Endoscopic Ultrasound-Guided Hepaticogastrostomy in Malignant Biliary Obstruction: A Comprehensive Review on Technical Tips and Clinical Outcomes
Abstract
1. Introduction
2. Current Indication for EUS-Guided HGS in Malignant Biliary Obstruction
3. Technical Aspects
3.1. Preoperative Evaluations
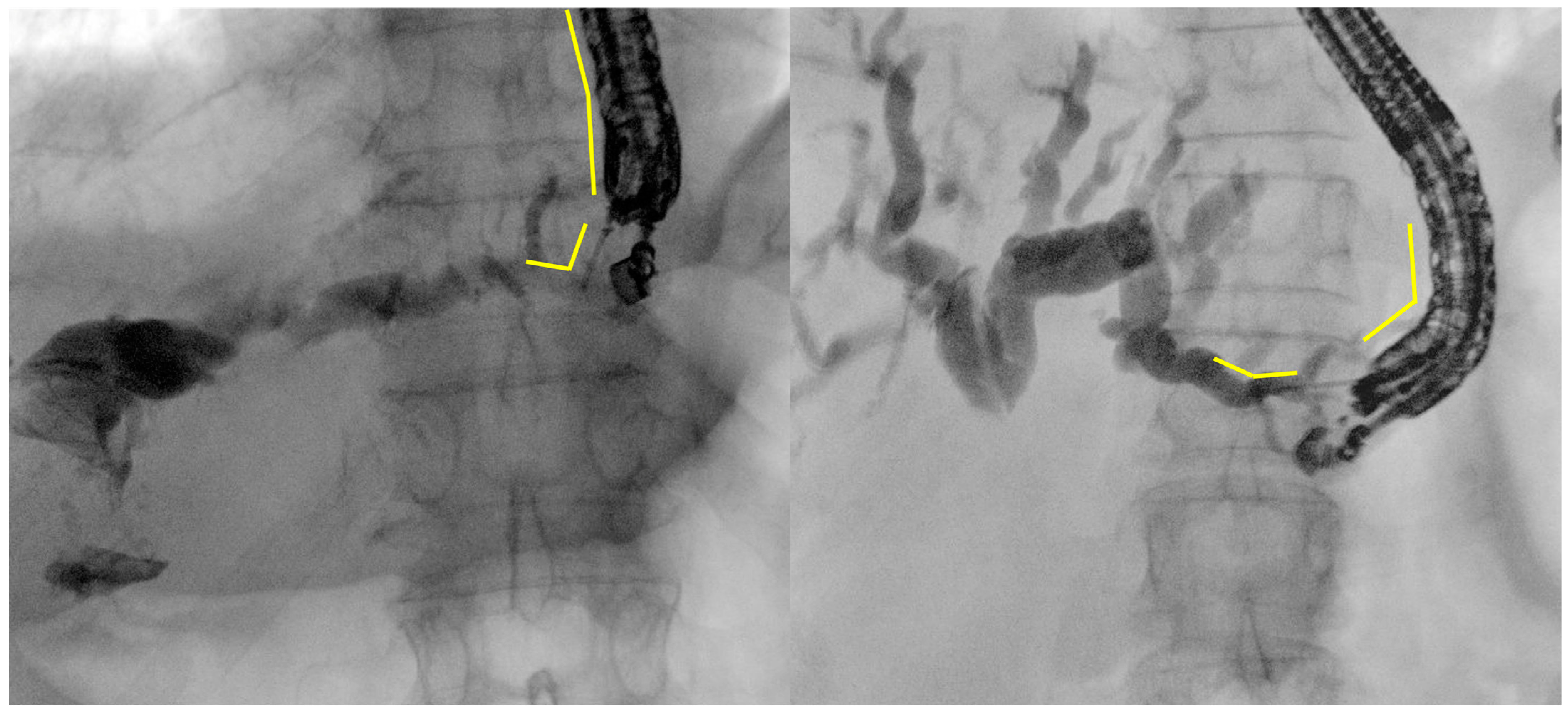
3.2. Intraprocedural Aspects
- Intrahepatic bile duct puncture
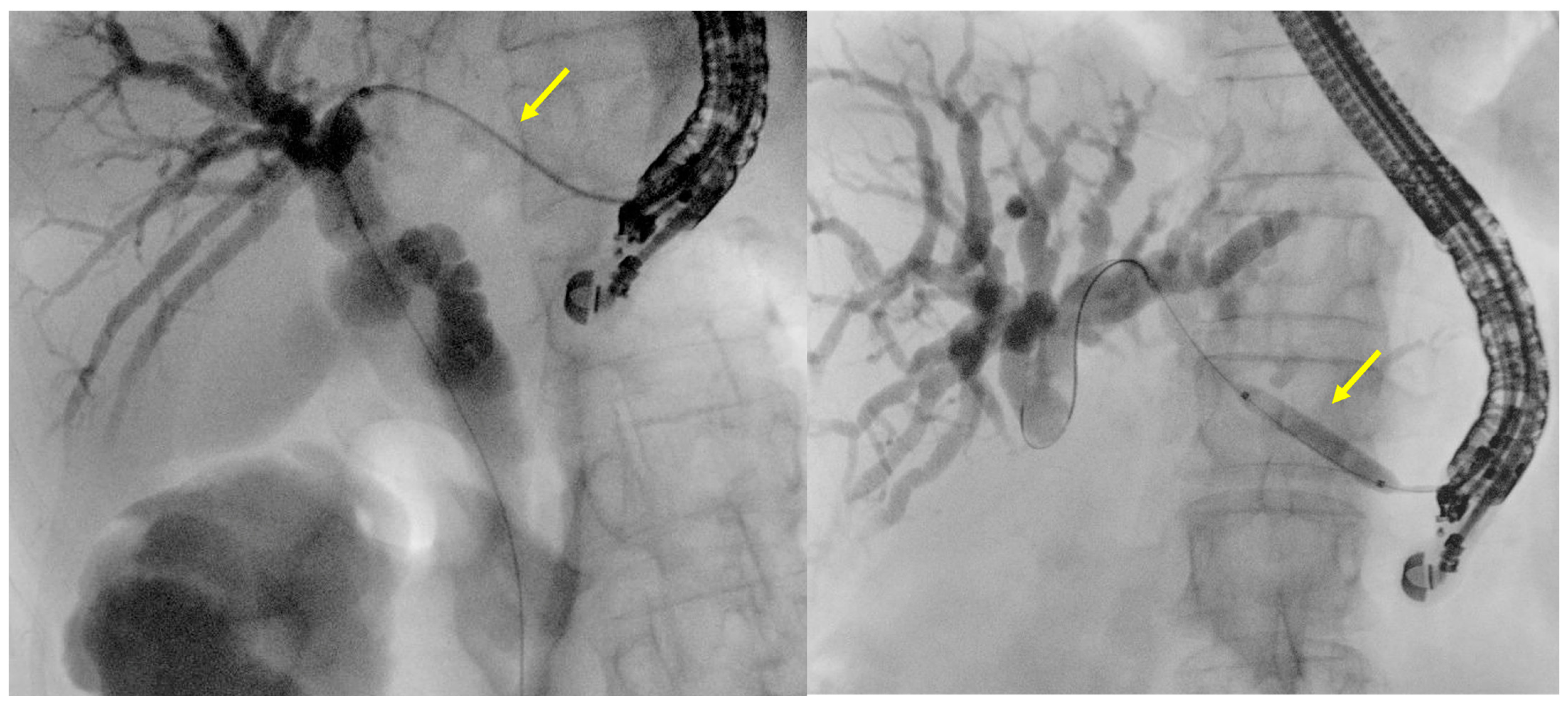
- Fluoroscopy and guidewire manipulation into the biliary tree
- Tract dilation
- Stent positioning
4. Efficacy of EUS-Guided HGS
| First Author Year | Design | No. of Patients | Condition | Obstruction Site | Stent | Technical Success % | Clinical Success % |
|---|---|---|---|---|---|---|---|
| Artifon 2015 [53] | RCT | 49 | Malignant | Distal | PC-SEMS | 96 | 91 |
| Park 2015 [56] | RCT | 32 | Malignant | Proximal/Distal | Dedicated SEMS | 199 | 94 |
| Paik 2018 [57] | RCT | 64 | Malignant | Proximal/Distal | Dedicated SEMS | 97 | 81 |
| Minaga 2019 [54] | RCT | 47 | Malignant | Distal | Dedicated SEMS | 88 | 100 |
| Marx 2022 [55] | Randomized phase II trial | 35 | Malignant/Benign | Proximal/Distal | Dedicated SEMS | 94 | 80 |
| Moryoussef 2017 [61] | Prospective | 18 | Malignant | Proximal | SEMS | 94 | 72 |
| Okuno 2018 [41] | Prospective | 20 | Malignant | Distal | SEMS | 100 | 95 |
| Jagielski 2021 [60] | Prospective | 53 | Malignant | Proximal/Distal | Dedicated SEMS | 98 | 87 |
| Anderloni 2022 [44] | Prospective | 22 | Malignant | Proximal/Distal | Dedicated SEMS | 100 | 91 |
| Vila 2012 [63] | Retrospective | 34 | Malignant | Proximal/Distal | Not specified | 65 | Not assessed |
| Poincloux 2015 [64] | Retrospective | 66 | Malignant/Benign | Proximal/Distal | Plastic, SEMS, Dedicated SEMS | 98 | 94 |
| Khashab 2016 [39] | Retrospective | 61 | Malignant/Benign | Distal | Plastic, SEMS | 92 | 82 |
| Nakai 2016 [43] | Retrospective | 33 | Malignant | Proximal/Distal | Dedicated SEMS | 100 | 100 |
| Sportes 2017 [65] | Retrospective | 31 | Malignant | Proximal/Distal | SEMS | 100 | 86 |
| Oh 2017 [27] | Retrospective | 129 | Malignant/Benign | Proximal/Distal | Plastic, Dedicated SEMS | 93 | 81 |
| Honjo 2018 [34] | Retrospective | 49 | Malignant/Benign | Proximal/Distal | Plastic, SEMS | 100 | Not assessed |
| Miyano 2018 [48] | Retrospective | 41 | Malignant/Benign | Proximal/Distal | SEMS | 100 | 100 |
| Nakai 2020 [42] | Retrospective | 110 | Malignant | Proximal/Distal | Dedicated SEMS | 100 | 94 |
5. Safety of EUS-Guided HGS
5.1. Bleeding
5.2. Bile Leakage
5.3. Infection
5.4. Stent Migration
5.5. Recurrent Biliary Obstruction
6. Comparison of EUS-Guided HGS with Other Biliary Drainage Methods
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sharaiha, R.Z.; Kumta, N.A.; Desai, A.P.; DeFilippis, E.M.; Gabr, M.; Sarkisian, A.M.; Salgado, S.; Millman, J.; Benvenuto, A.; Cohen, M.; et al. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: Predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg. Endosc. 2016, 30, 5500–5505. [Google Scholar] [CrossRef] [PubMed]
- Hayat, U.; Bakker, C.; Dirweesh, A.; Khan, M.Y.; Adler, D.G.; Okut, H.; Leul, N.; Bilal, M.; Siddiqui, A.A. EUS-guided versus percutaneous transhepatic cholangiography biliary drainage for obstructed distal malignant biliary strictures in patients who have failed endoscopic retrograde cholangiopancreatography: A systematic review and meta-analysis. Endosc. Ultrasound 2022, 11, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Moutardier, V.; Pesenti, C.; Bories, E.; Lelong, B.; Delpero, J.R. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy 2001, 33, 898–900. [Google Scholar] [CrossRef] [PubMed]
- Bories, E.; Pesenti, C.; Caillol, F.; Lopes, C.; Giovannini, M. Transgastric endoscopic ultrasonography-guided biliary drainage: Results of a pilot study. Endoscopy 2007, 39, 287–291. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, S.W.; van Wanrooij, R.L.J.; Bronswijk, M.; Everett, S.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Kunda, R.; Badaoui, A.; Law, R.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 185–205. [Google Scholar] [CrossRef]
- Teoh, A.Y.B.; Dhir, V.; Kida, M.; Yasuda, I.; Jin, Z.D.; Seo, D.W.; Almadi, M.; Ang, T.L.; Hara, K.; Hilmi, I.; et al. Consensus guidelines on the optimal management in interventional EUS procedures: Results from the Asian EUS group RAND/UCLA expert panel. Gut 2018, 67, 1209–1228. [Google Scholar] [CrossRef]
- Marzioni, M.; Crinò, S.F.; Lisotti, A.; Fuccio, L.; Vanella, G.; Amato, A.; Bertani, H.; Binda, C.; Coluccio, C.; Forti, E.; et al. Biliary drainage in patients with malignant distal biliary obstruction: Results of an Italian consensus conference. Surg. Endosc. 2024, 38, 6207–6226. [Google Scholar] [CrossRef]
- Pawa, S.; Marya, N.B.; Thiruvengadam, N.R.; Ngamruengphong, S.; Baron, T.H.; Bun Teoh, A.Y.; Bent, C.K.; Abidi, W.; Alipour, O.; Amateau, S.K.; et al. American Society for Gastrointestinal Endoscopy guideline on the role of therapeutic EUS in the management of biliary tract disorders: Summary and recommendations. Gastrointest. Endosc. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Chen, Y.I.; Sahai, A.; Donatelli, G.; Lam, E.; Forbes, N.; Mosko, J.; Paquin, S.C.; Donnellan, F.; Chatterjee, A.; Telford, J.; et al. Endoscopic Ultrasound-Guided Biliary Drainage of First Intent With a Lumen-Apposing Metal Stent vs Endoscopic Retrograde Cholangiopancreatography in Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Study (ELEMENT Trial). Gastroenterology 2023, 165, 1249–1261.e5. [Google Scholar] [CrossRef]
- Teoh, A.Y.B.; Napoleon, B.; Kunda, R.; Arcidiacono, P.G.; Kongkam, P.; Larghi, A.; Van der Merwe, S.; Jacques, J.; Legros, R.; Thawee, R.E.; et al. EUS-Guided Choledocho-duodenostomy Using Lumen Apposing Stent Versus ERCP With Covered Metallic Stents in Patients With Unresectable Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Trial (DRA-MBO Trial). Gastroenterology 2023, 165, 473–482.e472. [Google Scholar] [CrossRef]
- Lauri, G.; Archibugi, L.; Arcidiacono, P.G.; Repici, A.; Hassan, C.; Capurso, G.; Facciorusso, A. Primary drainage of distal malignant biliary obstruction: A comparative network meta-analysis. Dig. Liver Dis. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Dumonceau, J.M.; Tringali, A.; Papanikolaou, I.S.; Blero, D.; Mangiavillano, B.; Schmidt, A.; Vanbiervliet, G.; Costamagna, G.; Devière, J.; García-Cano, J.; et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline—Updated October 2017. Endoscopy 2018, 50, 910–930. [Google Scholar] [CrossRef]
- Lee, T.H.; Kim, T.H.; Moon, J.H.; Lee, S.H.; Choi, H.J.; Hwangbo, Y.; Hyun, J.J.; Choi, J.H.; Jeong, S.; Kim, J.H.; et al. Bilateral versus unilateral placement of metal stents for inoperable high-grade malignant hilar biliary strictures: A multicenter, prospective, randomized study (with video). Gastrointest. Endosc. 2017, 86, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Toyonaga, H.; Hayashi, T.; Kin, T.; Hama, K.; Iwano, K.; Nakamura, R.; Katanuma, A. Combination of ERCP with endoscopic ultrasound-guided hepaticogastrostomy and hepaticoduodenostomy for biliary drainage in malignant hilar biliary obstruction. Endoscopy 2022, 54, E912–E913. [Google Scholar] [CrossRef] [PubMed]
- Kongkam, P.; Orprayoon, T.; Boonmee, C.; Sodarat, P.; Seabmuangsai, O.; Wachiramatharuch, C.; Auan-Klin, Y.; Pham, K.C.; Tasneem, A.A.; Kerr, S.J.; et al. ERCP plus endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage for malignant hilar biliary obstruction: A multicenter observational open-label study. Endoscopy 2021, 53, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Hijioka, S.; Nagashio, Y.; Sugawara, S.; Nara, S.; Sone, M.; Esaki, M.; Arai, Y.; Okusaka, T.; Nakajima, A. Use of endoscopic ultrasound-guided biliary drainage as a rescue of re-intervention after the placement of multiple metallic stents for malignant hilar biliary obstruction. J. Hepatobiliary Pancreat. Sci. 2022, 29, 404–414. [Google Scholar] [CrossRef]
- van Wanrooij, R.L.J.; Bronswijk, M.; Kunda, R.; Everett, S.M.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Badaoui, A.; Law, R.; Arcidiacono, P.G.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy 2022, 54, 310–332. [Google Scholar] [CrossRef]
- Ogura, T.; Higuchi, K. Technical tips for endoscopic ultrasound-guided hepaticogastrostomy. World J. Gastroenterol. 2016, 22, 3945–3951. [Google Scholar] [CrossRef]
- Matsubara, S.; Nakagawa, K.; Suda, K.; Otsuka, T.; Oka, M.; Nagoshi, S. Practical Tips for Safe and Successful Endoscopic Ultrasound-Guided Hepaticogastrostomy: A State-of-the-Art Technical Review. J. Clin. Med. 2022, 11, 1591. [Google Scholar] [CrossRef]
- Ogura, T.; Nishioka, N.; Ueno, S.; Yamada, T.; Yamada, M.; Ueshima, K.; Matsuno, J.; Okuda, A.; Yamamoto, Y.; Higuchi, K. Antiplatelet and/or anticoagulant treatment does not increase hemorrhagic adverse events during EUS-guided biliary drainage. Gastrointest. Endosc. 2020, 92, 659–666. [Google Scholar] [CrossRef]
- Sidhu, R.; Turnbull, D.; Haboubi, H.; Leeds, J.S.; Healey, C.; Hebbar, S.; Collins, P.; Jones, W.; Peerally, M.F.; Brogden, S.; et al. British Society of Gastroenterology guidelines on sedation in gastrointestinal endoscopy. Gut 2024, 73, 219–245. [Google Scholar] [CrossRef]
- Spadaccini, M.; Binda, C.; Fugazza, A.; Repici, A.; Tarantino, I.; Fabbri, C.; Cugia, L.; Anderloni, A.; On Behalf Of The Interventional Endoscopy Amp Ultra Sound I-Eus Group. Informed Consent for Endoscopic Biliary Drainage: Time for a New Paradigm. Medicina 2022, 58, 331. [Google Scholar] [CrossRef]
- Sekine, M.; Hashimoto, Y.; Shibuki, T.; Okumura, K.; Kobori, I.; Miyagaki, A.; Sasaki, Y.; Takano, Y.; Matsumoto, K.; Mashima, H. A retrospective multicenter study comparing the punctures to B2 and B3 in endoscopic ultrasound-guided hepaticogastrostomy. DEN Open 2023, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Higuchi, K. Endoscopic Ultrasound-Guided Hepaticogastrostomy: Technical Review and Tips to Prevent Adverse Events. Gut Liver 2021, 15, 196–205. [Google Scholar] [CrossRef]
- Kaneko, J.; Ishiwatari, H.; Takizawa, K.; Satoh, T.; Sato, J.; Matsubayashi, H.; Ono, H. Mediastinitis due to perforation by a metal stent after endoscopic ultrasound-guided hepaticogastrostomy: A rare complication. Endoscopy 2020, 52, 519–521. [Google Scholar] [CrossRef]
- Ogura, T.; Nishioka, N.; Ueno, S.; Yamada, T.; Yamada, M.; Imoto, A.; Hakoda, A.; Higuchi, K. Effect of echoendoscope angle on success of guidewire manipulation during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2021, 53, 369–375. [Google Scholar] [CrossRef]
- Oh, D.; Park, D.H.; Song, T.J.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap. Adv. Gastroenterol. 2017, 10, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Ogura, T.; Nishioka, N.; Yamada, T.; Yamada, M.; Ueno, S.; Higuchi, K. Risk factors for adverse events associated with bile leak during EUS-guided hepaticogastrostomy. Endosc. Ultrasound 2020, 9, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Ueno, S.; Okuda, A.; Nishioka, N.; Yamada, M.; Ueshima, K.; Matsuno, J.; Yamamoto, Y.; Higuchi, K. Expanding indications for endoscopic ultrasound-guided hepaticogastrostomy for patients with insufficient dilatation of the intrahepatic bile duct using a 22G needle combined with a novel 0.018-inch guidewire (with video). Dig. Endosc. 2022, 34, 222–227. [Google Scholar] [CrossRef]
- Takahashi, K.; Ohyama, H.; Ouchi, M.; Kan, M.; Nagashima, H.; Iino, Y.; Kusakabe, Y.; Okitsu, K.; Ohno, I.; Takiguchi, Y.; et al. Feasibility of endoscopic ultrasound-guided hepaticogastrostomy using a 22-gauge needle. Medicine 2022, 101, e31545. [Google Scholar] [CrossRef]
- Hwang, J.H.; Aslanian, H.R.; Thosani, N.; Goodman, A.; Manfredi, M.; Navaneethan, U.; Pannala, R.; Parsi, M.A.; Smith, Z.L.; Sullivan, S.A.; et al. Devices for use with EUS. VideoGIE 2017, 2, 35–45. [Google Scholar] [CrossRef][Green Version]
- Ryou, M.; Benias, P.C.; Kumbhari, V. Initial clinical experience of a steerable access device for EUS-guided biliary drainage. Gastrointest. Endosc. 2020, 91, 178–184. [Google Scholar] [CrossRef]
- Ogura, T.; Masuda, D.; Takeuchi, T.; Fukunishi, S.; Higuchi, K. Liver impaction technique to prevent shearing of the guidewire during endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy 2015, 47, E583–E584. [Google Scholar] [CrossRef] [PubMed]
- Honjo, M.; Itoi, T.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Mukai, S.; Sofuni, A.; Nagakawa, Y.; Iwasaki, H.; Kanai, T. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video). Endosc. Ultrasound 2018, 7, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Uba, Y.; Yamamura, M.; Kawai, J.; Nishikawa, H. Successful endoscopic ultrasound-guided hepaticogastrostomy with use of a novel drill dilator for challenging tract dilation. Endoscopy 2023, 55, E149–E150. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Itoi, T.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Yamamoto, K.; Nagai, K.; Matsunami, Y.; Kojima, H.; Sofuni, A. A novel technique for one-step dilation followed by bile aspiration using an ultra-tapered bougie dilator with side holes to minimize bile leakage during EUS-guided hepaticogastrostomy. J. Hepatobiliary Pancreat. Sci. 2024, 31, e17–e19. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Ito, K.; Sakai, T.; Okano, H. Novel combination of a 0.018-inch guidewire, dedicated thin dilator, and 22-gauge needle for EUS-guided hepaticogastrostomy. VideoGIE 2020, 5, 355–358. [Google Scholar] [CrossRef]
- Park, D.H.; Jang, J.W.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest. Endosc. 2011, 74, 1276–1284. [Google Scholar] [CrossRef]
- Khashab, M.A.; Messallam, A.A.; Penas, I.; Nakai, Y.; Modayil, R.J.; De la Serna, C.; Hara, K.; El Zein, M.; Stavropoulos, S.N.; Perez-Miranda, M.; et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc. Int. Open 2016, 4, E175–E181. [Google Scholar] [CrossRef]
- Vanella, G.; Bronswijk, M.; Maleux, G.; van Malenstein, H.; Laleman, W.; Van der Merwe, S. EUS-guided intrahepatic biliary drainage: A large retrospective series and subgroup comparison between percutaneous drainage in hilar stenoses or postsurgical anatomy. Endosc. Int. Open 2020, 8, E1782–E1794. [Google Scholar] [CrossRef]
- Okuno, N.; Hara, K.; Mizuno, N.; Kuwahara, T.; Iwaya, H.; Ito, A.; Kuraoka, N.; Matsumoto, S.; Polmanee, P.; Niwa, Y. Efficacy of the 6-mm fully covered self-expandable metal stent during endoscopic ultrasound-guided hepaticogastrostomy as a primary biliary drainage for the cases estimated difficult endoscopic retrograde cholangiopancreatography: A prospective clinical study. J. Gastroenterol. Hepatol. 2018, 33, 1413–1421. [Google Scholar] [CrossRef]
- Nakai, Y.; Sato, T.; Hakuta, R.; Ishigaki, K.; Saito, K.; Saito, T.; Takahara, N.; Hamada, T.; Mizuno, S.; Kogure, H.; et al. Long-term outcomes of a long, partially covered metal stent for EUS-guided hepaticogastrostomy in patients with malignant biliary obstruction (with video). Gastrointest. Endosc. 2020, 92, 623–631.e1. [Google Scholar] [CrossRef]
- Nakai, Y.; Isayama, H.; Yamamoto, N.; Matsubara, S.; Ito, Y.; Sasahira, N.; Hakuta, R.; Umefune, G.; Takahara, N.; Hamada, T.; et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy 2016, 48, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Anderloni, A.; Fugazza, A.; Spadaccini, M.; Colombo, M.; Capogreco, A.; Carrara, S.; Maselli, R.; Ferrara, E.C.; Galtieri, P.A.; Pellegatta, G.; et al. Feasibility and safety of a new dedicated biliary stent for EUS-guided hepaticogastrostomy: The FIT study (with video). Endosc. Ultrasound 2023, 12, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Isayama, H.; Nakai, Y.; Itoi, T.; Yasuda, I.; Kawakami, H.; Ryozawa, S.; Kitano, M.; Irisawa, A.; Katanuma, A.; Hara, K.; et al. Clinical practice guidelines for safe performance of endoscopic ultrasound/ultrasonography-guided biliary drainage: 2018. J. Hepatobiliary Pancreat. Sci. 2019, 26, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Yamamoto, K.; Sano, T.; Onda, S.; Imoto, A.; Masuda, D.; Takagi, W.; Fukunishi, S.; Higuchi, K. Stent length is impact factor associated with stent patency in endoscopic ultrasound-guided hepaticogastrostomy. J. Gastroenterol. Hepatol. 2015, 30, 1748–1752. [Google Scholar] [CrossRef]
- Paik, W.H.; Park, D.H.; Choi, J.H.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H.; Lee, J.B. Simplified fistula dilation technique and modified stent deployment maneuver for EUS-guided hepaticogastrostomy. World J. Gastroenterol. 2014, 20, 5051–5059. [Google Scholar] [CrossRef]
- Miyano, A.; Ogura, T.; Yamamoto, K.; Okuda, A.; Nishioka, N.; Higuchi, K. Clinical Impact of the Intra-scope Channel Stent Release Technique in Preventing Stent Migration During EUS-Guided Hepaticogastrostomy. J. Gastrointest. Surg. 2018, 22, 1312–1318. [Google Scholar] [CrossRef]
- Anderloni, A.; Attili, F.; Carrara, S.; Galasso, D.; Di Leo, M.; Costamagna, G.; Repici, A.; Kunda, R.; Larghi, A. Intra-channel stent release technique for fluoroless endoscopic ultrasound-guided lumen-apposing metal stent placement: Changing the paradigm. Endosc. Int. Open 2017, 5, E25–E29. [Google Scholar] [CrossRef]
- Niiya, F.; Ishiwatari, H.; Sato, J.; Matsubayashi, H.; Ono, H. Endoscopic ultrasound-guided hepaticogastrostomy with bridging as reintervention for stent occlusion in malignant hilar biliary obstruction. Endoscopy 2023, 55, E1213–E1214. [Google Scholar] [CrossRef]
- Ishiwatari, H.; Satoh, T.; Sato, J.; Kaneko, J.; Matsubayashi, H.; Ono, H. Double-guidewire technique facilitates endoscopic ultrasound-guided biliary drainage for hilar biliary obstruction. Endoscopy 2019, 51, E321–E322. [Google Scholar] [CrossRef]
- Ogura, T.; Sano, T.; Onda, S.; Imoto, A.; Masuda, D.; Yamamoto, K.; Kitano, M.; Takeuchi, T.; Inoue, T.; Higuchi, K. Endoscopic ultrasound-guided biliary drainage for right hepatic bile duct obstruction: Novel technical tips. Endoscopy 2015, 47, 72–75. [Google Scholar] [CrossRef]
- Artifon, E.L.; Marson, F.P.; Gaidhane, M.; Kahaleh, M.; Otoch, J.P. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: Is there any difference? Gastrointest. Endosc. 2015, 81, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Minaga, K.; Ogura, T.; Shiomi, H.; Imai, H.; Hoki, N.; Takenaka, M.; Nishikiori, H.; Yamashita, Y.; Hisa, T.; Kato, H.; et al. Comparison of the efficacy and safety of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for malignant distal biliary obstruction: Multicenter, randomized, clinical trial. Dig. Endosc. 2019, 31, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Marx, M.; Caillol, F.; Autret, A.; Ratone, J.P.; Zemmour, C.; Boher, J.M.; Pesenti, C.; Bories, E.; Barthet, M.; Napoléon, B.; et al. EUS-guided hepaticogastrostomy in patients with obstructive jaundice after failed or impossible endoscopic retrograde drainage: A multicenter, randomized phase II Study. Endosc. Ultrasound 2022, 11, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Lee, T.H.; Paik, W.H.; Choi, J.H.; Song, T.J.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. Feasibility and safety of a novel dedicated device for one-step EUS-guided biliary drainage: A randomized trial. J. Gastroenterol. Hepatol. 2015, 30, 1461–1466. [Google Scholar] [CrossRef]
- Paik, W.H.; Lee, T.H.; Park, D.H.; Choi, J.H.; Kim, S.O.; Jang, S.; Kim, D.U.; Shim, J.H.; Song, T.J.; Lee, S.S.; et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2018, 113, 987–997. [Google Scholar] [CrossRef]
- Binda, C.; Dajti, E.; Giuffrida, P.; Trebbi, M.; Coluccio, C.; Cucchetti, A.; Fugazza, A.; Perini, B.; Gibiino, G.; Anderloni, A.; et al. Efficacy and safety of endoscopic ultrasound-guided hepaticogastrostomy: A meta-regression analysis. Endoscopy 2024, 56, 694–705. [Google Scholar] [CrossRef]
- Moond, V.; Loganathan, P.; Koyani, B.; Khan, S.R.; Kassab, L.L.; Chandan, S.; Mohan, B.P.; Broder, A.; Adler, D.G. Efficacy and safety of EUS-guided hepatogastrostomy: A systematic review and meta-analysis. Endosc. Ultrasound 2024, 13, 171–182. [Google Scholar] [CrossRef]
- Jagielski, M.; Zieliński, M.; Piątkowski, J.; Jackowski, M. Outcomes and limitations of endoscopic ultrasound-guided hepaticogastrostomy in malignant biliary obstruction. BMC Gastroenterol. 2021, 21, 202. [Google Scholar] [CrossRef]
- Moryoussef, F.; Sportes, A.; Leblanc, S.; Bachet, J.B.; Chaussade, S.; Prat, F. Is EUS-guided drainage a suitable alternative technique in case of proximal biliary obstruction? Therap. Adv. Gastroenterol. 2017, 10, 537–544. [Google Scholar] [CrossRef]
- Vargas-Madrigal, J.; Chan, S.M.; Dhar, J.; Teoh, A.Y.B.; Samanta, J.; Lakhtakia, S.; Giovannini, M. Dedicated cautery-enhanced tubular self-expandable metal stent for endoscopic ultrasound-guided hepaticogastrostomy: Feasibility study. Endoscopy 2024, 56, 864–869. [Google Scholar] [CrossRef]
- Vila, J.J.; Pérez-Miranda, M.; Vazquez-Sequeiros, E.; Abadia, M.A.; Pérez-Millán, A.; González-Huix, F.; Gornals, J.; Iglesias-Garcia, J.; De la Serna, C.; Aparicio, J.R.; et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest Endosc 2012, 76, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Poincloux, L.; Rouquette, O.; Buc, E.; Privat, J.; Pezet, D.; Dapoigny, M.; Bommelaer, G.; Abergel, A. Endoscopic ultrasound-guided biliary drainage after failed ERCP: Cumulative experience of 101 procedures at a single center. Endoscopy 2015, 47, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Sportes, A.; Camus, M.; Greget, M.; Leblanc, S.; Coriat, R.; Hochberger, J.; Chaussade, S.; Grabar, S.; Prat, F. Endoscopic ultrasound-guided hepaticogastrostomy versus percutaneous transhepatic drainage for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography: A retrospective expertise-based study from two centers. Therap. Adv. Gastroenterol. 2017, 10, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Minaga, K.; Takenaka, M.; Kitano, M.; Chiba, Y.; Imai, H.; Yamao, K.; Kamata, K.; Miyata, T.; Omoto, S.; Sakurai, T.; et al. Rescue EUS-guided intrahepatic biliary drainage for malignant hilar biliary stricture after failed transpapillary re-intervention. Surg. Endosc. 2017, 31, 4764–4772. [Google Scholar] [CrossRef]
- Ochiai, K.; Fujisawa, T.; Ishii, S.; Suzuki, A.; Saito, H.; Takasaki, Y.; Ushio, M.; Takahashi, S.; Yamagata, W.; Tomishima, K.; et al. Risk Factors for Stent Migration into the Abdominal Cavity after Endoscopic Ultrasound-Guided Hepaticogastrostomy. J. Clin. Med. 2021, 10, 3111. [Google Scholar] [CrossRef]
- Schoch, A.; Lisotti, A.; Walter, T.; Fumex, F.; Leblanc, S.; Artru, P.; Desramé, J.; Brighi, N.; Marsot, J.; Souquet, J.C.; et al. Efficacy of EUS-guided hepaticogastrostomy in prolonging survival of patients with perihilar cholangiocarcinoma. Endosc. Ultrasound 2022, 11, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Fugazza, A.; Colombo, M.; Spadaccini, M.; Vespa, E.; Gabbiadini, R.; Capogreco, A.; Repici, A.; Anderloni, A. Relief of jaundice in malignant biliary obstruction: When should we consider endoscopic ultrasonography-guided hepaticogastrostomy as an option? Hepatobiliary Pancreat. Dis. Int. 2022, 21, 234–240. [Google Scholar] [CrossRef]
- Yamazaki, H.; Yamashita, Y.; Shimokawa, T.; Minaga, K.; Ogura, T.; Kitano, M. Endoscopic ultrasound-guided hepaticogastrostomy versus choledochoduodenostomy for malignant biliary obstruction: A meta-analysis. DEN Open 2024, 4, e274. [Google Scholar] [CrossRef]
- Giri, S.; Mohan, B.P.; Jearth, V.; Kale, A.; Angadi, S.; Afzalpurkar, S.; Harindranath, S.; Sundaram, S. Adverse events with EUS-guided biliary drainage: A systematic review and meta-analysis. Gastrointest. Endosc. 2023, 98, 515–523.e8. [Google Scholar] [CrossRef]
- Fabbri, C.; Scalvini, D.; Paolo, G.; Binda, C.; Mauro, A.; Coluccio, C.; Mazza, S.; Trebbi, M.; Torello Viera, F.; Anderloni, A. Complications and management of interventional endoscopic ultrasound: A critical review. Best Pract. Res. Clin. Gastroenterol. 2024, 69, 101888. [Google Scholar] [CrossRef]
- Ishiwatari, H.; Ishikawa, K.; Niiya, F.; Matsubayashi, H.; Kishida, Y.; Yoshida, M.; Kawata, N.; Imai, K.; Hotta, K.; Ono, H. Endoscopic ultrasound-guided hepaticogastrostomy versus hepaticogastrostomy with antegrade stenting for malignant distal biliary obstruction. J. Hepatobiliary Pancreat. Sci. 2022, 29, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Uemura, R.S.; Khan, M.A.; Otoch, J.P.; Kahaleh, M.; Montero, E.F.; Artifon, E.L.A. EUS-guided Choledochoduodenostomy Versus Hepaticogastrostomy: A Systematic Review and Meta-analysis. J. Clin. Gastroenterol. 2018, 52, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, J.; Liu, F.; Fang, J. Comparison of Choledochoduodenostomy and Hepaticogastrostomy for EUS-Guided Biliary Drainage: A Meta-Analysis. Front. Surg. 2022, 9, 811005. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Hu, B.; Sun, F.; Wan, K. Choledochoduodenostomy Versus Hepaticogastrostomy in Endoscopic Ultrasound-guided Drainage for Malignant Biliary Obstruction: A Meta-analysis and Systematic Review. Surg. Laparosc. Endosc. Percutan. Tech. 2021, 32, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Mangiavillano, B.; Paduano, D.; Binda, C.; Crinò, S.F.; Gkolfakis, P.; Ramai, D.; Fugazza, A.; Tarantino, I.; Lisotti, A.; et al. Methods for Drainage of Distal Malignant Biliary Obstruction after ERCP Failure: A Systematic Review and Network Meta-Analysis. Cancers 2022, 14, 3291. [Google Scholar] [CrossRef] [PubMed]
- Hedjoudje, A.; Sportes, A.; Grabar, S.; Zhang, A.; Koch, S.; Vuitton, L.; Prat, F. Outcomes of endoscopic ultrasound-guided biliary drainage: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2019, 7, 60–68. [Google Scholar] [CrossRef]
- Khan, M.A.; Akbar, A.; Baron, T.H.; Khan, S.; Kocak, M.; Alastal, Y.; Hammad, T.; Lee, W.M.; Sofi, A.; Artifon, E.L.; et al. Endoscopic Ultrasound-Guided Biliary Drainage: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2016, 61, 684–703. [Google Scholar] [CrossRef]
- Koutlas, N.J.; Pawa, S.; Russell, G.; Ferris, T.; Ponnatapura, J.; Pawa, R. EUS-guided hepaticogastrostomy versus percutaneous transhepatic biliary drainage after failed ERCP: A propensity score-matched analysis. Endosc. Int. Open 2024, 12, E108–E115. [Google Scholar] [CrossRef]

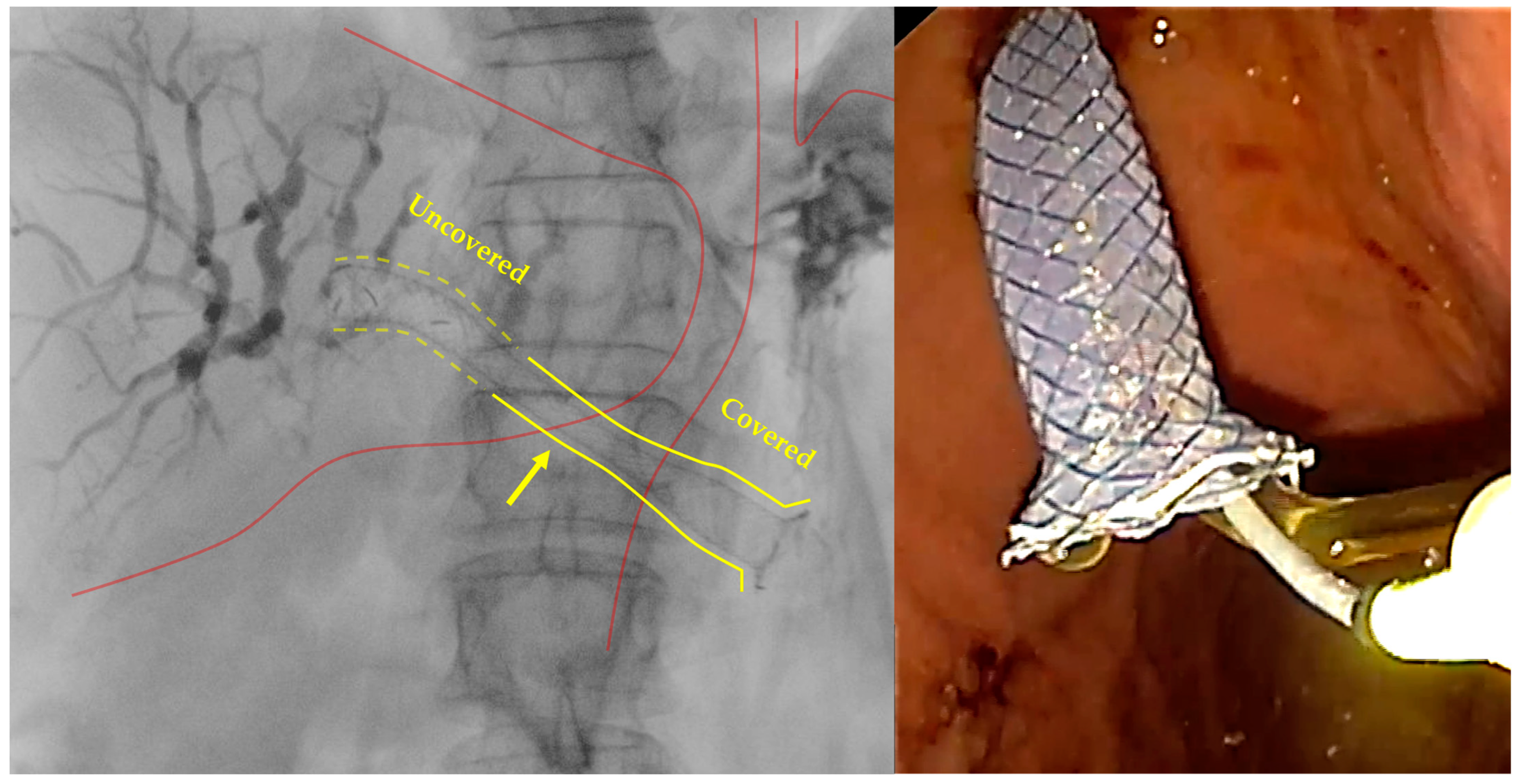
| Stent Name | Company | Available Diameters | Available Lengths | Covering | Anti-Migration Flared Ends | Anti-Migration Flaps | Radiopaque Markers | Electrocautery-Enhanced Catheter |
|---|---|---|---|---|---|---|---|---|
| GIOBOR (Niti-S Biliary Covered Stent) | Taewoong Medical, Seoul, Republic of Korea | 8, 10 mm | 6, 8, 10, 12 cm (for each diameter) | Partially covered 30% Uncovered portion 70% Covered portion | Yes, proximal end | No | Yes, at both stent ends and at the end of covered part | No |
| HANAROSTENT Biliary | M.I. Tech, Seoul, Republic of Korea | 10 mm | 4, 5, 6, 7, 8, 9, 10, 11, 12 cm | Partially covered 3 cm Uncovered portion The rest covered | Yes, proximal end | BPD: No BPF: Yes, at both ends | Yes, at both stent ends and at the end of covered part | No |
| BONASTENT—Hybrid stent | Standard Sci Tech, Seoul, Republic of Korea | 8, 10 mm | 5, 6, 7, 8, 9, 10 cm (for each diameter) | Partially covered 35 mm Covered portion The rest uncovered | No | Yes, at the covered/uncovered passage, and at proximal end | No | No |
| BileRush Advance | Piolax Medical Devices, Kanagawa, Japan | 8 mm | 10, 12 cm | Partially covered 20 mm Uncovered portion The rest covered | Yes, proximal end | No | Yes, at both ends | No |
| HOT GIOBOR | Taewoong Medical, Seoul, Republic of Korea | 8, 10 mm | 10 cm | Partially covered 3 cm proximal uncovered portion The rest covered | Yes, proximal end | No | Yes, at both stent ends and at the end of covered part | Yes |
| First Author Year | Design | No. of Patients | Condition | Obstruction Site | Stent | Overall AEs % | Profile of AEs |
|---|---|---|---|---|---|---|---|
| Park 2015 [56] | RCT | 32 | Malignant | Proximal/Distal | Dedicated SEMS | 25 | Cholangitis (1/16) Leak (1/16) Bleeding (1/16) Pneumoperitoneum (4/16) |
| Paik 2018 [57] | RCT | 64 | Malignant | Proximal/Distal | SEMS | 10 | Pneumoperitoneum (2/7) Leak (1/7) Cholangitis (4/7) |
| Okuno 2018 [41] | Prospective | 20 | Malignant | Distal | SEMS | 15 | Cholangitis (2/20) Migration (6/20) Occlusion (4/20) |
| Sportes 2017 [65] | Retrospective | 31 | Malignant | Proximal/Distal | SEMS | 16 | Cholangitis (2/16) Bleeding (1/16) Leak (2/16) Mortality (2/16) |
| Miyano 2018 [48] | Prospective | 41 | Malignant | Proximal/Distal | SEMS | 40 | Leak (3/20) Cholangitis (1/20) Migration (1/20) |
| Minaga 2017 [66] | Retrospective | 30 | Malignant | Proximal | SEMS-Plastic | 33 (10) | Leak (3/10) Cholangitis (7/10) |
| Ochiai 2021 [67] | Retrospective | 48 | Malignant | Proximal/Distal | SEMS | 17 | Migration (5/8) Leak (3/8) |
| Anderloni 2022 [44] | Prospective | 22 | Malignant | Proximal/Distal | Dedicated SEMS | 13 | Hepatic abscess (3/3) |
| Nakai 2020 [42] | Retrospective | 111 | Malignant | Proximal/Distal | Dedicated SEMS | 25 | Leak (4/27) Cholangitis (3/27) Bleeding (1/27) |
| Schoch 2022 [68] | Retrospective | 34 | Malignant | Proximal | SEMS | 26 | Cholangitis (5/9) Bleeding (3/9) Leak (1/9) Mortality (1/9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazza, S.; Masciangelo, G.; Mauro, A.; Scalvini, D.; Torello Viera, F.; Bardone, M.; Veronese, L.; Rovedatti, L.; Agazzi, S.; Strada, E.; et al. Endoscopic Ultrasound-Guided Hepaticogastrostomy in Malignant Biliary Obstruction: A Comprehensive Review on Technical Tips and Clinical Outcomes. Diagnostics 2024, 14, 2644. https://doi.org/10.3390/diagnostics14232644
Mazza S, Masciangelo G, Mauro A, Scalvini D, Torello Viera F, Bardone M, Veronese L, Rovedatti L, Agazzi S, Strada E, et al. Endoscopic Ultrasound-Guided Hepaticogastrostomy in Malignant Biliary Obstruction: A Comprehensive Review on Technical Tips and Clinical Outcomes. Diagnostics. 2024; 14(23):2644. https://doi.org/10.3390/diagnostics14232644
Chicago/Turabian StyleMazza, Stefano, Graziella Masciangelo, Aurelio Mauro, Davide Scalvini, Francesca Torello Viera, Marco Bardone, Letizia Veronese, Laura Rovedatti, Simona Agazzi, Elena Strada, and et al. 2024. "Endoscopic Ultrasound-Guided Hepaticogastrostomy in Malignant Biliary Obstruction: A Comprehensive Review on Technical Tips and Clinical Outcomes" Diagnostics 14, no. 23: 2644. https://doi.org/10.3390/diagnostics14232644
APA StyleMazza, S., Masciangelo, G., Mauro, A., Scalvini, D., Torello Viera, F., Bardone, M., Veronese, L., Rovedatti, L., Agazzi, S., Strada, E., Pozzi, L., Barteselli, C., Sgarlata, C., Ravetta, V., Fusaroli, P., & Anderloni, A. (2024). Endoscopic Ultrasound-Guided Hepaticogastrostomy in Malignant Biliary Obstruction: A Comprehensive Review on Technical Tips and Clinical Outcomes. Diagnostics, 14(23), 2644. https://doi.org/10.3390/diagnostics14232644






