Quantification of Replacement Fibrosis in Aortic Stenosis: A Narrative Review on the Utility of Cardiovascular Magnetic Resonance Imaging
Abstract
1. Introduction
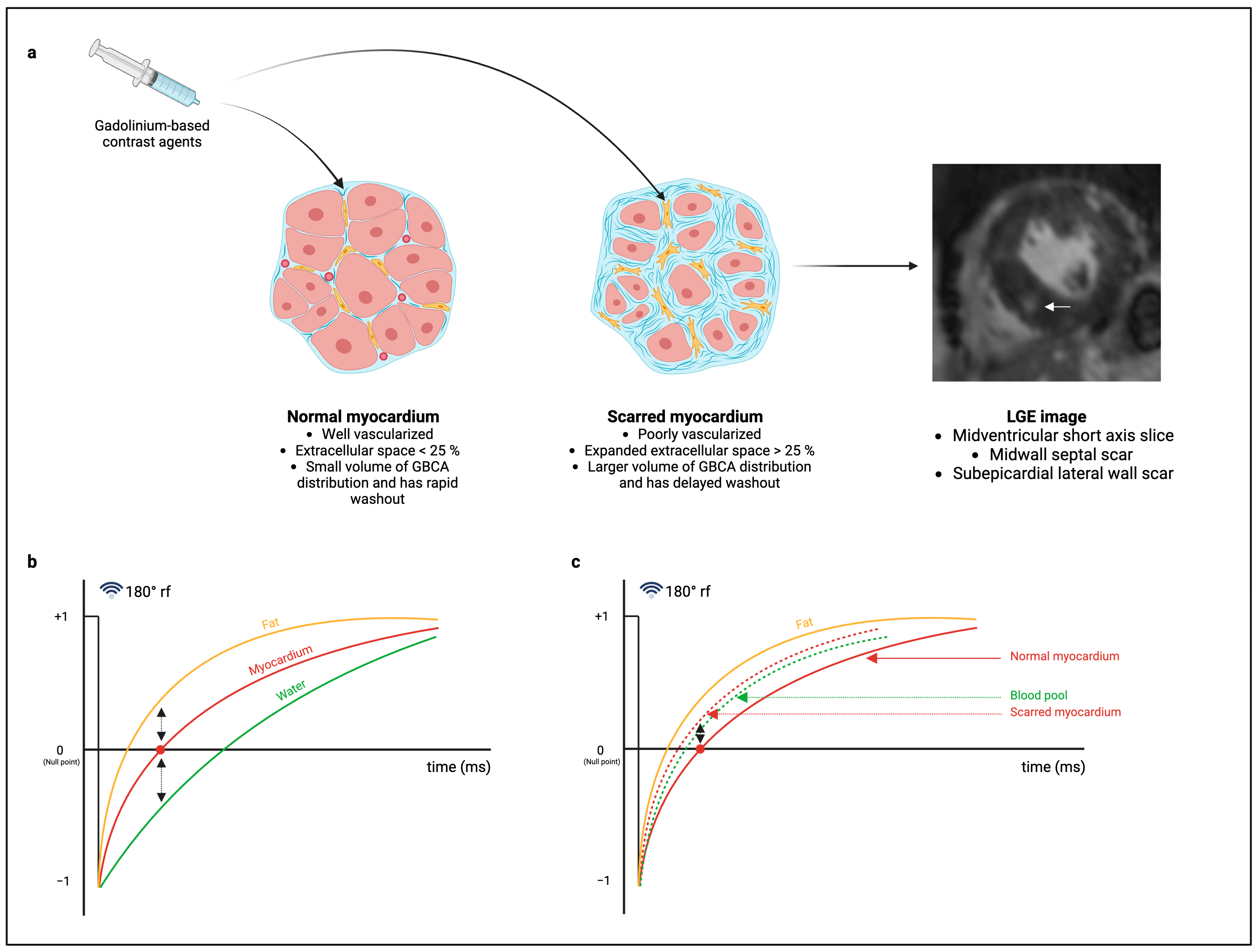
1.1. CMR Image Acquisition for the Detection of Replacement Fibrosis
1.2. Post-Processing Methods for the Quantification of Replacement Fibrosis in AS
1.2.1. Visual LGE Quantification Method
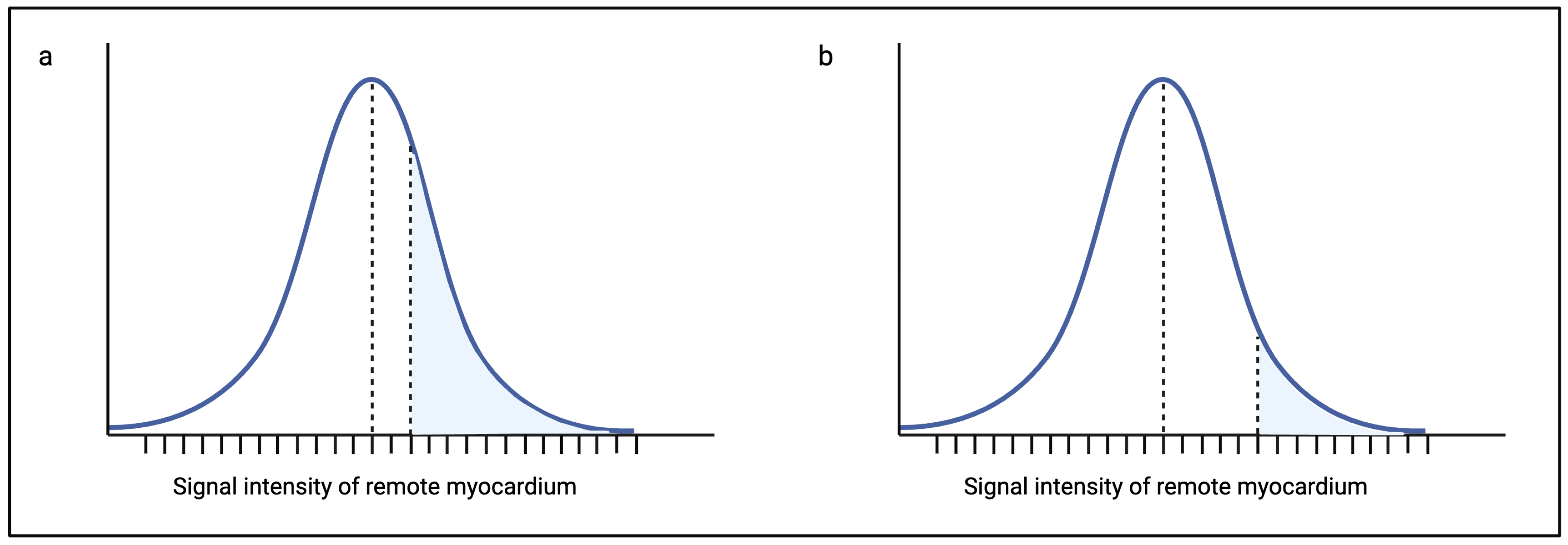
1.2.2. Signal Threshold Versus Reference Mean (STRM) Method
1.2.3. Full-Width Half-Maximum (FWHM) Method
1.2.4. Fully Automated Method
1.3. Validation of LGE Quantification
1.4. Towards a Consensus
2. Future Directions
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AS | aortic stenosis |
| AVR | aortic valve replacement |
| AHA | American Heart Association |
| ESC | European Society of Cardiology |
| LV | left ventricle/left ventricular |
| CMR | cardiovascular magnetic resonance imaging |
| GBCAs | gadolinium-based contrast agents |
| LGE | late gadolinium enhancement |
| STRM | signal threshold versus reference mean |
| SD | standard deviation(s) |
| FWHM | full-width half-maximum |
| H&E | haemotoxylin and eosin |
| DCM | dilated cardiomyopathy |
| ECV | extracellular volume |
References
- Thaden, J.J.; Nkomo, V.T.; Enriquez-Sarano, M. The global burden of aortic stenosis. Prog. Cardiovasc. Dis. 2014, 56, 565–571. [Google Scholar] [CrossRef]
- Cupido, B.J.; Peters, F.; Ntusi, N.A.B. An approach to the diagnosis and management of valvular heart disease. S. Afr. Med. J. 2016, 106, 39–42. [Google Scholar] [CrossRef]
- Coffey, S.; Roberts-Thomson, R.; Brown, A.; Carapetis, J.; Chen, M.; Enriquez-Sarano, M.; Zühlke, L.; Prendergast, B.D. Global epidemiology of valvular heart disease. Nat. Rev. Cardiol. 2021, 18, 853–864. [Google Scholar] [CrossRef]
- Okor, I.; Bob-Manual, T.; Garikapati, K.; Baldawi, H.; Gillies, C.; Ibebuogu, U.N. Trancatheter aortic valve replacement in rheumatic aortic stenosis: A comprehensive review. Curr. Probl. Cardiol. 2021, 46, 100843. [Google Scholar] [CrossRef]
- Marquis-Gravel, G.; Redfors, B.; Leon, M.B.; Généreux, P. Medical Treatment of Aortic Stenosis. Circulation 2016, 134, 1766–1784. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2021, 143, e27–e227. [Google Scholar]
- Lee, G.; Chikwe, J.; Milojevic, M.; Wijeysundera, H.C.; Biondi-Zoccai, G.; Flather, M.; Gaudino, M.F.L.; Fremes, S.E.; Tam, D.Y. ESC/EACTS vs. ACC/AHA guidelines for the management of severe aortic stenosis. Eur. Heart J. 2023, 44, 796–812. [Google Scholar] [CrossRef]
- Wagenar, M.; Reuthebuch, O.; Heg, D.; Tüller, D.; Ferrari, E.; Grünenfelder, J.; Huber, C.; Moarof, I.; Muller, O.; Nietlispach, F.; et al. Clinical outcomes in high-gradient, classical low-flow, low-gradient, and paradoxical low-flow, low-gradient aortic stenosis after transcatheter aortic valve implantation: A report from the SwissTAVI registry. J. Am. Heart Assoc. 2023, 12, e029489. [Google Scholar] [CrossRef]
- Puls, M.; Korte, K.P.; Bleckmann, A.; Huenlich, M.; Danner, B.; Schoendube, F.; Hasenfuß, G.; Jacobshagen, C.; Schillinger, W. Long-term outcomes after TAVI in patients with different types of aortic stenosis: The conundrum of low flow, low gradient and low ejection fraction. EuroIntervention 2017, 13, 286–293. [Google Scholar] [CrossRef]
- Bohbot, Y.; de Meester de Ravenstein, C.; Chadha, G.; Rusinaru, D.; Belkhir, K.; Trouillet, C.; Pasquet, A.; Marechaux, S.; Vanoverschelde, J.-L.; Tribouilloy, C. Relationship between left ventricular ejection fraction and mortality in asymptomatic and minimally symptomatic patients with severe aortic stenosis. JACC Cardiovasc. Imaging 2019, 12, 38–48. [Google Scholar] [CrossRef]
- Jacquemyn, X.; Strom, J.B.; Strange, G.; Playford, D.; Stewart, S.; Kutty, S.; Bhatt, D.L.; Bleiziffer, S.; Grubb, K.J.; Pellikka, P.A.; et al. Moderate aortic valve stenosis is associated with increased mortality rate and lifetime loss: Systematic review and meta-analysis of reconstructed time-to-event data of 409,680 patients. J. Am. Heart Assoc. 2024, 13, e033872. [Google Scholar] [CrossRef]
- Puls, M.; Beuthner, B.E.; Topci, R.; Vogelgesang, A.; Bleckmann, A.; Sitte, M.; Lange, T.; Backhaus, S.J.; Schuster, A.; Seidler, T.; et al. Impact of myocardial fibrosis on left ventricular remodelling, recovery, and outcome after transcatheter aortic valve implantation in different haemodynamic subtypes of severe aortic stenosis. Eur. Heart J. 2020, 41, 1903–1914. [Google Scholar] [CrossRef]
- Treibel, T.A.; Kozor, R.; Schofield, R.; Benedetti, G.; Fontana, M.; Bhuva, A.N.; Sheikh, A.; López, B.; González, A.; Manisty, C.; et al. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J. Am. Coll. Cardiol. 2018, 71, 860–871. [Google Scholar] [CrossRef]
- Călin, A.; Roşca, M.; Beladan, C.C.; Enache, R.; Mateescu, A.D.; Ginghină, C.; Popescu, B.A. The left ventricle in aortic stenosis—Imaging assessment and clinical implications. Cardiovasc. Ultrasound. 2015, 13, 22. [Google Scholar] [CrossRef]
- Calin, A.; Mateescu, A.D.; Popescu, A.C.; Bing, R.; Dweck, M.R.; Popescu, B.A. Role of advanced left ventricular imaging in adults with aortic stenosis. Heart 2020, 106, 962–969. [Google Scholar] [CrossRef]
- Bing, R.; Cavalcante, J.L.; Everett, R.J.; Clavel, M.A.; Newby, D.E.; Dweck, M.R. Imaging and impact of myocardial fibrosis in aortic stenosis. JACC Cardiovasc. Imaging 2019, 12, 283–296. [Google Scholar] [CrossRef]
- Krayenbuehl, H.P.; Hess, O.M.; Monrad, E.S.; Schneider, J.; Mall, G.; Turina, M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation 1989, 79, 744–755. [Google Scholar] [CrossRef]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A.C. Assessment of myocardial fibrosis with cardiac magnetic resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef]
- Treibel, T.A.; López, B.; González, A.; Menacho, K.; Schofield, R.S.; Ravassa, S.; Fontana, M.; White, S.K.; DiSalvo, C.; Roberts, N.; et al. Reappraising myocardial fibrosis in severe aortic stenosis: An invasive and non-invasive study in 133 patients. Eur. Heart J. 2018, 39, 699–709. [Google Scholar] [CrossRef]
- Weidemann, F.; Herrmann, S.; Störk, S.; Niemann, M.; Frantz, S.; Lange, V.; Beer, M.; Gattenlöhner, S.; Voelker, W.; Ertl, G.; et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009, 120, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Thornton, G.D.; Vassiliou, V.S.; A Musa, T.; Aziminia, N.; Craig, N.; Dattani, A.; Davies, R.H.; Captur, G.; Moon, J.C.; Dweck, M.R.; et al. Myocardial scar and remodelling predict long-term mortality in severe aortic stenosis beyond 10 years. Eur. Heart J. 2024, 45, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Barone-Rochette, G.; Piérard, S.; de Meester de Ravenstein, C.; Seldrum, S.; Melchior, J.; Maes, F.; Pouleur, A.-C.; Vancraeynest, D.; Pasquet, A.; Vanoverschelde, J.-L.; et al. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J. Am. Coll. Cardiol. 2014, 64, 144–154. [Google Scholar] [CrossRef]
- Dweck, M.R.; Joshi, S.; Murigu, T.; Alpendurada, F.; Jabbour, A.; Melina, G.; Banya, W.; Gulati, A.; Roussin, I.; Raza, S.; et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J. Am. Coll. Cardiol. 2011, 58, 1271–1279. [Google Scholar] [CrossRef]
- Musa, T.A.; Treibel, T.A.; Vassiliou, V.S.; Captur, G.; Singh, A.; Chin, C.; Dobson, L.E.; Pica, S.; Loudon, M.; Malley, T.; et al. Myocardial scar and mortality in severe aortic stenosis. Circulation 2018, 138, 1935–1947. [Google Scholar] [CrossRef]
- Rajesh, G.N.; Thottian, J.J.; Subramaniam, G.; Desabandhu, V.; Sajeev, C.G.; Krishnan, M.N. Prevalence and prognostic significance of left ventricular myocardial late gadolinium enhancement in severe aortic stenosis. Indian Heart J. 2017, 69, 742–750. [Google Scholar] [CrossRef]
- Quarto, C.; Dweck, M.R.; Murigu, T.; Joshi, S.; Melina, G.; Angeloni, E.; Prasad, S.K.; Pepper, J.R. Late gadolinium enhancement as a potential marker of increased perioperative risk in aortic valve replacement. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 45–50. [Google Scholar] [CrossRef][Green Version]
- Azevedo, C.F.; Nigri, M.; Higuchi, M.L.; Pomerantzeff, P.M.; Spina, G.S.; Sampaio, R.O.; Tarasoutchi, F.; Grinberg, M.; Rochitte, C.E. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J. Am. Coll. Cardiol. 2010, 56, 278–287. [Google Scholar] [CrossRef]
- Chin, C.W.L.; Everett, R.J.; Kwiecinski, J.; Vesey, A.T.; Yeung, E.; Esson, G. Myocardial fibrosis and cardiac decompensation in aortic stenosis. J. Am. Coll. Cardiol. 2017, 10, 1320–1333. [Google Scholar] [CrossRef]
- Hinderer, S.; Schenke-Layland, K. Cardiac fibrosis—A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82. [Google Scholar] [CrossRef]
- Bohbot, Y.; Renard, C.; Manrique, A.; Levy, F.; Maréchaux, S.; Gerber, B.L.; Tribouilloy, C. Usefulness of cardiac magnetic resonance imaging in aortic stenosis. Circ. Cardiovasc. Imaging 2020, 13, e010356. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Menger, J.; Bleumke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of trustees task force on standardized post-processing. J. Cardiovasc. Magn. Reson. 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Biglands, J.D.; Radjenovic, A.; Ridgway, J.P. Cardiovascular magnetic resonance physics for clinicians: Part II. J. Cardiovasc. Magn. Reson. 2012, 14, 66. [Google Scholar] [CrossRef]
- Selvanayagam, J.; Nucifora, G. Early and late gadolinium enhancement. In The EACVI Textbook of Cardiovascular Magnetic Resonance; Lombardi, M., Plein, S., Petersen, S., Bucciarelli-Ducci, C., Buechel, E.R.V., Basso, C., Ferrari, V., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 119–132. [Google Scholar]
- Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Klem, I.; Judd, R.M.; Kim, R.J.; Kim, H.W. Revisiting how we perform late gadolinium enhancement CMR: Insights gleaned over 25 years of clinical practice. J. Cardiovasc. Magn. Reson. 2023, 25, 18. [Google Scholar] [CrossRef] [PubMed]
- Holtackers, R.J.; Emrich, T.; Botnar, R.M.; Kooi, M.E.; Wildberger, J.E.; Kreitner, K.F. Late gadolinium enhancement cardiac magnetic resonance imaging: From basic concepts to emerging methods. Rofo 2022, 194, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.L.; Finn, J.P. MRI contrast agents. In The EACVI Textbook of Cardiovascular Magnetic Resonance; Lombardi, M., Plein, S., Petersen, S., Bucciarelli-Ducci, C., Buechel, E.R.V., Basso, C., Ferrari, V., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 63–69. [Google Scholar]
- Kwong, R.Y.; Farzaneh-Far, A. Measuring myocardial scar by CMR. J. Am. Coll. Cardiol. 2011, 4, 157–160. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Zhao, S.; Lu, M. Detection of myocardial fibrosis: Where we stand. Front. Cardiovasc. Med. 2022, 9, 926378. [Google Scholar] [CrossRef]
- de Meester de Ravenstein, C.; Bouzin, C.; Lazam, S.; Boulif, J.; Amzulescu, M.; Melchio, J.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.-C.; Vanoverschelde, J.-L.J.; et al. Histological validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3T. J. Cardiovasc. Magn. Reson. 2015, 17, 48. [Google Scholar] [CrossRef]
- Kellman, P.; Xue, H.; Olivieri, L.J.; Cross, R.R.; Grant, E.K.; Fontana, M.; Ugander, M.; Moon, J.C.; Hansen, M.S. Dark blood late enhancement imaging. J. Cardiovasc. Magn. Reson. 2016, 18, 77. [Google Scholar] [CrossRef]
- Holtackers, R.J.; Van De Heyning, C.M.; Chiribiri, A.; Wildberger, J.E.; Botnar, R.M.; Kooi, M.E. Dark-blood late gadolinium enhancement cardiovascular magnetic resonance for improved detection of subendocardial scar: A review of current techniques. J. Cardiovasc. Magn. Reson. 2021, 23, 96. [Google Scholar] [CrossRef]
- Flett, A.S.; Hasleton, J.; Cook, C.; Hausenloy, D.; Quarta, G.; Ariti, C.; Muthurangu, V.; Moon, J.C. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc. Imaging 2011, 4, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Dusenberry, S.M.; Jerosch-Herold, M.; Rickers, C.; Colan, S.D.; Geva, T.; Newburger, J.W. Myocardial extracellular remodeling is associated with ventricular diastolic dysfunction in children and young adults with congenital aortic stenosis. J. Am. Coll. Cardiol. 2014, 63, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.W.L.; Shah, A.S.V.; McAllister, D.A.; Cowell, S.J.; Alam, S.; Langrish, J.P.; Strachan, F.E.; Hunter, A.L.; Choy, A.M.; Lang, C.C.; et al. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur. Heart J. 2014, 35, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.V.; Chin, C.W.L.; Vassiliou, V.; Cowell, S.J.; Doris, M.; Kwok, T.C.; Semple, S.; Zamvar, V.; White, A.C.; McKillop, G.; et al. Left ventricular hypertrophy with strain and aortic stenosis. Circulation 2014, 130, 1607–1616. [Google Scholar] [CrossRef]
- Rudolph, A.; Abdel-Aty, H.; Bohl, S.; Boyé, P.; Zagrosek, A.; Dietz, R.; Schulz-Menger, J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy. J. Am. Coll. Cardiol. 2009, 53, 284–291. [Google Scholar] [CrossRef]
- Debl, K.; Djavidani, B.; Buchner, S.; Lipke, C.; Nitz, W.; Feuerbach, S.; Riegger, G.; Luchner, A. Delayed hyperenhancement in magnetic resonance imaging of left ventricular hypertrophy caused by aortic stenosis and hypertrophic cardiomyopathy: Visualization of focal fibrosis. Heart 2006, 92, 1447–1451. [Google Scholar] [CrossRef]
- Everett, R.J.; Tastet, L.; Clavel, M.-A.; Chin, C.W.L.; Capoulade, R.; Vassiliou, V.S.; Kwiecinski, J.; Gomez, M.; van Beek, E.J.; White, A.C.; et al. Progression of hypertrophy and myocardial fibrosis in aortic stenosis. Circ. Cardiovasc. Imaging 2018, 11, e007451. [Google Scholar] [CrossRef]
- Tastet, L.; Kwiecinski, J.; Pibarot, P.; Capoulade, R.; Everett, R.J.; Newby, D.E.; Shen, M.; Guzzetti, E.; Arsenault, M.; Bédard, É.; et al. Sex-related differences in the extent of myocardial fibrosis in patients with aortic valve stenosis. J. Am. Coll. Cardiol. 2020, 13, 699–711. [Google Scholar] [CrossRef]
- Child, N.; Suna, G.; Dabir, D.; Yap, M.-L.; Rogers, T.; Kathirgamanathan, M.; Arroyo-Ucar, E.; Hinojar, R.; Mahmoud, I.; Young, C.; et al. Comparison of MOLLI, shMOLLI, and SASHA in discrimination between health and disease and relationship with histologically derived collagen volume fraction. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 768–776. [Google Scholar] [CrossRef]
- Singh, A.; Chan, D.C.S.; Kanagala, P.; Hogrefe, K.; Kelly, D.J.; Khoo, J.P. Short term adverse remodeling progression in asymptomatic aortic stenosis. Eur. Radiol. 2021, 31, 3923–3930. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.-B.; Yoon, Y.E.; Park, E.-A.; Kim, H.-K.; Lee, W.; Kim, Y.-J.; Cho, G.-Y.; Sohn, D.-W.; Greiser, A.; et al. Noncontrast myocardial T1 mapping by cardiac magnetic resonance predicts outcome in patients with aortic stenosis. J. Am. Coll. Cardiol. 2018, 11, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Maltes, S.; Abecasis, J.; Santos, R.R.; Oliveira, L.; Mendes, G.S.; Guerreiro, S.; Lima, T.; Freitas, P.; Ferreira, A.; Cardim, N.; et al. Late gadolinium enhancement patterns in severe symptomatic high-gradient aortic stenosis. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544.233. [Google Scholar] [CrossRef]
- Maltes, S.; Abecasis, J.; Santos, R.R.; Lopes, P.; Oliveira, L.; Guerreiro, S.; Freitas, P.; Ferreira, A.; Nolasco, T.; Gil, V.; et al. LGE prevalence and patterns in severe aortic stenosis: When “junctional” means the same. Int. J. Cardiol. 2023, 378, 159–163. [Google Scholar] [CrossRef]
- Hwang, I.-C.; Kim, H.-K.; Park, J.-B.; Park, E.-A.; Lee, W.; Lee, S.-P.; Kim, Y.-J.; Sohn, D.-W.; Oh, J.K. Aortic valve replacement-induced changes in native T1 are related to prognosis in severe aortic stenosis: T1 mapping cardiac magnetic resonance imaging study. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 653–663. [Google Scholar] [CrossRef]
- Balčiūnaitė, G.; Besusparis, J.; Palionis, D.; Žurauskas, E.; Skorniakov, V.; Janušauskas, V.; Zorinas, A.; Zaremba, T.; Valevičienė, N.; Šerpytis, P.; et al. Exploring myocardial fibrosis in severe aortic stenosis: Echo, CMR and histology data from FIB-AS study. Int. J. Cardiovasc. Imaging 2022, 38, 1555–1568. [Google Scholar] [CrossRef]
- Hoffmann, R.; Altiok, E.; Friedman, Z.; Becker, M.; Frick, M. Myocardial deformation imaging by two-dimensional speckle-tracking echocardiography in comparison to late gadolinium enhancement cardiac magnetic resonance for analysis of myocardial fibrosis in severe aortic stenosis. Am. J. Cardiol. 2014, 114, 1083–1088. [Google Scholar] [CrossRef]
- Fairbairn, T.A.; Steadman, C.D.; Mather, A.N.; Motwani, M.; Blackman, D.J.; Plein, S.; McCann, G.P.; Greenwood, J.P. Assessment of valve haemodynamics, reverse ventricular remodelling and myocardial fibrosis following transcatheter aortic valve implantation compared to surgical aortic valve replacement: A cardiovascular magnetic resonance study. Heart 2013, 99, 1185–1191. [Google Scholar] [CrossRef]
- Everett, R.J.; Treibel, T.A.; Fukui, M.; Lee, H.; Rigolli, M.; Singh, A.; Bijsterveld, P.; Tastet, L.; Al Musa, T.; Dobson, L.; et al. Extracellular myocardial volume in patients with aortic stenosis. J. Am. Coll. Cardiol. 2020, 75, 304–316. [Google Scholar] [CrossRef]
- Maltes, S.; Abecasis, J.; Pinto, D.G.; Santos, R.R.; Oliveira, L.; Mendes, G.S.; Guerreiro, S.; Lima, T.; Freitas, P.; Ferreira, A.; et al. Histology-verified myocardial fibrosis and quantification in severe As patient: Correlation with non-invasive LV myocardial tissue assessment. Eur. Heart J. 2022, 43 (Suppl. S2), ehac544.2996. [Google Scholar] [CrossRef]
- Kwak, S.; Everett, R.J.; Treibel, T.A.; Yang, S.; Hwang, D.; Ko, T.; Williams, M.C.; Bing, R.; Singh, T.; Joshi, S.; et al. Markers of myocardial damage predicts mortality in patients with aortic stenosis. J. Am. Coll. Cardiol. 2021, 78, 545–558. [Google Scholar] [CrossRef]
- Fine, N.M.; Tandon, S.; Kim, H.W.; Shah, D.J.; Thompson, T.; Drangova, M.; White, J.A. Validation of sub-segmental visual scoring for the quantification of ischemic and noneschemic myocardial fibrosis using late gadolinium enhancement MRI. J. Cardiovasc. Magn. Reson. 2013, 38, 1369–1376. [Google Scholar]
- Spiewak, M.; Małek, L.A.; Misko, J.; Chojnowska, L.; Milosz, B.; Klopotowski, M.; Petryka, J.; Dabrowski, M.; Kepka, C.; Ruzyllo, W. Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur. J. Radiol. 2010, 74, e149–e153. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Coehlo-Filho, O.R.; Danik, S.B.; Shah, R.V.; Dodson, J.A.; Verdini, D.J.; Tokuda, M.; Daly, C.A.; Tedrow, U.B.; Stevenson, W.G.; et al. CMR quantification of myocardial scar provides additive prognostic information in nonischaemic cardiomyopathy. JACC Cardiovasc. Imaging 2013, 6, 944–954. [Google Scholar] [CrossRef]
- Gräni, C.; Eichhorn, C.; Bière, L.; Kaneko, K.; Murthy, V.L.; Agarwal, V.; Aghayev, A.; Steigner, M.; Blankstein, R.; Jerosch-Herold, M.; et al. Comparison of myocardial fibrosis quantification methods by cardiovascular magnetic resonance imaging for risk stratification of patients with suspected myocarditis. J. Cardiovasc. Magn. Reson. 2019, 21, 14. [Google Scholar] [CrossRef]
- Iles, L.M.; Ellims, A.H.; Llewellyn, H.; Hare, J.L.; Kaye, D.M.; McLean, C.A.; Taylor, A.J. Histological validation of cardiac magnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 14–22. [Google Scholar] [CrossRef]
- Dweck, M.R.; Boon, N.A.; Newby, D.E. Calcific aortic stenosis. J. Am. Coll. Cardiol. 2012, 60, 1854–1863. [Google Scholar] [CrossRef]
- Ridgway, J.P. Cardiovascular magnetic resonance physics for clinicians: Part I. J. Cardiovasc. Magn. Reson. 2010, 12, 71. [Google Scholar] [CrossRef]
- Vermes, E.; Childs, H.; Carbone, I.; Barckow, P.; Friedrich, M.G. Auto-threshold quantification of late gadolinium enhancement in patients with acute heart disease. J. Cardiovasc. Magn. Reson. 2013, 37, 382–390. [Google Scholar] [CrossRef]
- Flett, A.S.; Hasleton, J.M.; Quarta, G.; Hausenloy, D.; Muthurangu, V.; Moon, J.C. The full with half maximum technique is superior for LGE quantification regardless of its aetiology. J. Cardiovasc. Magn. Reson. 2010, 12 (Suppl. S1), O41. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial tissue characterization and fibrosis by imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef]
- Makarov, I.; Voronkina, D.; Gurshchenkov, A.; Ryzhkov, A.; Starshinova, A.; Kudlay, D.; Mitrofanova, L. Are endomyocardial ventricular biopsies useful for assessing myocardial fibrosis? J. Clin. Med. 2024, 13, 3275. [Google Scholar] [CrossRef] [PubMed]
- Schipke, J.; Brandenberger, C.; Rajces, A.; Manninger, M.; Alogna, A.; Post, H.; Mühlfeld, C. Assessment of cardiac fibrosis: A morphometric method comparison for collagen quantification. J. Appl. Physiol. 2017, 122, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- de Jong, S.; van Veen, T.A.B.; de Bakker, J.M.T.; van Rijen, H.V.M. Monitoring cardiac fibrosis: A technical challenge. Neth. Heart J. 2012, 20, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Francone, M. Role of cardiac magnetic resonance in the evaluation of dilated cardiomyopathy: Diagnostic contribution and prognostic significance. ISRN Radiol. 2014, 2014, 365404. [Google Scholar] [CrossRef] [PubMed]
- Crossman, D.J.; Ruygrok, P.N.; Hou, Y.F.; Soeller, C. Next-generation endomyocardial biopsy: The potential of confocal and super-resolution microscopy. Heart Fail. Rev. 2015, 20, 203–214. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar]
- Jathanna, N.; Podlasek, A.; Sokol, A.; Auer, D.; Chen, X.; Jamil-Copley, S. Diagnostic utility of artificial intelligence for left ventricular scar identification using cardiac magnetic resonance imaging—A systematic review. Cardiovasc. Digit. Health J. 2021, 2 (Suppl. S6), S21–S29. [Google Scholar] [CrossRef]
- Fadil, H.; Totman, J.J.; Hausenloy, D.J.; Ho, H.H.; Joseph, P.; Low, A.F.H.; Richards, A.M.; Chan, M.Y.; Marchesseau, S. A deep learning pipeline for automatic analysis of multi-scancardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2021, 23, 47. [Google Scholar] [CrossRef]
- Carminati, M.C.; Boniotti, C.; Fusini, L.; Andreini, D.; Pontone, G.; Pepi, M.; Caiani, E.G. Comparison of image processing techniques for nonviable tissue quantification in late gadolinium enhancement cardiac magnetic resonance imaging. J. Thorac. Imaging 2016, 31, 168–176. [Google Scholar] [CrossRef]
- López, B.; González, A.; Ravassa, S.; Beaumont, J.; Moreno, M.U.; San José, G.; Querejeta, R.; Díez, J. Circulating biomarkers of myocardial fibrosis: The need for a reappraisal. J. Am. Coll. Cardiol. 2015, 65, 2449–2456. [Google Scholar] [CrossRef]
- Fan, D.; Takawale, A.; Lee, J.; Kassiri, Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012, 5, 15. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
| Study | LGE Quantification Method | Endomyocardial Biopsy for Validation of LGE Quantification |
|---|---|---|
| Weidemann et al. (2009) [21] | Counted segments | Yes |
| Dusenberry et al. (2014) [44] | Counted segments | No |
| Chin et al. (2014) [45] | 2 SD STRM method | No |
| Shah et al. (2014) [46] | 2 SD STRM method | No |
| Rudolph et al. (2009) [47] | 2 SD STRM method | No |
| Debl et al. (2006) [48] | 2 SD STRM method | No |
| Barone-Rochette et al. (2014) [23] | 2.4 SD STRM method | No |
| Rajesh et al. (2017) [26] | 2.4 SD STRM method and counted segments | No |
| De Meester de Ravenstein et al. (2015) [40] | 2.4 SD STRM method | Yes |
| Treibel et al. (2018) [20] | 3 SD STRM method | Yes |
| Treibel et al. (2018) [14] | 3 SD STRM method | No (Published in previous article) |
| Everett et al. (2018) [49] | 3 SD STRM method | No |
| Tastet et al. (2020) [50] | 3 SD STRM method and FWHM | No |
| Puls et al. (2020) [13] | 3 SD STRM method | No |
| Child et al. (2018) [51] | 4 SD STRM method | No |
| Singh et al. (2021) [52] | 5 SD STRM method | No |
| Lee et al. (2018) [53] | 5 SD STRM method | No |
| Maltes et al. (2022) [54] | 5 SD STRM method | No |
| Maltes et al. (2023) [55] | 5 SD STRM method | No |
| Hwang et al. (2020) [56] | 5 SD STRM method | No |
| Balčiūnaitė et al. (2021) [57] | 5 SD STRM method | No |
| Hoffmann et al. (2014) [58] | 6 SD STRM method | No |
| Quarto et al. (2012) [27] | FWHM | No |
| Dweck et al. (2011) [24] | FWHM | No |
| Musa et al. (2018) [25] | FWHM | No |
| Fairbairn et al. (2013) [59] | FWHM | No |
| Everett et al. (2020) [60] | FWHM | No |
| Azevedo et al. (2010) [28] | Modified 2 SD STRM method | Yes |
| Maltes et al. (2022) [61] | Unknown | Yes |
| Kwak et al. (2021) [62] | Unknown | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajah, M.R.; Doubell, A.F.; Herbst, P.G. Quantification of Replacement Fibrosis in Aortic Stenosis: A Narrative Review on the Utility of Cardiovascular Magnetic Resonance Imaging. Diagnostics 2024, 14, 2435. https://doi.org/10.3390/diagnostics14212435
Rajah MR, Doubell AF, Herbst PG. Quantification of Replacement Fibrosis in Aortic Stenosis: A Narrative Review on the Utility of Cardiovascular Magnetic Resonance Imaging. Diagnostics. 2024; 14(21):2435. https://doi.org/10.3390/diagnostics14212435
Chicago/Turabian StyleRajah, Megan R., Anton F. Doubell, and Philip G. Herbst. 2024. "Quantification of Replacement Fibrosis in Aortic Stenosis: A Narrative Review on the Utility of Cardiovascular Magnetic Resonance Imaging" Diagnostics 14, no. 21: 2435. https://doi.org/10.3390/diagnostics14212435
APA StyleRajah, M. R., Doubell, A. F., & Herbst, P. G. (2024). Quantification of Replacement Fibrosis in Aortic Stenosis: A Narrative Review on the Utility of Cardiovascular Magnetic Resonance Imaging. Diagnostics, 14(21), 2435. https://doi.org/10.3390/diagnostics14212435






