Somatostatin Receptor Imaging in the Diagnosis and Management of Parathyroid Neuroendocrine Neoplasia
Abstract
1. Introduction
2. Materials and Methods
3. Results
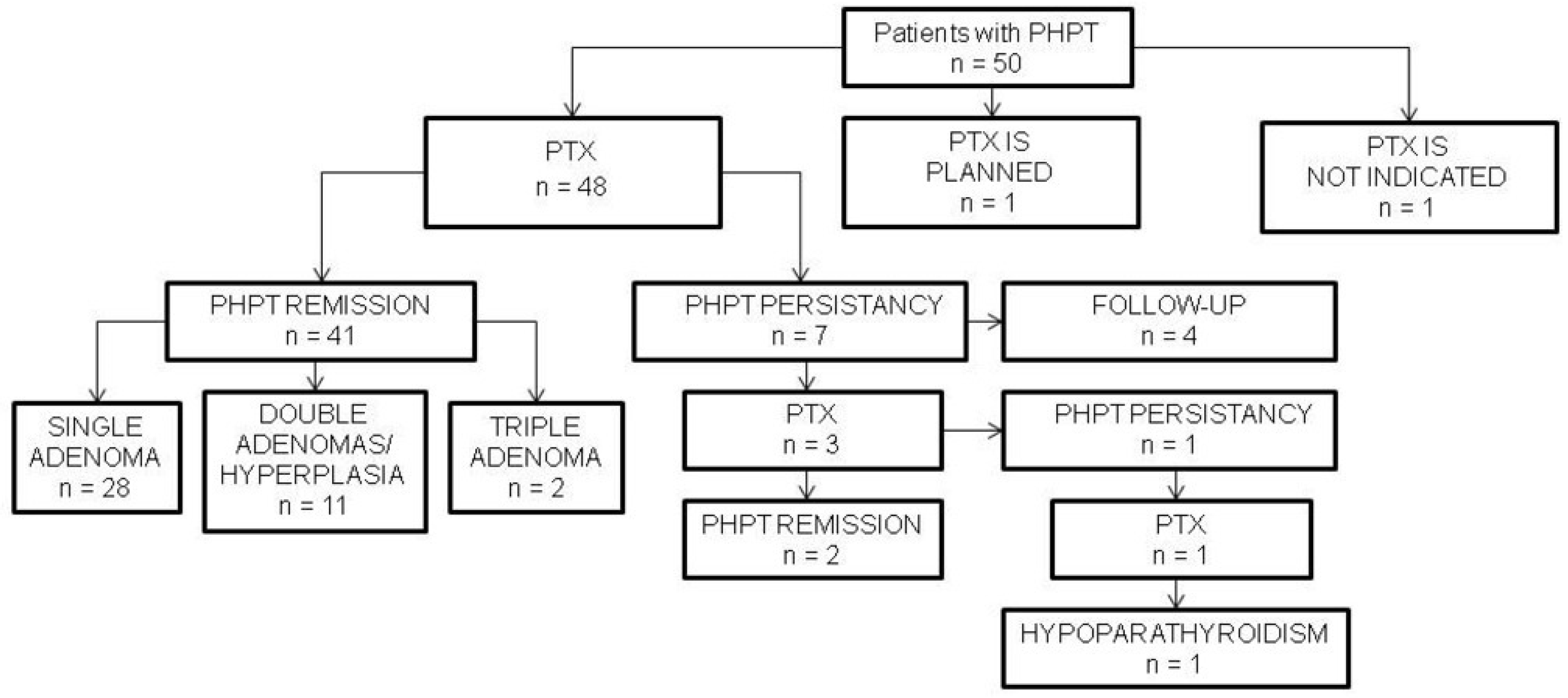
3.1. Patients’ Clinical Characteristics
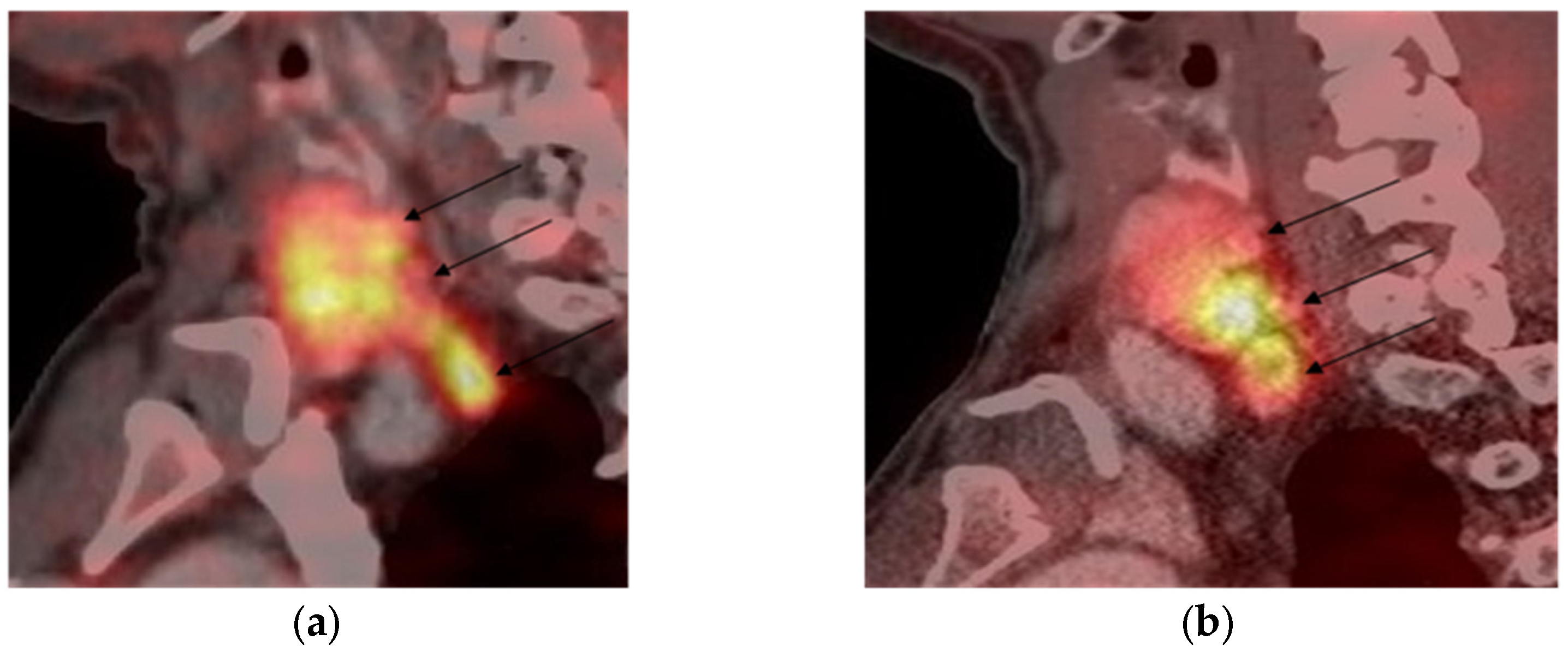
3.2. [68Ga]-DOTA-Peptide PET/CT Results
3.3. SST2 and SST5 Staining Results
3.4. First-Generation Somatostatin Analogs’ Effects on Calcium and Parathyroid Hormone Values in PHPT Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Patient № * | Gender, Age 1 | PHPT Status | Other MEN1 Manifestations |
|---|---|---|---|
| 1 | M, 51 y.o. | remission after subtotal PTX | multiple duodenal and non-functioning pancreatic NETs, chemodectoma, non-functioning adrenal adenoma |
| 2 | F, 38 y.o. | recurrence after subtotal PTX | prolactinoma, insulinoma, multipal doudenal and non-functioning pancreatic NETs, non-functioning adrenal adenoma |
| 3 | F, 41 y.o. | recurrence after subtotal PTX | prolactinoma, multiple doudenal and non-functioning pancreatic NETs |
| 4 | F, 36 y.o. | remission after subtotal PTX | prolactinoma, multiple doudenal and non-functioning pancreatic NETs |
| 5 | F, 55 y.o. | hypoparathyroidism after subtotal PTX | non-functioning pituitary adenoma, multiple non-functioning pancreatic NETs |
| 6 | M, 53 y.o. | remission after subtotal PTX | duodenal gastrinoma, multiple non-functioning pancreatic, gastric NETs, non-functioning adrenal adenoma |
| 7 | F, 21 y.o. | remission after subtotal PTX | prolactinoma, multiple non-functioning pancreatic NETs |
| 8 | M, 31 y.o. | recurrence after 1 adenoma removal | prolactinoma, multiple non-functioning pancreatic insulinoma, non-functioning adrenal adenomas |
| 9 | M, 61 y.o. | recurrence after 1 adenoma removal | non-functioning pancreatic NETs, non-functioning adrenal adenomas |
| 10 | M, 66 y.o. | remission after subtotal PTX | prolactinoma, multiple non-functioning pancreatic NETs |
References
- Rindi, G.; Inzani, F. Neuroendocrine neoplasm update: Toward universal nomenclature. Endocr. Relat. Cancer 2020, 27, R211–R218. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Hanley, D.A.; Rizzoli, R.; Bollerslev, J.; Young, J.E.; Rejnmark, L.; Thakker, R.; D’Amour, P.; Paul, T.; Van Uum, S.; et al. Primary hyperparathyroidism: Review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos. Int. 2017, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gokozan, H.N.; Scognamiglio, T. Advances and updates in parathyroid pathology. Adv. Anat. Pathol. 2023, 30, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Guilmette, J.; Sadow, P.M. Parathyroid pathology. Surg. Pathol. Clin. 2019, 12, 1007–1019. [Google Scholar] [CrossRef]
- Wermers, R.A. Incidence of primary hyperparathyroidism in the current era: Have we finally reached a steady state? J. Clin. Endocrinol. Metab. 2023, 108, e1749–e1750. [Google Scholar] [CrossRef]
- Clifton-Bligh, P.B.; Nery, M.L.; Supramaniam, R.; Reeve, T.S.; Delbridge, L.; Stiel, J.N.; McElduff, A.; Wilmshurst, E.G.; Robinson, B.G.; Fulcher, G.R.; et al. Mortality associated with primary hyperparathyroidism. Bone 2015, 74, 121–124. [Google Scholar] [CrossRef]
- Silva, B.C.; Cusano, N.E.; Bilezikian, J.P. Primary hyperparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2024, 38, 101247. [Google Scholar] [CrossRef]
- English, K.A.; Lines, K.E.; Thakker, R.V. Genetics of hereditary forms of primary hyperparathyroidism. Hormones 2024, 23, 3–14. [Google Scholar] [CrossRef]
- Jha, S.; Simonds, W.F. Molecular and clinical spectrum of primary hyperparathyroidism. Endocr. Rev. 2023, 44, 779–818. [Google Scholar] [CrossRef]
- Simonds, W.F. Clinical and molecular genetics of primary hyperparathyroidism. Horm. Metab. Res. 2020, 52, 578–587. [Google Scholar] [CrossRef]
- Brandi, M.L.; Agarwal, S.K.; Perrier, N.D.; Lines, K.E.; Valk, G.D.; Thakker, R.V. Multiple endocrine neoplasia type 1: Latest insights. Endocr. Rev. 2021, 42, 133–170. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, J.S.; Effraimidis, G.; Rossing, M.; Rasmussen, Å.K.; Hoejberg, L.; Bastholt, L.; Godballe, C.; Oturai, P.; Feldt-Rasmussen, U. Multiple endocrine neoplasia type 2: A review. Semin. Cancer Biol. 2022, 79, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Bonazzi, N.; Zanoni, L.; Fanti, S.; Ambrosini, V. Molecular imaging theranostics of neuroendocrine tumors. Semin. Nucl. Med. 2023, 53, 539–554. [Google Scholar] [CrossRef]
- Bodei, L.; Ambrosini, V.; Herrmann, K.; Modlin, I. Current concepts in 68Ga-DOTATATE imaging of neuroendocrine neoplasms: Interpretation, biodistribution, dosimetry, and molecular strategies. J. Nucl. Med. 2017, 58, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.A.; Goroshi, M.R.; Shah, H.; Malhotra, G.; Hira, P.; Sarathi, V.; Lele, V.; Jadhav, S.; Lila, A.; Bandgar, T.; et al. Comparison of [68Ga]-DOTA-NaI3-Octreotide/tyr3-octreotate positron emission tomography/computed tomography and contrast-enhanced computed tomography in localization of tumors in multiple endocrine neoplasia 1 syndrome. World J. Nucl. Med. 2020, 19, 99–105. [Google Scholar] [CrossRef]
- Werenski, H.E.; Nguyen, C.J.; Johansson, E.D.; Bunch, P.M.; Randle, R.W. Value of old imaging for patients undergoing parathyroidectomy for primary hyperparathyroidism. J. Surg. Res. 2023, 282, 147–154. [Google Scholar] [CrossRef]
- Broome, D.T.; Naples, R.; Bailey, R.; Tekin, Z.; Hamidi, M.; Bena, J.F.; Morrison, S.L.; Berber, E.; Siperstein, A.E.; Scharpf, J.; et al. Use of preoperative imaging in primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2021, 106, e328–e337. [Google Scholar] [CrossRef]
- Pogosian, K.; Karonova, T.; Ryzhkova, D.; Yanevskaya, L.; Tsoy, U.; Yudina, O.; Berkovich, G.; Dalmatova, A.; Grineva, E. [11C]-methionine PET/CT and conventional imaging techniques in the diagnosis of primary hyperparathyroidism. Quant. Imaging Med. Surg. 2023, 13, 2352–2363. [Google Scholar] [CrossRef]
- Kuzminski, S.J.; Sosa, J.A.; Hoang, J.K. Update in parathyroid imaging. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 151–166. [Google Scholar] [CrossRef]
- Morris, M.A.; Saboury, B.; Ahlman, M.; Malayeri, A.A.; Jones, E.C.; Chen, C.C.; Millo, C. Parathyroid imaging: Past, present, and future. Front. Endocrinol. 2022, 12, 760419. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Khan, A.A.; Silverberg, S.J.; Fuleihan, G.E.; Marcocci, C.; Minisola, S.; Perrier, N.; Sitges-Serra, A.; Thakker, R.V.; Guyatt, G.; et al. Evaluation and management of primary hyperparathyroidism: Summary statement and guidelines from the fifth international workshop. J. Bone Miner. Res. 2022, 37, 2293–2314. [Google Scholar] [CrossRef] [PubMed]
- Cusano, N.E.; Cetani, F. Normocalcemic primary hyperparathyroidism. Arch. Endocrinol. Metab. 2022, 66, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Thakker, R.V.; Newey, P.J.; Walls, G.V.; Bilezikian, J.; Dralle, H.; Ebeling, P.R.; Melmed, S.; Sakurai, A.; Tonelli, F.; Brandi, M.L. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J. Clin. Endocrinol. Metab. 2012, 97, 2990–3011. [Google Scholar] [CrossRef] [PubMed]
- Hoang, J.K.; Sung, W.K.; Bahl, M.; Phillips, C.D. How to perform parathyroid 4D CT: Tips and traps for technique and interpretation. Radiology 2014, 270, 15–24. [Google Scholar] [CrossRef]
- Sutter, C.W.; Joshi, M.J.; Stadalnik, R.C. Noncardiac uptake of technetium-99m MIBI. Semin. Nucl. Med. 1994, 24, 84–86. [Google Scholar] [CrossRef]
- Harris, S.M.; Davis, J.C.; Snyder, S.E.; Butch, E.R.; Vāvere, A.L.; Kocak, M.; Shulkin, B.L. Evaluation of the biodistribution of 11C-methionine in children and young adults. J. Nucl. Med. 2013, 54, 1902–1908. [Google Scholar] [CrossRef]
- Mehrotra, M.; Reddy, V.; Singh, P. Neuroanatomy, Stellate Ganglion; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sharma, P.; Mukherjee, A.; Karunanithi, S.; Naswa, N.; Kumar, R.; Ammini, A.C.; Bal, C. Accuracy of [68Ga] DOTANOC PET/CT imaging in patients with multiple endocrine neoplasia syndromes. Clin. Nucl. Med. 2015, 40, e351–e356. [Google Scholar] [CrossRef]
- Lastoria, S.; Marciello, F.; Faggiano, A.; Aloj, L.; Caracò, C.; Aurilio, M.; D’Ambrosio, L.; Di Gennaro, F.; Ramundo, V.; Camera, L.; et al. Role of (68)Ga-DOTATATE PET/CT in patients with multiple endocrine neoplasia type 1 (MEN1). Endocrine 2016, 52, 488–494. [Google Scholar] [CrossRef]
- Storvall, S.; Leijon, H.; Ryhänen, E.; Louhimo, J.; Haglund, C.; Schalin-Jäntti, C.; Arola, J. Somatostatin receptor expression in parathyroid neoplasms. Endocr. Connect. 2019, 8, 1213–1223. [Google Scholar] [CrossRef]
- Majala, S.; Vesterinen, T.; Seppänen, H.; Mustonen, H.; Sundström, J.; Schalin-Jäntti, C.; Gullichsen, R.; Schildt, J.; Kemppainen, J.; Arola, J.; et al. Correlation of somatostatin receptor 1-5 expression, [68Ga]Ga-DOTANOC, [18F]F-FDG PET/CT and clinical outcome in a prospective cohort of pancreatic neuroendocrine neoplasms. Cancers 2021, 14, 162. [Google Scholar] [CrossRef]
- Rufini, V.; Lorusso, M.; Inzani, F.; Pasciuto, T.; Triumbari, E.K.A.; Grillo, L.R.; Locco, F.; Margaritora, S.; Pescarmona, E.; Rindi, G. Correlation of somatostatin receptor PET/CT imaging features and immunohistochemistry in neuroendocrine tumors of the lung: A retrospective observational study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4182–4193. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, Y.; Suzuki, T.; Mikami, Y.; Moriya, T.; Satomi, S.; Sasano, H. Systemic distribution of somatostatin receptor subtypes in human: An immunohistochemical study. Endocr. J. 2005, 52, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Hasse, C.; Zielke, A.; Bruns, C.; Künneke, M.; Ehlenz, K.; Bachem, M.G.; Hey, A.; Kaffarnik, H.; Gressner, A.; Rothmund, M. Influence of somatostatin to biochemical parameters in patients with primary hyperparathyroidism. Exp. Clin. Endocrinol. Diabetes 1995, 103, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.S.; Madsen, K.; Price, M.; Søe, K.; Omata, Y.; Zaiss, M.M.; Gorvin, C.M.; Frost, M.; Rauch, A. Transcriptional reprogramming during human osteoclast differentiation identifies regulators of osteoclast activity. Bone Res. 2024, 12, 5. [Google Scholar] [CrossRef]



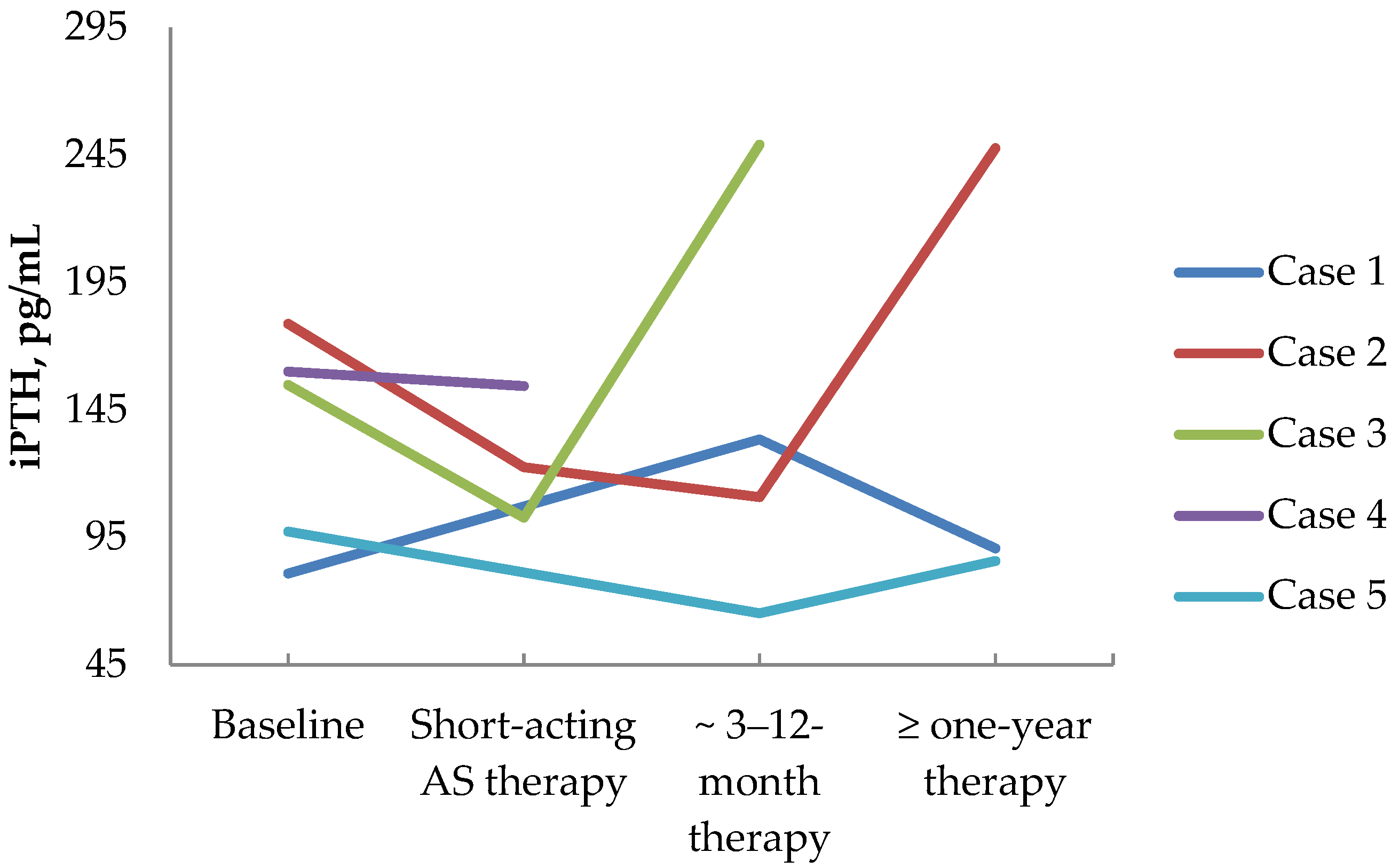
| Variables | Me (Min–Max) |
|---|---|
| age (years) | 65 (21–87) |
| weight (kg) | 74 (45–107) |
| height (cm) | 162 (147–192) |
| BMI (kg/m2) | 26 (16–40) |
| iPTH (pmol/L) | 199 (73–1456) |
| serum iCa (mmol/L) | 1.6 (1.3–2.1) |
| serum total Ca (mmol/L) | 2.9 (2.5–3.6) |
| 24 h calciura (mmol/24 h) | 6.3 (4.3–24.1) |
| serum P (mmol/L) | 0.8 (0.5–1.3) |
| 25(OH)D (ng/mL) | 28 (11–87) |
| eGFR 1 (mL/min/1.73 m2) | 81 (47–123) |
| Patient № 1 | Gender, Age | Histology Result | Size, mm | [68Ga]-DOTA- Peptides Uptake |
|---|---|---|---|---|
| 1 | M, 51 y.o. | adenoma | 6.5 × 7 × 21 | Neg. |
| 2 | F, 41 y.o. | NA | 13 × 7 × 14 | Pos. |
| 3 | F, 45 y.o. | NA | 14 × 6.5 × 29 | Pos. |
| 9.2 × 5.4 × 13.2 | Neg. | |||
| 4 | F, 36 y.o. | adenoma | 14 × 11 × 9 | Pos. |
| atypical tumor | 7 × 6 × 12 | Neg. | ||
| adenoma | 4 × 3 × 3 | Neg. | ||
| 5 | F, 56 y.o. | adenoma | 8.5 × 5.5 × 7 | Pos. |
| 6 | M, 53 y.o. | adenoma | 10 × 5 × 7 | Pos. |
| double adenoma | 15 × 10 × 23 | Pos. | ||
| adenoma | 14 × 10 × 24 | Pos. | ||
| 7 | F, 21 y.o. | adenoma | 8 × 4 × 13 | Neg. |
| adenoma | 5 × 6 × 6 | Neg. | ||
| adenoma | 6 × 7 × 8 | Neg. | ||
| 8 | M, 53 y.o. | adenoma | 15 × 10 × 18 | Pos. |
| adenoma | 27 × 9 × 12 | Pos. | ||
| 9 | F, 81 y.o. | adenoma | 8 × 7 × 13 | Pos. |
| 10 | M, 66 y.o. | adenoma | 22 × 13 × 16 | Pos. |
| atypical tumor | 44 × 48 × 54 | Pos. | ||
| 11 | F, 81 y.o. | adenoma | 13 × 12 × 8 | Pos. |
| adenoma | 8.5 × 7 × 5 | Pos. | ||
| 12 | F, 63 y.o. | NA | 5.1 × 5 × 6.7 | Pos. |
| NA | 5.9 × 3.7 × 5.3 | Pos. | ||
| 13 | F, 54 y.o. | adenoma | 18 × 22 × 27 | Pos. |
| 14 | F, 65 y.o. | NA | 20 × 11 × 44 | Pos. |
| 15 | F, 71 y.o. | adenoma | 18 × 10 × 22 | Pos. |
| 16 | F, 71 y.o. | atypical tumor | 27 × 26 × 41 | Pos. |
| Case № | Gender, Age | Indications for the Prescription of Somatostatin Analogs | Somatostatin Analogs’ Treatment Regimen | PHPT History |
|---|---|---|---|---|
| 1 | M, 51 y.o. | Multiple duodenal and non-functioning pancreatic NETs | Lanreotide 120 mg/28 days | Persistent PHPT after PTX (2 adenomas removed) |
| 2 | F, 41 y.o. | Multiple duodenal and non-functioning G2 pancreatic | Initially: Octreotide 200 mcg/3 times daily. Long-term: Lanreotide 120 mg/28 days | Persistent PHPT after subtotal PTX |
| 3 | F, 45 y.o. | Non-functioning pancreatic NET operated on in 2003 with local recurrence and metastasis in parapancreatic lymph node | Initially: Octreotide 300 mcg/2 times daily Long-term: Lanreotide 120 mg/28 days | Persistent PHPT after subtotal PTX |
| 4 | M, 53 y.o. | Duodenal gastrinoma, multiple non-functioning pancreatic and gastric NETs | Initially: Octreotide 100 mcg/2 times daily Long-term: Lanreotide 120 mg/28 days | PHPT before the surgery (3 adenomas) |
| 5 | M, 52 y.o. | Growth hormone-secreting pituitary adenoma’s persistence after surgery in 2022 | Lanreotide 120 mg/28 days | PHPT before the surgery (2 adenomas) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoy, U.; Pogosian, K.; Ryzhkova, D.; Yudina, O.; Yakovenko, K.; Ryazanov, P.; Matsueva, I.; Sokolnikova, P.; Salov, M.; Karonova, T.; et al. Somatostatin Receptor Imaging in the Diagnosis and Management of Parathyroid Neuroendocrine Neoplasia. Diagnostics 2024, 14, 2718. https://doi.org/10.3390/diagnostics14232718
Tsoy U, Pogosian K, Ryzhkova D, Yudina O, Yakovenko K, Ryazanov P, Matsueva I, Sokolnikova P, Salov M, Karonova T, et al. Somatostatin Receptor Imaging in the Diagnosis and Management of Parathyroid Neuroendocrine Neoplasia. Diagnostics. 2024; 14(23):2718. https://doi.org/10.3390/diagnostics14232718
Chicago/Turabian StyleTsoy, Uliana, Karina Pogosian, Daria Ryzhkova, Olga Yudina, Ksenia Yakovenko, Pavel Ryazanov, Irina Matsueva, Polina Sokolnikova, Maksim Salov, Tatiana Karonova, and et al. 2024. "Somatostatin Receptor Imaging in the Diagnosis and Management of Parathyroid Neuroendocrine Neoplasia" Diagnostics 14, no. 23: 2718. https://doi.org/10.3390/diagnostics14232718
APA StyleTsoy, U., Pogosian, K., Ryzhkova, D., Yudina, O., Yakovenko, K., Ryazanov, P., Matsueva, I., Sokolnikova, P., Salov, M., Karonova, T., & Grineva, E. (2024). Somatostatin Receptor Imaging in the Diagnosis and Management of Parathyroid Neuroendocrine Neoplasia. Diagnostics, 14(23), 2718. https://doi.org/10.3390/diagnostics14232718






