mFFE CT-like MRI Sequences for the Assessment of Vertebral Fractures
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Population
2.2. Imaging Protocol and Analysis
2.3. Statistical Analysis
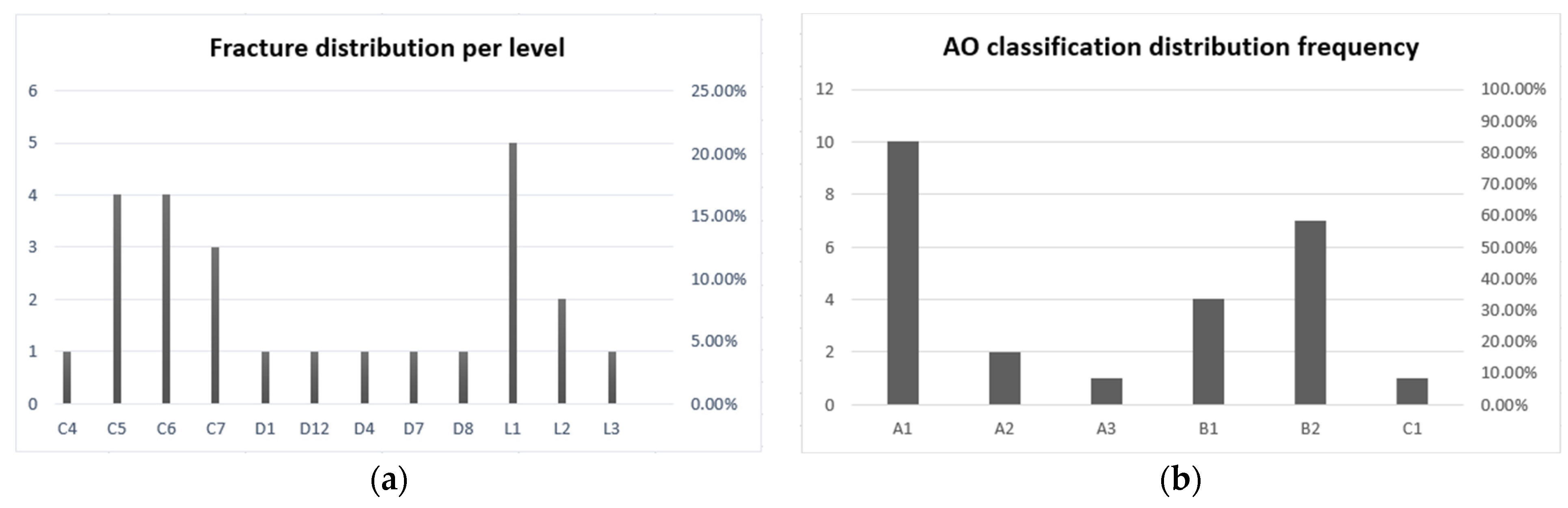
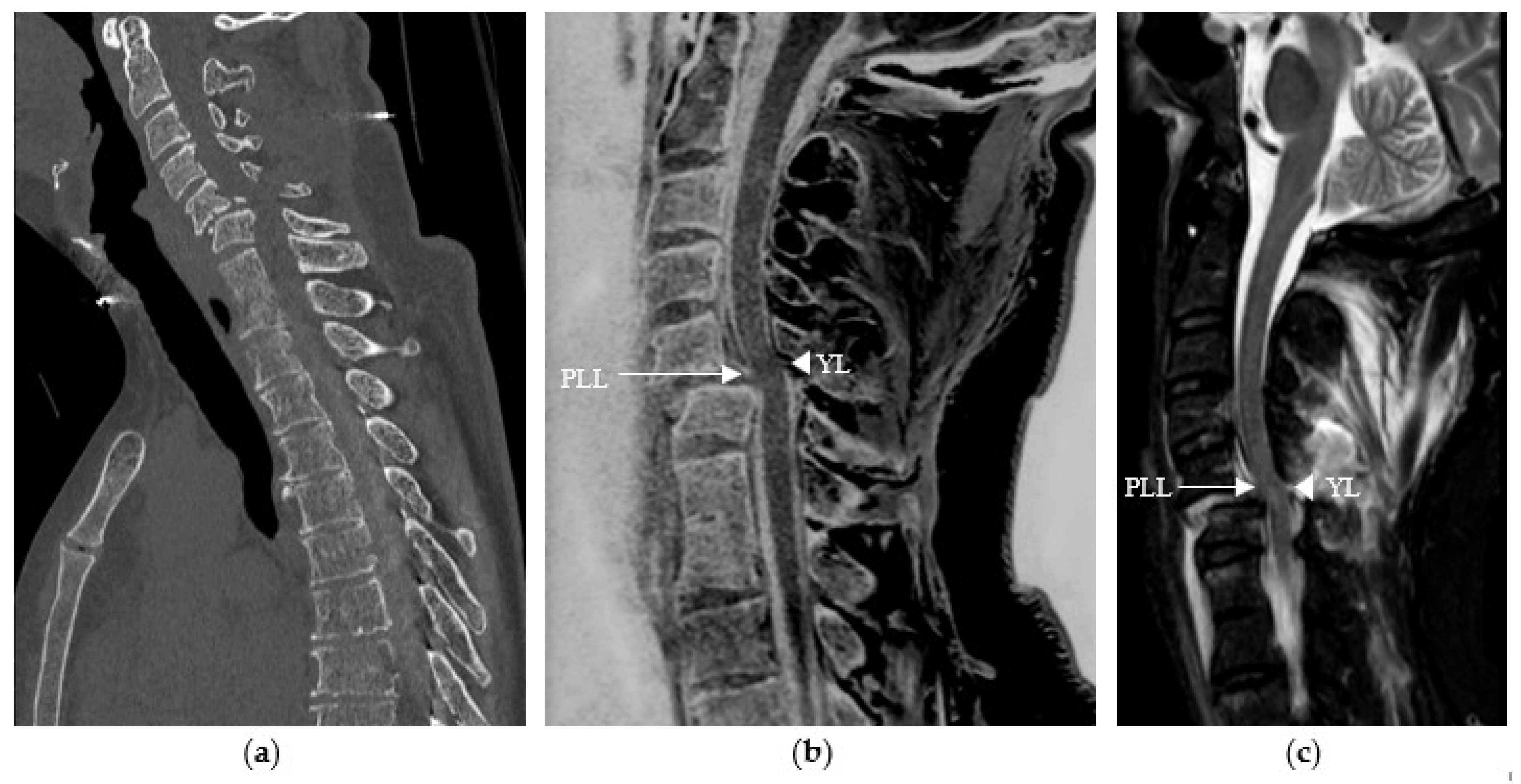
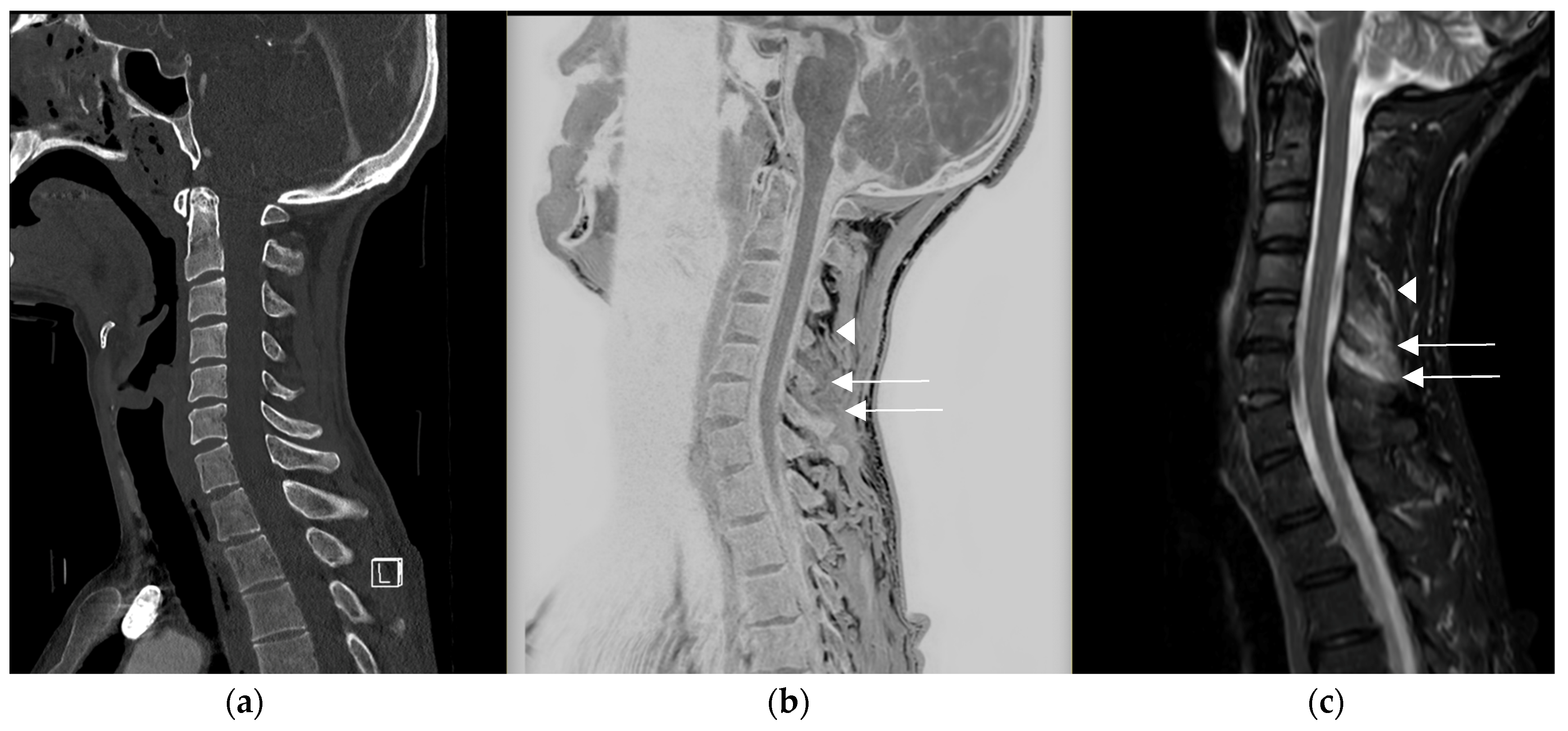
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burger, H.; Van Daele, P.L.; Grashuis, K.; Hofman, A.; Grobbee, D.E.; Schütte, H.E.; Birkenhäger, J.C.; Pols, H.A. Vertebral deformities and functional impairment in men and women. J. Bone Miner. Res. 1997, 12, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, B.; Black, D.M.; Nevitt, M.C.; Rundle, A.C.; Cauley, J.A.; Cummings, S.R.; Genant, H.K. Contribution of vertebral deformities to chronic back pain and disability. The Study of Osteoporotic Fractures Research Group. J. Bone Miner. Res. 1992, 7, 449–456. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, T.W.; Cockerill, W.; Matthis, C.; Raspe, H.H.; Lunt, M.; Cooper, C.; Banzer, D.; Cannata, J.B.; Naves, M.; Felsch, B.; et al. Back pain, disability, and radiographic vertebral fracture in European women: A prospective study. Osteoporos. Int. 2004, 15, 760–765. [Google Scholar] [CrossRef]
- Ioannidis, G.; Papaioannou, A.; Hopman, W.M.; Akhtar-Danesh, N.; Anastassiades, T.; Pickard, L.; Kennedy, C.C.; Prior, J.C.; Olszynski, W.P.; Davison, K.S.; et al. Relation between fractures and mortality: Results from the Canadian Multicentre Osteoporosis Study. CMAJ 2009, 181, 265–271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bliuc, D.; Nguyen, N.D.; Milch, V.E.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009, 301, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Sistrom, C.L.; McKay, N.L. Costs, charges, and revenues for hospital diagnostic imaging procedures: Differences by modality and hospital characteristics. J. Am. Coll. Radiol. 2005, 2, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Schegerer, A.; Loose, R.; Heuser, L.J.; Brix, G. Diagnostic Reference Levels for Diagnostic and Interventional X-Ray Procedures in Germany: Update and Handling. RöFo 2019, 191, 739–751, English, German. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, B.J.; Schneider, C.; Kronthaler, S.; Gassert, F.T.; Böhm, C.; Pfeiffer, D.; Baum, T.; Kirschke, J.S.; Karampinos, D.C.; Makowski, M.R.; et al. CT-like images based on T1 spoiled gradient-echo and ultra-short echo time MRI sequences for the assessment of vertebral fractures and degenerative bone changes of the spine. Eur. Radiol. 2021, 31, 4680–4689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feuerriegel, G.C.; Kronthaler, S.; Boehm, C.; Renz, M.; Leonhardt, Y.; Gassert, F.; Foreman, S.C.; Weiss, K.; Wurm, M.; Liebig, T.; et al. Diagnostic value of water-fat-separated images and CT-like susceptibility-weighted images extracted from a single ultrashort echo time sequence for the evaluation of vertebral fractures and degenerative changes of the spine. Eur. Radiol. 2023, 33, 1445–1455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gassert, F.T.; Kufner, A.; Renz, M.; Gassert, F.G.; Bollwein, C.; Kronthaler, S.; Feuerriegel, G.C.; Kirschke, J.S.; Ganter, C.; Makowski, M.R.; et al. Comparing CT-like Images Based on Ultra-Short Echo Time and Gradient Echo T1-Weighted MRI Sequences for the Assessment of Vertebral Disorders Using Histology and True CT as the Reference Standard. J. Magn. Reson. Imaging. 2024, 59, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Gersing, A.S.; Pfeiffer, D.; Kopp, F.K.; Schwaiger, B.J.; Knebel, C.; Haller, B.; Noël, P.B.; Settles, M.; Rummeny, E.J.; Woertler, K. Evaluation of MR-derived CT-like images and simulated radiographs compared to conventional radiography in patients with benign and malignant bone tumors. Eur. Radiol. 2019, 29, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Alizai, H.; Dempsey, M. Fast field echo resembling a CT using restricted echo-spacing (FRACTURE): A novel MRI technique with superior bone contrast. Skelet. Radiol. 2021, 50, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Gomyo, M.; Katase, S.; Hiraoka, S.; Tateishi, H. Magnetic resonance bone imaging: Applications to vertebral lesions. Jpn. J. Radiol. 2023, 41, 1173–1185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Z.Z.; Shan, H.; Li, X. Fat-suppressed 3D T1-weighted gradient-echo imaging of the cartilage with a volumetric interpolated breath-hold examination. Am. J. Roentgenol. 2010, 194, W414–W419. [Google Scholar] [CrossRef] [PubMed]
- Florkow, M.C.; Willemsen, K.; Mascarenhas, V.V.; Oei, E.H.G.; van Stralen, M.; Seevinck, P.R. Magnetic Resonance Imaging Versus Computed Tomography for Three-Dimensional Bone Imaging of Musculoskeletal Pathologies: A Review. J. Magn. Reson. Imaging 2022, 56, 11–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lafforgue, P.F.; Chagnaud, C.J.; Daver, L.M.; Daumen-Legré, V.M.; Peragut, J.C.; Kasbarian, M.J.; Volot, F.; Acquaviva, P.C. Intervertebral disk vacuum phenomenon secondary to vertebral collapse: Prevalence and significance. Radiology 1994, 193, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Schnake, K.J.; Schroeder, G.D.; Vaccaro, A.R.; Oner, C. AOSpine Classification Systems (Subaxial, Thoracolumbar). J. Orthop. Trauma 2017, 31, S14–S23. [Google Scholar] [CrossRef] [PubMed]
- Beeharry, M.W.; Moqeem, K.; Rohilla, M.U. Management of Cervical Spine Fractures: A Literature Review. Cureus 2021, 13, e14418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joaquim, A.F.; Patel, A.A.; Schroeder, G.D.; Vaccaro, A.R. A simplified treatment algorithm for treating thoracic and lumbar spine trauma. J. Spinal Cord. Med. 2019, 42, 416–422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- So, A.; Nicolaou, S. Spectral Computed Tomography: Fundamental Principles and Recent Developments. Korean J. Radiol. 2021, 22, 86–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gosangi, B.; Mandell, J.C.; Weaver, M.J.; Uyeda, J.W.; Smith, S.E.; Sodickson, A.D.; Khurana, B. Bone Marrow Edema at Dual-Energy CT: A Game Changer in the Emergency Department. Radiographics 2020, 40, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Yun, S.J.; Jin, W.; Lee, S.H.; Park, S.Y.; Ryu, C.W. Diagnostic performance of dual-energy CT for the detection of bone marrow oedema: A systematic review and meta-analysis. Eur. Radiol. 2018, 28, 4182–4194. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wu, G.; Chang, X. Diagnostic accuracy of dual-energy computed tomography in bone marrow edema with vertebral compression fractures: A meta-analysis. Eur. J. Radiol. 2018, 99, 124–129. [Google Scholar] [CrossRef] [PubMed]

| 3D mFFE Cervical Spine | 3D mFFE Thoracic or Lumbar Spine | |
|---|---|---|
| Field of view | 320 × 250 mm | 320 × 250 mm |
| Acquisition voxel size | 0.8 × 0.8 × 1.5 mm | 0.8 × 0.8 × 1.5 mm |
| Reconstruction voxel size | 0.42 × 0.42 × 0.75 mm | 0.42 × 0.42 × 0.75 mm |
| Parallel imaging | CS-SENSE factor 2 | CS-SENSE factor 2 |
| Echo time (ms) | 4.6 ms | 4.6 ms |
| Repetition time (ms) | 31 ms | 35 ms |
| Delta echo time (ms) | 5.8 ms | 5.8 ms |
| Acquisition time | 3 min 46 s | 5 min 09 s |
| Parameter | Description | Grading Scale |
|---|---|---|
| Genant classification | Semiquantitative visual grading of vertebral deformities | Grade 1 (20–25%) Grade 2 (25–40%) Grade 3 (>40%) |
| Anterior vertebral body height | Measured in the median sagittal plane, from the anterosuperior to the anteroinferior corner of the vertebral body, excluding osteophytes or fragments | mm |
| Posterior vertebral body height | Measured in the median sagittal plane, from the posterosuperior to the posteroinferior corner of the vertebral body, excluding osteophytes or fragments | mm |
| AO/Magerl classification | Classification of fractures in compression, distraction, and translation injuries according to Magerl et al. and Vaccaro et al. | A1: wedge compression A2: split A3 + 4: incomplete and complete burst B: distraction C: displacement or dislocation |
| Disc fracture | Fracture or traumatic displacement | Yes or no |
| Ligamentous injury | Yes or no | |
| Diagnostic quality of the images | Likert scale | 1: inadequate 2: poor 3: moderate 4: good 5: excellent |
| Parameters | Inter-Observer Agreement for CT Images | Inter-Observer Agreement for CT-like Images | Intra-Observer Agreement Between CT and CT-like Images (Observer 1) |
|---|---|---|---|
| AO classification (weighted kappa) | 0.93321 95%CI (0.83866–1.0000) | 0.91289 95%CI (0.81294–1.0000) | 0.87027 95%CI (0.73079–1.0000) |
| Genant classification (weighted kappa) | 0.94783 95%CI (0.84661–1.0000) | 0.94643 95%CI (0.84161–1.0000) | 0.79039 95%CI (0.60741–0.97338) |
| Anterior vertebral body height (ICC) | 0.9684 95%CI (0.9284–0.9862) | 0.9474 95%CI (0.8824–0.9769) | 0.9600 95%CI (0.9098–0.9825) |
| Posterior vertebral body height (ICC) | 0.9795 95%CI (0.9533–0.9911) | 0.9692 95%CI (0.9302–0.9866) | 0.9757 95%CI (0.9447–0.9894) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira Branco, D.; Bouredoucen, H.; Hamard, M.; Gorican, K.; Poletti, P.-A.; Delattre, B.M.A.; Boudabbous, S. mFFE CT-like MRI Sequences for the Assessment of Vertebral Fractures. Diagnostics 2024, 14, 2434. https://doi.org/10.3390/diagnostics14212434
Ferreira Branco D, Bouredoucen H, Hamard M, Gorican K, Poletti P-A, Delattre BMA, Boudabbous S. mFFE CT-like MRI Sequences for the Assessment of Vertebral Fractures. Diagnostics. 2024; 14(21):2434. https://doi.org/10.3390/diagnostics14212434
Chicago/Turabian StyleFerreira Branco, David, Hicham Bouredoucen, Marion Hamard, Karel Gorican, Pierre-Alexandre Poletti, Bénédicte Marie Anne Delattre, and Sana Boudabbous. 2024. "mFFE CT-like MRI Sequences for the Assessment of Vertebral Fractures" Diagnostics 14, no. 21: 2434. https://doi.org/10.3390/diagnostics14212434
APA StyleFerreira Branco, D., Bouredoucen, H., Hamard, M., Gorican, K., Poletti, P.-A., Delattre, B. M. A., & Boudabbous, S. (2024). mFFE CT-like MRI Sequences for the Assessment of Vertebral Fractures. Diagnostics, 14(21), 2434. https://doi.org/10.3390/diagnostics14212434





