Early Experience with Inner Branch Stent–Graft System for Endovascular Repair of Thoraco-Abdominal and Pararenal Abdominal Aortic Aneurysm
Abstract
1. Introduction
2. Materials and Methods
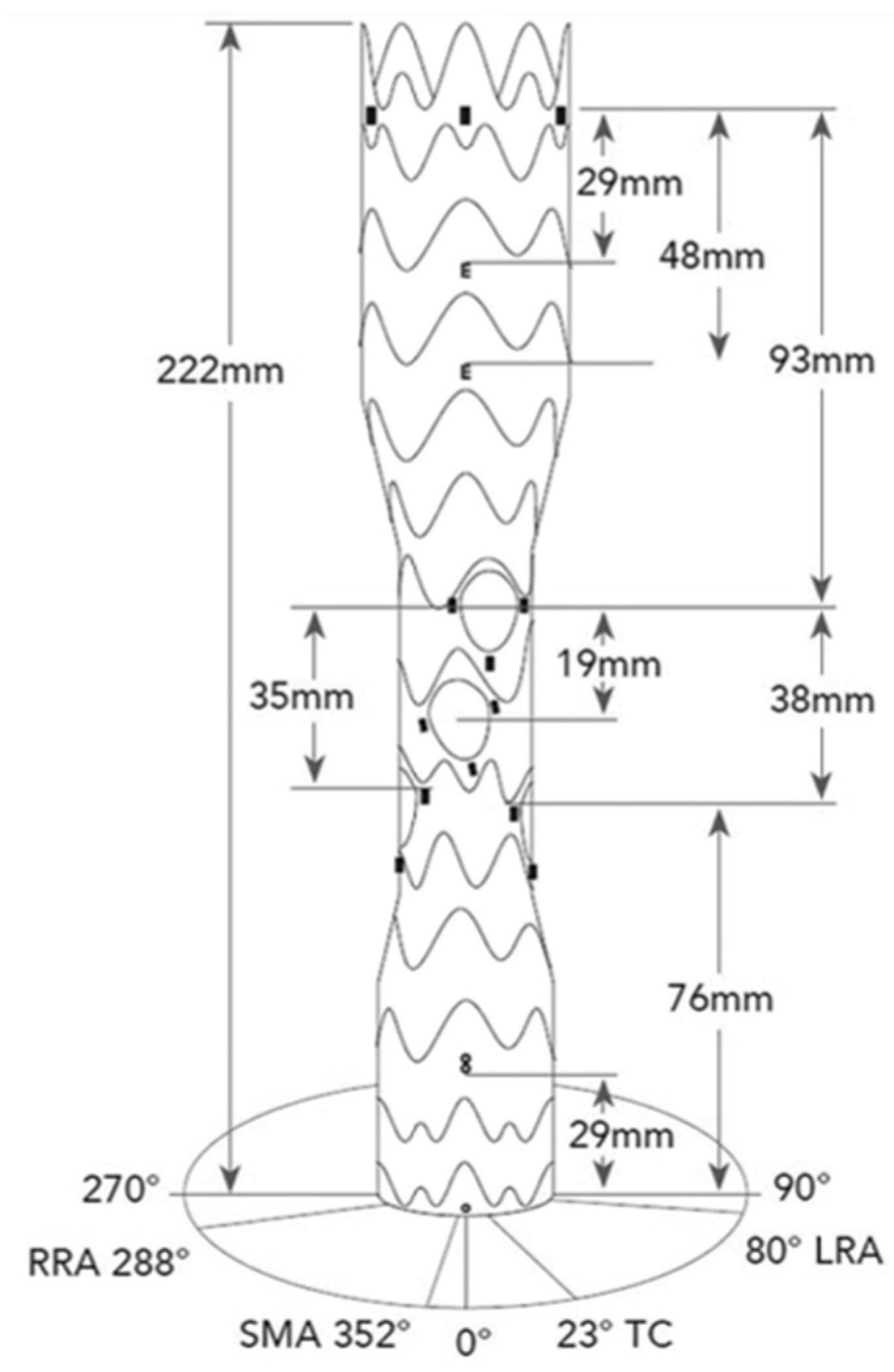
2.1. Device Description
2.2. Procedural Details
2.3. Endpoints
3. Results
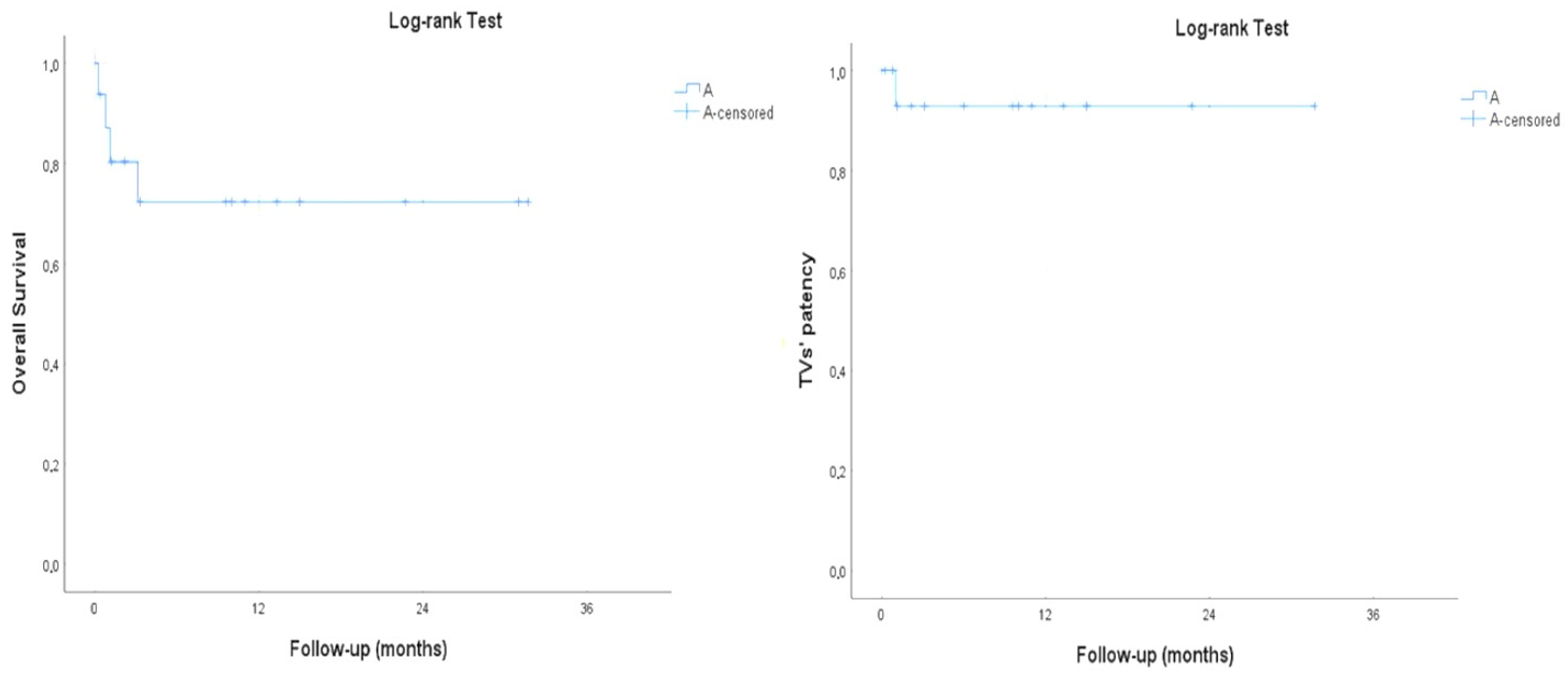
3.1. Perioperative Outcomes
3.2. Technical Metrics
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuozzo, S.; Sbarigia, E.; Jabbour, J.; Marzano, A.; D’Amico, C.; Brizzi, V.; Martinelli, O. Impact of frailty on outcomes of patients undergoing elective endovascular thoraco-abdominal aortic aneurysm repair. J. Cardiovasc. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Menges, A.L.; Rancic, Z.; Meuli, L.; Dueppers, P.; Reutersberg, B. E-nside Off-the-Shelf Inner BranchStentGraft: Technical Aspects of Planning and Implantation. J. Endovasc. Ther. 2022, 29, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kapalla, M.; Busch, A.; Lutz, B.; Nebelung, H.; Wolk, S.; Reeps, C. Single-center initial experience with inner-branch complex EVAR in 44 patients. Front. Cardiovasc. Med. 2023, 10, 1188501. [Google Scholar] [CrossRef] [PubMed]
- Toro, D.; Ohrlander, T.; Resch, T. Experience with inner branch off the shelf device for thoracoabdominal aneurysms. J. Cardiovasc. Surg. 2023, 64, 475–480. [Google Scholar] [CrossRef]
- Jónsson, G.G.; Mani, K.; Mosavi, F.; D’Oria, M.; Semenas, E.; Wanhainen, A.; Lindström, D. Spinal drain-related complications after complex endovascular aortic repair using a prophylactic automated volume-directed drainage protocol. J. Vasc. Surg. 2023, 78, 575–583.e572. [Google Scholar] [CrossRef]
- Riambau, V.; Böckler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G. Editor’s choice—Management of descending thoracic aorta diseases: Clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef]
- Kasprzak, P.M.; Gallis, K.; Cucuruz, B.; Pfister, K.; Janotta, M.; Kopp, R. Editor’s choice—Temporary aneurysm sac perfusion as an adjunct for prevention of spinal cord ischemia after branched endovascular repair of thoracoabdominal aneurysms. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 258–265. [Google Scholar] [CrossRef]
- Verzini, F.; Loschi, D.; De Rango, P.; Ferrer, C.; Simonte, G.; Coscarella, C.; Pogany, G.; Cao, P. Current results of total endovascular repair of thoracoabdominal aortic aneurysms. J. Cardiovasc. Surg. 2014, 55, 9–19. [Google Scholar]
- Piccinelli, M.; Veneziani, A.; Steinman, D.A.; Remuzzi, A.; Antiga, L. A framework for geometric analysis of vascular structures: Application to cerebral aneurysms. IEEE Trans. Med. Imaging 2009, 28, 1141–1155. [Google Scholar] [CrossRef]
- Feezor, R.J.; Martin, T.D.; Hess Jr, P.J.; Daniels, M.J.; Beaver, T.M.; Klodell, C.T.; Lee, W.A. Extent of aortic coverage and incidence of spinal cord ischemia after thoracic endovascular aneurysm repair. Ann. Thorac. Surg. 2008, 86, 1809–1814; discussion 1814. [Google Scholar] [CrossRef]
- Diamond, K.R.; Simons, J.P.; Crawford, A.S.; Arous, E.J.; Judelson, D.R.; Aiello, F.; Jones, D.W.; Messina, L.; Schanzer, A. Effect of thoracoabdominal aortic aneurysm extent on outcomes in patients undergoing fenestrated/branched endovascular aneurysm repair. J. Vasc. Surg. 2021, 74, 833–842.e2. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M.; Squizzato, F.; Ferri, M.; Pratesi, G.; Gatta, E.; Orrico, M.; Giudice, R.; Antonello, M.; Spezia, M.; Grego, F.; et al. INBREED Investigators. Outcomes of off-the-shelf preloaded inner branch device for urgent endovascular thoraco-abdominal aortic repair in the ItaliaN Branched Registry of E-nside EnDograft. J. Vasc. Surg. 2024, 80, 1350–1360.e4. [Google Scholar] [CrossRef] [PubMed]
- Cuozzo, S.; Martinelli, O.; Brizzi, V.; Miceli, F.; Flora, F.; Sbarigia, E.; Gattuso, R. Early Experience with Ovation Alto Stent-Graft. Ann. Vasc. Surg. 2023, 88, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, L.; Cambiaghi, T.; Ferrer, C.; Baccellieri, D.; Verzini, F.; Melissano, G.; Chiesa, R.; Tshomba, Y. Comparison of sacrificed healthy aorta during thoracoabdominal aortic aneurysm repair using off-the-shelf endovascular branched devices and open surgery. J. Vasc. Surg. 2018, 67, 695–702. [Google Scholar] [CrossRef]
- Spath, P.; Tsilimparis, N.; Furlan, F.; Hamwi, T.; Prendes, C.F.; Stana, J. Additional Aortic Coverage with an Off The Shelf, Multibranched Endograft Compared with Custom Made Devices For Endovascular Repair of Pararenal Abdominal Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 710–718. [Google Scholar] [CrossRef]
- Piazza, M.; Squizzato, F.; Pratesi, G.; Tshomba, Y.; Gaggiano, A.; Gatta, E.; Simonte, G.; Piffaretti, G.; Frigatti, P.; Veraldi, G.F.; et al. INBREED Investigators. Editor’s Choice—Early Outcomes of a Novel Off the Shelf Preloaded Inner Branch Endograft for the Treatment of Complex Aortic Pathologies in the Italian Branched Registry of E-nside EnDograft (INBREED). Eur. J. Vasc. Endovasc. Surg. 2023, 65, 811–817. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Wang, S.; D’Oria, M.; Zhang, X.; Bi, J.; Cui, D.; Dai, X. Systematic Review and Meta-analysis of Short-term and Mid-term Outcomes After Use of t-Branch Off-the-shelf Multibranched Endograft for Elective and Urgent Treatment of Thoracoabdominal Aortic Aneurysms. J. Endovasc. Ther. 2023. [Google Scholar] [CrossRef]
- Konstantinou, N.; Antonopoulos, C.N.; Jerkku, T.; Banafsche, R.; Kölbel, T.; Fiorucci, B.; Tsilimparis, N. Systematic review and meta-analysis of published studies on endovascular repair of thoracoabdominal aortic aneurysms with the t-Branch off-the-shelf multibranched endograft. J. Vasc. Surg. 2020, 72, 716–725.e1. [Google Scholar] [CrossRef]
- Abisi, S.; Zymvragoudakis, V.; Gkoutzios, P.; Sallam, M.; Donati, T.; Saha, P.; Zayed, H. Early outcomes of Jotec inner-branched endografts in complex endovascular aortic aneurysm repair. J. Vasc. Surg. 2021, 74, 871–879. [Google Scholar] [CrossRef]
- Bilman, V.; Cambiaghi, T.; Grandi, A.; Carta, N.; Melissano, G.; Chiesa, R.; Bertoglio, L. Anatomical feasibility of a new off-the-shelf inner branch stent graft (E-nside) for endovascular treatment of thoraco-abdominal aneurysms. Eur. J. Cardiothorac. Surg. 2020, 58, 1296–1303. [Google Scholar] [CrossRef]
- Silverberg, D.; Bar-Dayan, A.; Hater, H.; Khaitovich, B.; Halak, M. Short-term outcomes of inner branches for endovascular repair of complex abdominal and thoracoabdominal aortic aneurysms. Vascular 2021, 29, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.; Deglise, S.; Szopinski, P.; Jost-Philipp, S.; Jomha, A.; Vahl, C.F.; Koshty, A. A multicenter experience with a new fenestrated-branched device for endovascular repair of thoracoabdominal aortic aneurysms. J. Endovasc. Ther. 2018, 25, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Katsargyris, A.; de Marino, P.M.; Mufty, H.; Pedro, L.M.; Fernandes, R.; Verhoeven, E.L. Early experience with the use of inner branches in endovascular repair of complex abdominal and thoraco-abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Oderich, G.S.; Greenberg, R.K.; Farber, M.; Lyden, S.; Sanchez, L.; Fairman, R.; Jia, F.; Bharadwaj, P.; Zenith Fenestrated Study Investigators. Results of the United States multicenter prospective study evaluating the Zenith fenestrated endovascular graft for treatment of juxtarenal abdominal aortic aneurysms. J. Vasc. Surg. 2014, 60, 1420–1428.e5. [Google Scholar] [CrossRef] [PubMed]
- Doonan, R.J.; Bin-Ayeed, S.; Charbonneau, P.; Hongku, K.; Mackenzie, K.; Steinmetz, O.; Bayne, J.; Abraham, C.; Obrand, D.; Girsowicz, E.; et al. Mortality and major adverse events improve with increased institutional experience for fenestrated and branched endovascular aortic repair. J. Endovasc. Ther. 2021, 29, 746–754. [Google Scholar] [CrossRef]
- Spinella, G.; Pane, B.; Finotello, A.; Bastianon, M.; Mena Vera, J.M.; Di Gregorio, S.; Pratesi, G. Early Experience of Inner Branch Retrograde Cannulation With E-nside Branch Stent Graft for Thoracoabdominal Aortic Aneurysms. J. Endovasc. Ther. 2023, 8, 15266028231163067. [Google Scholar] [CrossRef]
- Yazar, O.; Huysmans, M.; Lacquet, M.; Salemans, P.B.; Wong, C.Y.; Bouwman, L.H. Single-Center Experience with Inner-Branched Endograft for the Treatment of Pararenal Abdominal Aortic Aneurysms. J. Endovasc. Ther. 2023, 16, 15266028231204286. [Google Scholar] [CrossRef]
- Silverberg, D.; Bar Dayan, A.; Speter, C.; Fish, M.; Halak, M. The Use of the Off-the-Shelf Inner Branch E-nside Endograft for the Treatment of Elective and Emergent Complex Aortic Aneurysms-A Single-Center Experience. Ann. Vasc. Surg. 2024, 104, 132–138. [Google Scholar] [CrossRef]
| Age-y (Mean, SD, Range) | 75.9 ± 5.5 (69–88) |
|---|---|
| Male (n, %) | 16 (72.7) |
| Arterial hypertension (n, %) | 22 (100) |
| Diabetes (n, %) | 3 (13.6) |
| Dyslipidemia (n, %) | 21 (95.4) |
| Smoking habit (n, %) | 15 (68.2) |
| Coronary artery disease (n, %) | 12 (54.5) |
| Chronic renal failure (n, %) | 10 (45.4) |
| COPD (n, %) | 12 (54.5) |
| Malignancy (n, %) | 6 (27.3) |
| Type I TAAA n = 4 | Type II TAAA n = 0 | Type III TAAA n = 8 | Type IV TAAA n = 5 | Type V TAAA n = 1 | PAAA n = 4 | |
|---|---|---|---|---|---|---|
| Outer-to-outer diameter (mm) at the level of proximal sealing | / | / | 33.2 ± 3.8 | 31 ± 2.5 | / | 31.5 ± 0.7 |
| Mean Aortic Diameter at Target Vessel Ostium Level (mm, Range) | Mean Time of Target Vessel Catheterization (min, Range) | |
|---|---|---|
| CT | 30.4 (24.5–44.2) | 20.7 (10–60) |
| SMA | 28.7 (21.3–40.4) | 15.5 (7–24) |
| RRA | 29.4 (19.7–54) | 21.1 (8–39) |
| LRA | 31.0 (19–54) | 21.1 (10–41) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuozzo, S.; Marzano, A.; Martinelli, O.; Jabbour, J.; Molinari, A.; Brizzi, V.; Sbarigia, E. Early Experience with Inner Branch Stent–Graft System for Endovascular Repair of Thoraco-Abdominal and Pararenal Abdominal Aortic Aneurysm. Diagnostics 2024, 14, 2612. https://doi.org/10.3390/diagnostics14232612
Cuozzo S, Marzano A, Martinelli O, Jabbour J, Molinari A, Brizzi V, Sbarigia E. Early Experience with Inner Branch Stent–Graft System for Endovascular Repair of Thoraco-Abdominal and Pararenal Abdominal Aortic Aneurysm. Diagnostics. 2024; 14(23):2612. https://doi.org/10.3390/diagnostics14232612
Chicago/Turabian StyleCuozzo, Simone, Antonio Marzano, Ombretta Martinelli, Jihad Jabbour, Andrea Molinari, Vincenzo Brizzi, and Enrico Sbarigia. 2024. "Early Experience with Inner Branch Stent–Graft System for Endovascular Repair of Thoraco-Abdominal and Pararenal Abdominal Aortic Aneurysm" Diagnostics 14, no. 23: 2612. https://doi.org/10.3390/diagnostics14232612
APA StyleCuozzo, S., Marzano, A., Martinelli, O., Jabbour, J., Molinari, A., Brizzi, V., & Sbarigia, E. (2024). Early Experience with Inner Branch Stent–Graft System for Endovascular Repair of Thoraco-Abdominal and Pararenal Abdominal Aortic Aneurysm. Diagnostics, 14(23), 2612. https://doi.org/10.3390/diagnostics14232612





