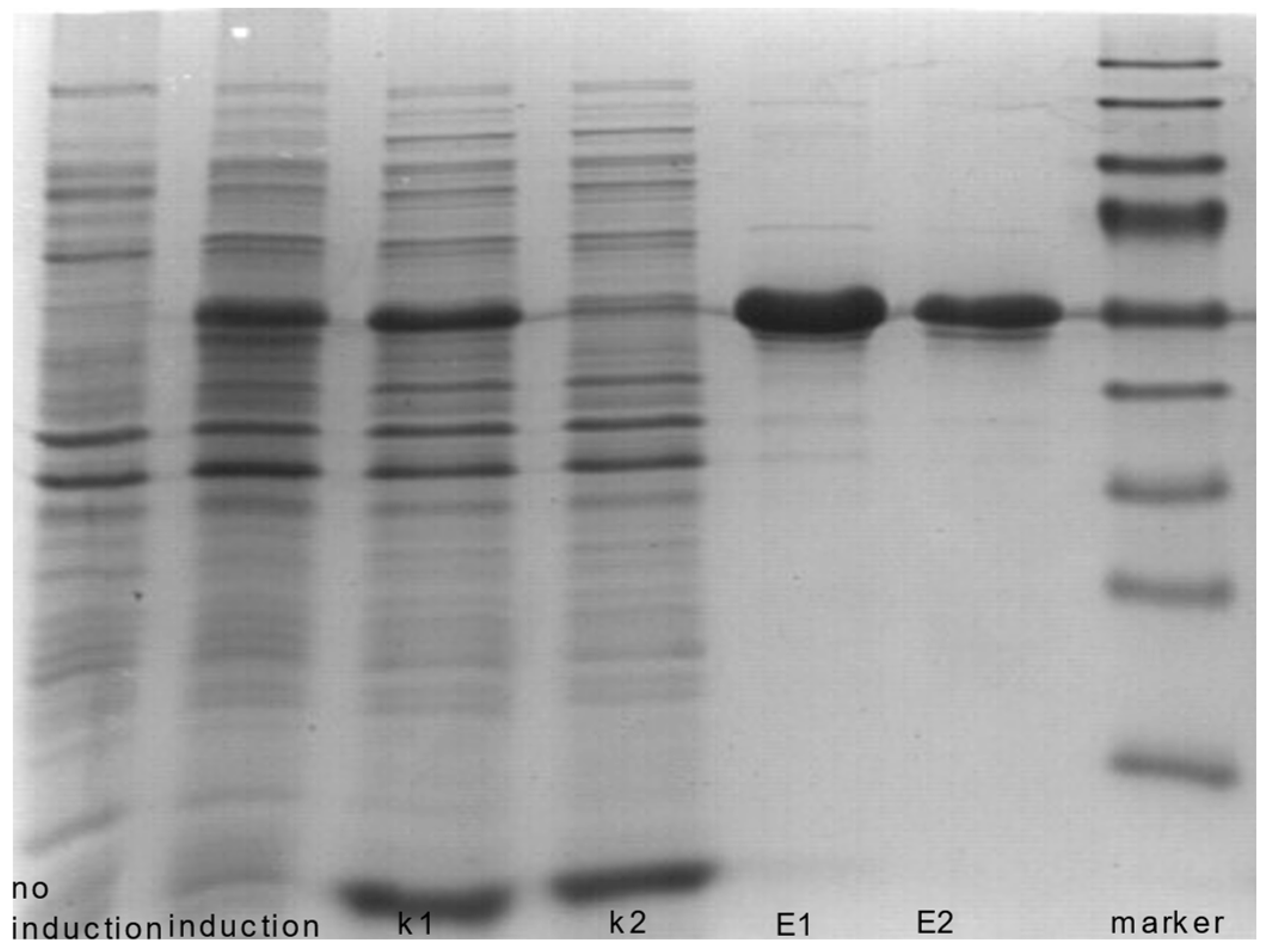
2.2. Recombinant ALDH1A1
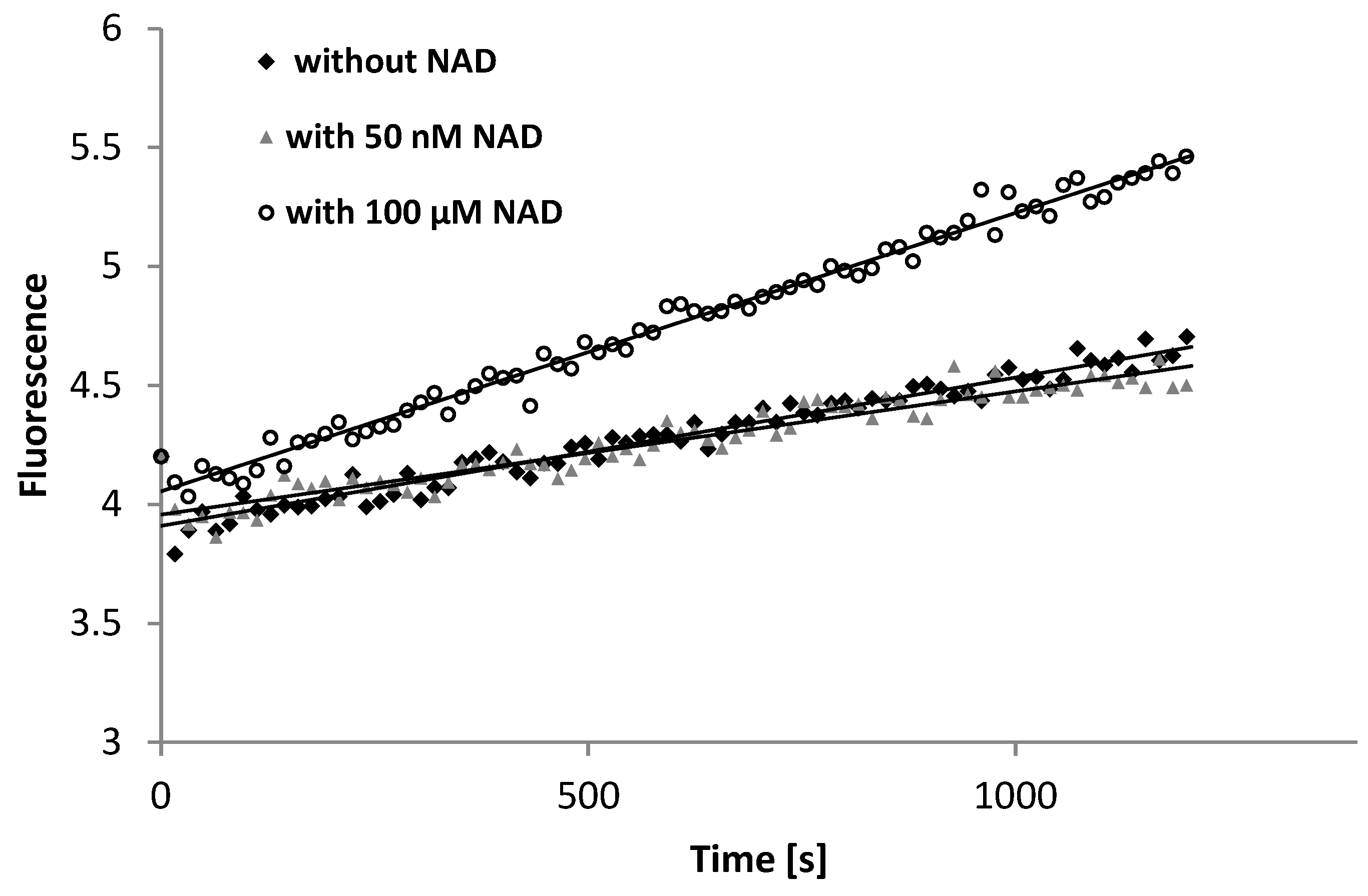
2.3. Spectrofluorometric Method of ALDH1 Determination
2.4. Chromatographic Method of ALDH1 Determination
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xanthis, V.; Mantso, T.; Dimtsi, A.; Pappa, A.; Fadouloglou, V.E. Human Aldehyde Dehydrogenases: A Superfamily of Similar Yet Different Proteins Highly Related to Cancer. Cancers 2023, 15, 4419. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Li, S.; Liu, S.; Zhang, L. Aldehyde dehydrogenase in solid tumors and other diseases: Potential biomarkers and therapeutic targets. MedComm 2023, 4, e195. [Google Scholar] [CrossRef] [PubMed]
- Seike, T.; Chen, C.-H.; Mochly-Rosen, D. Impact of common ALDH2 inactivating mutation and alcohol consumption on Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1223977. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Zhao, X.; Pang, J.; Pan, C.; Wang, J.; Wei, S.; Yu, X.; Zhang, C.; Chen, Y. The role of aldehyde dehydrogenase 2 in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 495–509. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yang, L.-F.; Lai, C.-L.; Yin, S.-J. Acetaldehyde Enhances Alcohol Sensitivity and Protects against Alcoholism: Evidence from Alcohol Metabolism in Subjects with Variant ALDH2*2 Gene Allele. Biomolecules 2021, 11, 1183. [Google Scholar] [CrossRef]
- Zygula, A.; Kosinski, P.; Wroczynski, P.; Makarewicz-Wujec, M.; Pietrzak, B.; Wielgos, M.; Giebultowicz, J. Oxidative Stress Markers Differ in Two Placental Dysfunction Pathologies: Pregnancy-Induced Hypertension and Intrauterine Growth Restriction. Oxid. Med. Cell. Longev. 2020, 2020, 1323891. [Google Scholar] [CrossRef]
- Le, N.T.A.; Nguyen, H.L.T.; Trinh, B.N.; Dang, H.H. Sjögren–Larsson syndrome caused by novel mutations in ALDH3A2 gene. Int. J. Dermatol. 2023, 62, e2–e4. [Google Scholar] [CrossRef]
- Berdyński, M.; Krawczyk, P.; Safranow, K.; Borzemska, B.; Szaflik, J.P.; Nowakowska-Żawrocka, K.; Żekanowski, C.; Giebułtowicz, J. Common ALDH3A1 Gene Variant Associated with Keratoconus Risk in the Polish Population. J. Clin. Med. 2021, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Liu, Y.; Xie, Y. Association between ALDH2 polymorphisms and the risk of diabetes mellitus in hypertensive patients. Int. J. Gen. Med. 2023, 16, 5719–5727. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, W.; Pan, T.; Xie, J.; Xu, Z.; Fang, Y. ALDH2 rs671 polymorphism likely a risk factor for hemorrhagic stroke: A hospital-based study. Int. J. Gen. Med. 2023, 16, 1471–1478. [Google Scholar] [CrossRef]
- Cai, N.; Li, C.; Gu, X.; Zeng, W.; Liu, J.; Zeng, G.; Zhong, J.; Zhu, J.; Hong, H. ALDH2 rs671 and MTHFR rs1801133 polymorphisms are risk factors for arteriosclerosis in multiple arteries. BMC Cardiovasc. Disord. 2023, 23, 319. [Google Scholar] [CrossRef] [PubMed]
- Motte, J.; Fisse, A.L.; Grüter, T.; Schneider, R.; Breuer, T.; Lücke, T.; Krueger, S.; Nguyen, H.P.; Gold, R.; Ayzenberg, I.; et al. Novel variants in a patient with late-onset hyperprolinemia type II: Diagnostic key for status epilepticus and lactic acidosis. BMC Neurol. 2019, 19, 345. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, F.; Wang, H.; Lin, C.; Fan, Y.; Zhang, X.; Qian, H.; Xu, B. Stem Cell Marker Aldehyde Dehydrogenase 1 (ALDH1)-Expressing Cells are Enriched in Triple-Negative Breast Cancer. Int. J. Biol. Markers 2013, 28, 357–364. [Google Scholar] [CrossRef]
- Li, D.; Cao, Y.; Luo, C.-W.; Zhang, L.-P.; Zou, Y.-B. The clinical significance and prognostic value of ALDH1 expression in non-small cell lung cancer: A systematic review and meta-analysis. Recent. Pat. Anti-Cancer Drug Discov. 2024, 19, 599–609. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Wroczyński, P.; Samolczyk-Wanyura, D. Can lower aldehyde dehydrogenase activity in saliva be a risk factor for oral cavity cancer? Oral Dis. 2013, 19, 763–766. [Google Scholar] [CrossRef]
- Püschel, J.; Dubrovska, A.; Gorodetska, I. The Multifaceted Role of Aldehyde Dehydrogenases in Prostate Cancer Stem Cells. Cancers 2021, 13, 4703. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Song, Y.; Liu, S.; Peng, C.; Zhang, Y. Mechanisms underlying the changes in acetaldehyde dehydrogenase 1 in cholangiocarcinoma. J. Cancer 2023, 14, 3203–3213. [Google Scholar] [CrossRef]
- Wang, Z.; Mo, Y.; Tan, Y.; Wen, Z.; Dai, Z.; Zhang, H.; Zhang, X.; Feng, S.; Liang, X.; Song, T.; et al. The ALDH Family Contributes to Immunocyte Infiltration, Proliferation and Epithelial-Mesenchymal Transformation in Glioma. Front. Immunol. 2022, 12, 756606. [Google Scholar] [CrossRef]
- Zhou, L.; Bai, L.; Zhu, H.; Guo, C.; Liu, S.; Yin, L.; Sun, J. Establishing nomograms for predicting disease-free survival and overall survival in patients with breast cancer. J. Obstet. Gynaecol. 2024, 44, 2361435. [Google Scholar] [CrossRef]
- Gorodetska, I.; Offermann, A.; Püschel, J.; Lukiyanchuk, V.; Gaete, D.; Kurzyukova, A.; Freytag, V.; Haider, M.T.; Fjeldbo, C.S.; Di Gaetano, S.; et al. ALDH1A1 drives prostate cancer metastases and radioresistance by interplay with AR- and RAR-dependent transcription. Theranostics 2024, 14, 714–737. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, B.; Yuan, X. Expression of ALDH1 plays the important role during generation and progression in human cervical cancer. Biotechnol. Genet. Eng. Rev. 2022, 40, 3768–3779. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.A.G.; Dos Santos, O.P.; Saddi, V.A.; Pereira, J.X.; da Costa Machado, H.; Santos Carneiro, M.A.; de Paula, H.M.; Figueiredo-Alves, R.R.; Zeferino, L.C.; Rabelo-Santos, S.H. Association of CD133, ALDH1, CD117 and OCT4 expression with prognosis of patients with cervical cancer. Virchows Arch. 2024, 1–11. [Google Scholar] [CrossRef]
- Fahmi, M.N.; Kusuma, F.; Hellyanti, T.; Kekalih, A.; Sekarutami, S.M.; Nurrana, L.; Purwoto, G.; Anggraeni, T.D. High ALDH-1 Expression Predicts Non-Complete Response of Radiotherapy in Stage III Squamous Cell Cervical Carcinoma Patients. Asian Pac. J. Cancer Prev. 2023, 24, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Jelski, W.; Szmitkowski, M. Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) in the cancer diseases. Clin. Chim. Acta 2008, 395, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.-L.; Prince, G.M.S.H.; Batzorig, U.; Huang, C.-Y.; Chang, Y.-J. ALDH2 promotes cancer stemness and metastasis in colorectal cancer through activating β-catenin signaling. J. Cell. Biochem. 2023, 124, 907–920. [Google Scholar] [CrossRef]
- Markowska, A.; Kojs, Z.; Twardawa, D.; Pietras, J.; Markowska, J. Selected markers of ovarian cancer and their relation to targeted therapy (Review). Exp. Ther. Med. 2024, 27, 236. [Google Scholar] [CrossRef]
- Izycka, N.; Rucinski, M.; Andrzejewska, M.; Szubert, S.; Nowak-Markwitz, E.; Sterzynska, K. The Prognostic Value of Cancer Stem Cell Markers (CSCs) Expression—ALDH1A1, CD133, CD44—For Survival and Long-Term Follow-Up of Ovarian Cancer Patients. Int. J. Mol. Sci. 2023, 24, 2400. [Google Scholar] [CrossRef]
- Mochizuki-Kashio, M.; Shiozaki, H.; Suda, T.; Nakamura-Ishizu, A. Mitochondria turnover and lysosomal function in hematopoietic stem cell metabolism. Int. J. Mol. Sci. 2021, 22, 4627. [Google Scholar] [CrossRef]
- Anastasiou, V.; Ninou, E.; Alexopoulou, D.; Stertmann, J.; Müller, A.; Dahl, A.; Solimena, M.; Speier, S.; Serafimidis, I.; Gavalas, A. Aldehyde dehydrogenase activity is necessary for beta cell development and functionality in mice. Diabetologia 2016, 59, 139–150. [Google Scholar] [CrossRef]
- Honeth, G.; Lombardi, S.; Ginestier, C.; Hur, M.; Marlow, R.; Buchupalli, B.; Shinomiya, I.; Gazinska, P.; Bombelli, S.; Ramalingam, V. Aldehyde dehydrogenase and estrogen receptor define a hierarchy of cellular differentiation in the normal human mammary epithelium. Breast Cancer Res. 2014, 16, R52. [Google Scholar] [CrossRef]
- Serio, R.N.; Laursen, K.B.; Urvalek, A.M.; Gross, S.S.; Gudas, L.J. Ethanol promotes differentiation of embryonic stem cells through retinoic acid receptor-γ. J. Biol. Chem. 2019, 294, 5536–5548. [Google Scholar] [CrossRef] [PubMed]
- Dancik, G.M.; Varisli, L.; Vlahopoulos, S.A. The Molecular Context of Oxidant Stress Response in Cancer Establishes ALDH1A1 as a Critical Target: What This Means for Acute Myeloid Leukemia. Int. J. Mol. Sci. 2023, 24, 9372. [Google Scholar] [CrossRef] [PubMed]
- Mei, B.; Li, J.; He, S.; Wang, X.; Huang, J.; Zhang, G. 324P All-trans retinoic acid sensitizes ovarian cancer to niraparib by inhibiting ALDH1A1 activity. Ann. Oncol. 2023, 34, S1597. [Google Scholar] [CrossRef]
- Bu, J.; Zhang, Y.; Wu, S.; Li, H.; Sun, L.; Liu, Y.; Zhu, X.; Qiao, X.; Ma, Q.; Liu, C.; et al. KK-LC-1 as a therapeutic target to eliminate ALDH+ stem cells in triple negative breast cancer. Nat. Commun. 2023, 14, 2602. [Google Scholar] [CrossRef] [PubMed]
- Helander, A. Aldehyde dehydrogenase in blood: Distribution, characteristics and possible use as marker of alcohol misuse. Alcohol. Alcohol. 1993, 28, 135–145. [Google Scholar]
- Wierzchowski, J.; Interewicz, E.; Wroczynski, P.; Orlanska, I. Continuous fluorimetric assay for human aldehyde dehydrogenase and its application to blood analysis. Anal. Chim. Acta 1996, 319, 209–219. [Google Scholar] [CrossRef]
- Wroczyński, P.; Kwolek, A.; Barut-Cichocka, J.; Wierzchowski, J.; Bobilewicz, D. Badanie aktywności dehydrogenazy aldehydowej metodą fluorymetryczną. Diagn. Lab. 2000, 36, 281–295. [Google Scholar]
- Jelski, W.; Orywal, K.; Laniewska, M.; Szmitkowski, M. The diagnostic value of alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) measurement in the sera of gastric cancer patients. Clin. Exp. Med. 2010, 10, 215–219. [Google Scholar] [CrossRef]
- Orywal, K.; Jelski, W.; Werel, T.; Szmitkowski, M. The diagnostic significance of serum alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase activity in renal cell cancer patients. Exp. Mol. Pathol. 2016, 100, 416–420. [Google Scholar] [CrossRef]
- Orywal, K.; Jelski, W.; Werel, T.; Szmitkowski, M. The alterations in alcohol dehydrogenase and aldehyde dehydrogenase activities in the sera of patients with renal cell carcinoma. Adv. Med. Sci. 2018, 63, 1–4. [Google Scholar] [CrossRef]
- Orywal, K.; Jelski, W.; Werel, T.; Szmitkowski, M. The diagnostic significance of serum alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase activity in prostate cancer patients. Anticancer Res. 2017, 37, 4961–4965. [Google Scholar] [PubMed]
- Giebułtowicz, J.; Dziadek, M.; Wroczyński, P.; Woznicka, K.; Wojno, B.; Pietrzak, M.; Wierzchowski, J. Salivary aldehyde dehydrogenase—Temporal and population variability, correlations with drinking and smoking habits and activity towards aldehydes contained in food. Acta Biochim. Pol. 2010, 57, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Billington, R.A.; Bruzzone, S.; De Flora, A.; Genazzani, A.A.; Koch-Nolte, F.; Ziegler, M.; Zocchi, E. Emerging functions of extracellular pyridine nucleotides. Mol. Med. (Camb. Mass.) 2006, 12, 324–327. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, T.; Niven, D.F. Levels of nicotinamide adenine dinucleotide in extracellular body fluids of pigs may be growth-limiting for Actinobacillus pleuropneumoniae and Haemophilus parasuis. Can. J. Vet. Res. Rev. Can. Rech. Vet. 2003, 67, 229–231. [Google Scholar]
- Zocchi, E.; Usai, C.; Guida, L.; Franco, L.; Bruzzone, S.; Passalacqua, M.; de Flora, A. Ligand-induced internalization of CD38 results in intracellular Ca2+ mobilization: Role of NAD+ transport across cell membranes. FASEB J. 1999, 13, 273–283. [Google Scholar] [CrossRef]
- Guru, S.C.; Taranath Shetty, K. Methodological aspects of aldehyde dehydrogenase assay by spectrophotometric technique. Alcohol 1990, 7, 397–401. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Wolinowska, R.; Sztybor, A.; Pietrzak, M.; Wroczyński, P.; Wierzchowski, J. Salivary Aldehyde Dehydrogenase: Activity towards Aromatic Aldehydes and Comparison with Recombinant ALDH3A1. Molecules 2009, 14, 2363–2372. [Google Scholar] [CrossRef]
- Anderle, H.; Weber, A. Fluorescent rare earth solutions as intrinsic wavelength standards for protein fluorescence spectroscopy. Anal. Biochem. 2017, 518, 86–88. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michorowska, S.; Wiśniewska, A.; Wolinowska, R.; Wroczyński, P.; Giebułtowicz, J. The Applicability and Limitations of the Spectrofluorometric Method for Determination of ALDH1 Activity in Serum and Plasma. Diagnostics 2024, 14, 2721. https://doi.org/10.3390/diagnostics14232721
Michorowska S, Wiśniewska A, Wolinowska R, Wroczyński P, Giebułtowicz J. The Applicability and Limitations of the Spectrofluorometric Method for Determination of ALDH1 Activity in Serum and Plasma. Diagnostics. 2024; 14(23):2721. https://doi.org/10.3390/diagnostics14232721
Chicago/Turabian StyleMichorowska, Sylwia, Agnieszka Wiśniewska, Renata Wolinowska, Piotr Wroczyński, and Joanna Giebułtowicz. 2024. "The Applicability and Limitations of the Spectrofluorometric Method for Determination of ALDH1 Activity in Serum and Plasma" Diagnostics 14, no. 23: 2721. https://doi.org/10.3390/diagnostics14232721
APA StyleMichorowska, S., Wiśniewska, A., Wolinowska, R., Wroczyński, P., & Giebułtowicz, J. (2024). The Applicability and Limitations of the Spectrofluorometric Method for Determination of ALDH1 Activity in Serum and Plasma. Diagnostics, 14(23), 2721. https://doi.org/10.3390/diagnostics14232721




