Comparison of Serum and Cerebrospinal Fluid Neurofilament Light Chain Concentrations Measured by Ella™ and Lumipulse™ in Patients with Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. NfL Assays
2.3. Statistical Analysis
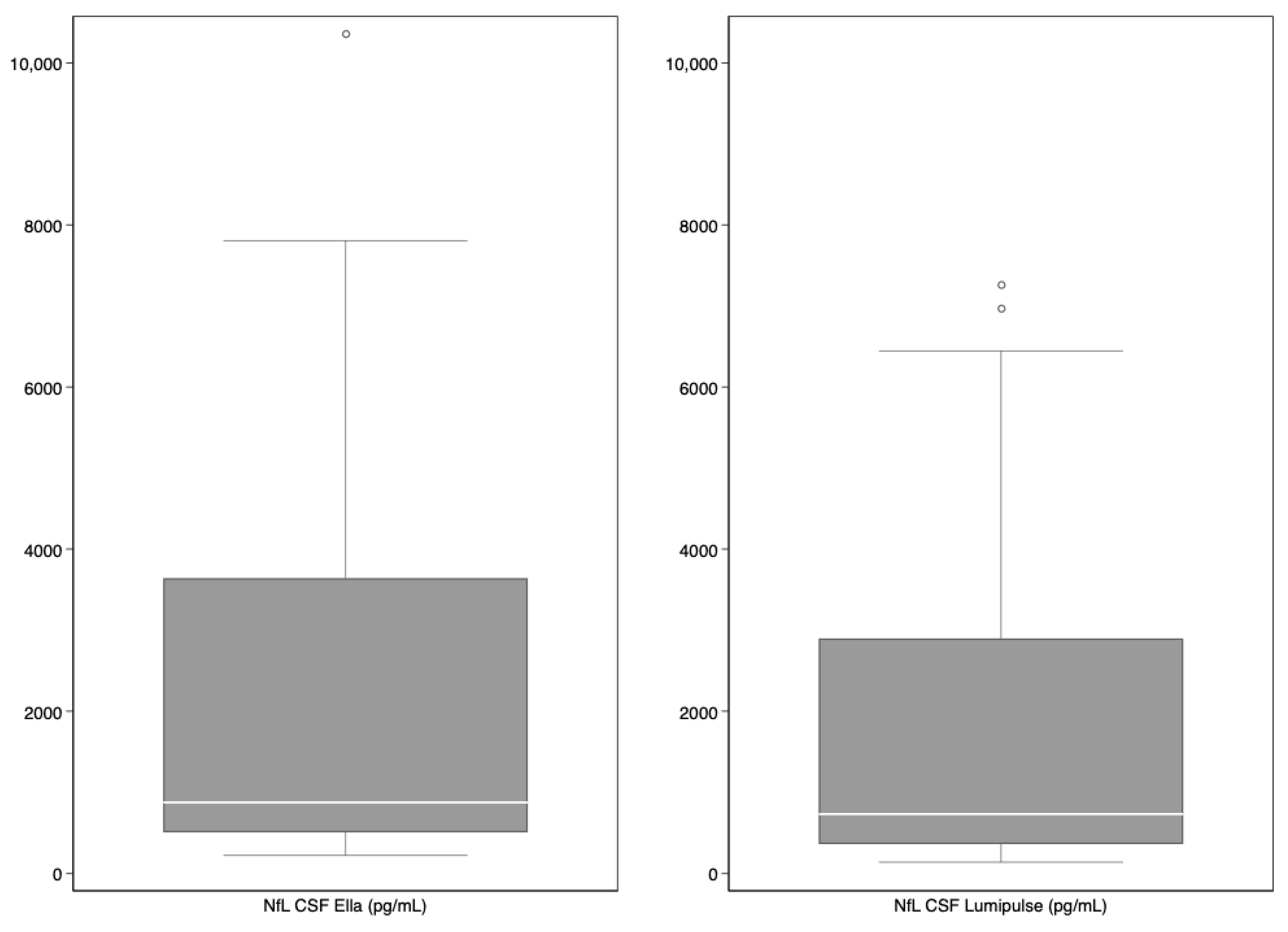
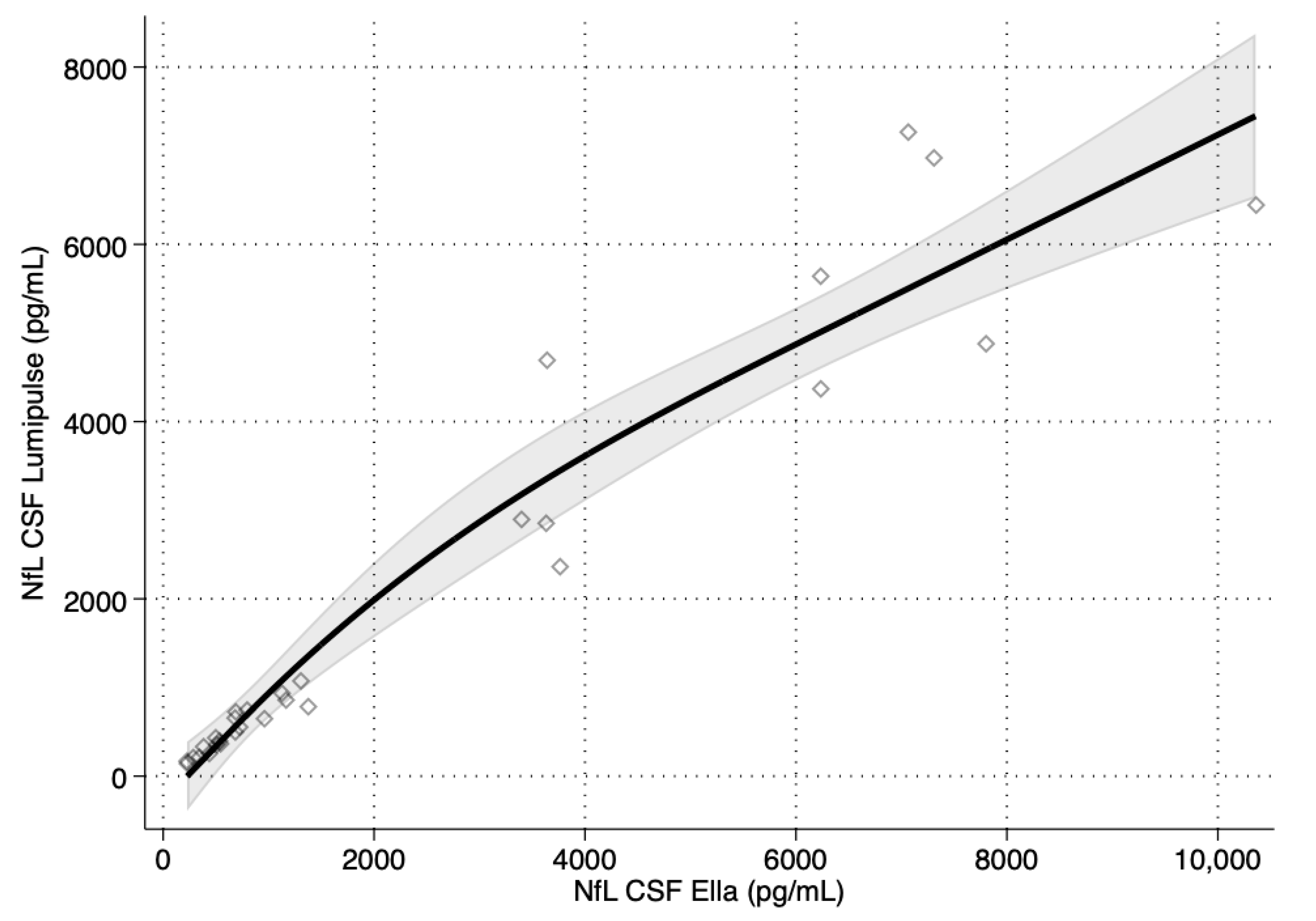
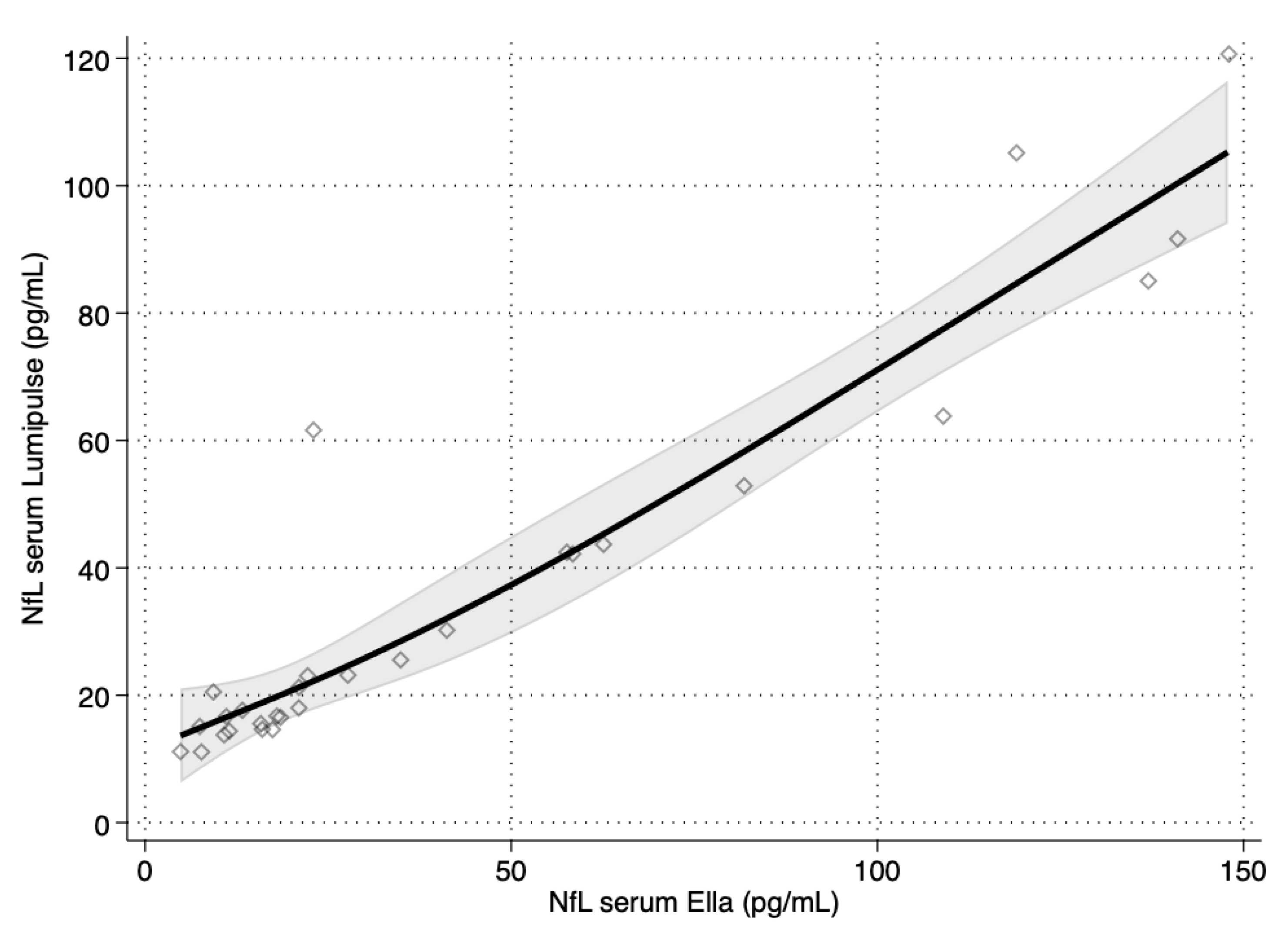
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bomont, P. The dazzling rise of neurofilaments: Physiological functions and roles as biomarkers. Curr. Opin. Cell Biol. 2021, 68, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Bridel, C.; van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; the NFL Group. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Barro, C.; Chitnis, T.; Weiner, H.L. Blood neurofilament light: A critical review of its application to neurologic disease. Ann. Clin. Transl. Neurol. 2020, 7, 2508–2523. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Yuan, A.; Nixon, R.A. Neurofilament proteins as biomarkers to monitor neurological diseases and the efficacy of therapies. Front. Neurosci. 2021, 15, 689938. [Google Scholar] [CrossRef]
- Kalm, M.; Bostrom, M.; Sandelius, A.; Eriksson, Y.; Ek, C.J.; Blennow, K.; Bjork-Eriksson, T.; Zetterberg, H. Serum concentrations of the axonal injury marker neurofilament light protein are not influenced by blood-brain barrier permeability. Brain Res. 2017, 1668, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Uher, T.; McComb, M.; Galkin, S.; Srpova, B.; Oechtering, J.; Barro, C.; Tyblova, M.; Bergsland, N.; Krasensky, J.; Dwyer, M.; et al. Neurofilament levels are associated with blood-brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult. Scler. 2021, 27, 220–231. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Abdelhak, A.; Foschi, M.; D’Anna, L.; Russo, M.; Steinacker, P.; Kuhle, J.; Tumani, H.; Blennow, K.; Otto, M. The multifaceted role of neurofilament light chain protein in non-primary neurological diseases. Brain 2023, 146, 421–437. [Google Scholar] [CrossRef]
- Lopes das Neves, P.; Duraes, J.; Silva-Spinola, A.; Lima, M.; Leitao, M.J.; Tabuas-Pereira, M.; Santana, I.; Baldeiras, I. Serum Neurofilament Light Chain in the Diagnostic Evaluation of Patients with Cognitive Symptoms in the Neurological Consultation of a Tertiary Center. J. Alzheimer’s Dis. 2023, 95, 391–397. [Google Scholar] [CrossRef]
- Notzel, M.; Werder, L.I.; Ziemssen, T.; Akgun, K. Ella versus Simoa Serum Neurofilament Assessment to Monitor Treatment Response in Highly Active Multiple Sclerosis Patients. Int. J. Mol. Sci. 2022, 23, 12361. [Google Scholar] [CrossRef]
- Vecchio, D.; Puricelli, C.; Malucchi, S.; Virgilio, E.; Martire, S.; Perga, S.; Passarelli, F.; Valentino, P.; Di Sapio, A.; Cantello, R.; et al. Serum and cerebrospinal fluid neurofilament light chains measured by SIMOA, Ella, and Lumipulse in multiple sclerosis naive patients. Mult. Scler. Relat. Disord. 2024, 82, 105412. [Google Scholar] [CrossRef] [PubMed]
- Truffi, M.; Garofalo, M.; Ricciardi, A.; Cotta Ramusino, M.; Perini, G.; Scaranzin, S.; Gastaldi, M.; Albasini, S.; Costa, A.; Chiavetta, V.; et al. Neurofilament-light chain quantification by Simoa and Ella in plasma from patients with dementia: A comparative study. Sci. Rep. 2023, 13, 4041. [Google Scholar] [CrossRef] [PubMed]
- Mersch, G.; Dauwe, M.; De Geyter, K.; Vandeponseele, P.; Le Bastard, N.; Vandenbroucke, I. Analytical performance of the Lumipulse® G NfL CSF. In Proceedings of the AD/PD 2023, Gothenburg, Sweden, 28 March–1 April 2023. [Google Scholar]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Nordberg, A.; Backman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment--beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Willemse, E.A.; Teunissen, C.E. Biobanking of cerebrospinal fluid for biomarker analysis in neurological diseases. In Biobanking in the 21st Century; Springer: Cham, Switzerland, 2015; pp. 79–93. [Google Scholar]
- Tondelli, M.; Salemme, S.; Vinceti, G.; Bedin, R.; Trenti, T.; Molinari, M.A.; Chiari, A.; Zamboni, G. Predictive value of phospho-tau/total-tau ratio in amyloid-negative Mild Cognitive Impairment. Neurosci. Lett. 2022, 787, 136811. [Google Scholar] [CrossRef]
- Urbano, T.; Chiari, A.; Malagoli, C.; Cherubini, A.; Bedin, R.; Costanzini, S.; Teggi, S.; Maffeis, G.; Vinceti, M.; Filippini, T. Particulate matter exposure from motorized traffic and risk of conversion from mild cognitive impairment to dementia: An Italian prospective cohort study. Environ. Res. 2023, 222, 115425. [Google Scholar] [CrossRef]
- Vinceti, M.; Urbano, T.; Chiari, A.; Filippini, T.; Wise, L.A.; Tondelli, M.; Michalke, B.; Shimizu, M.; Saito, Y. Selenoprotein P concentrations and risk of progression from mild cognitive impairment to dementia. Sci. Rep. 2023, 13, 8792. [Google Scholar] [CrossRef]
- Martinelli, I.; Zucchi, E.; Simonini, C.; Gianferrari, G.; Bedin, R.; Biral, C.; Ghezzi, A.; Fini, N.; Carra, S.; Mandrioli, J. SerpinA1 levels in amyotrophic lateral sclerosis patients: An exploratory study. Eur. J. Neurol. 2024, 31, e16054. [Google Scholar] [CrossRef]
- Vinceti, M.; Urbano, T.; Filippini, T.; Bedin, R.; Simonini, C.; Sorarù, G.; Trojsi, F.; Michalke, B.; Mandrioli, J. Changes in Cerebrospinal Fluid Concentrations of Selenium Species Induced by Tofersen Administration in Subjects with Amyotrophic Lateral Sclerosis Carrying SOD1 Gene Mutations. Biol. Trace Elem. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- The MathWorks Inc. MATLAB, R2022a; The MathWorks Inc.: Natick, MA, USA, 2022. [Google Scholar]
- Urbano, T.; Filippini, T.; Malavolti, M.; Fustinoni, S.; Michalke, B.; Wise, L.A.; Vinceti, M. Adherence to the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet and exposure to selenium species: A cross-sectional study. Nutr. Res. 2023, 122, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Urbano, T.; Verzelloni, P.; Malavolti, M.; Sucato, S.; Polledri, E.; Agnoli, C.; Sieri, S.; Natalini, N.; Marchesi, C.; Fustinoni, S.; et al. Influence of dietary patterns on urinary excretion of cadmium in an Italian population: A cross-sectional study. J. Trace Elem. Med. Biol. 2023, 80, 127298. [Google Scholar] [CrossRef] [PubMed]
- Alcolea, D.; Beeri, M.S.; Rojas, J.C.; Gardner, R.C.; Lleo, A. Blood Biomarkers in Neurodegenerative Diseases: Implications for the Clinical Neurologist. Neurology 2023, 101, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Arslan, B.; Zetterberg, H. Neurofilament light chain as neuronal injury marker—what is needed to facilitate implementation in clinical laboratory practice? Clin. Chem. Lab. Med. 2023, 61, 1140–1149. [Google Scholar] [CrossRef]
- Bayoumy, S.; Verberk, I.M.W.; Vermunt, L.; Willemse, E.; den Dulk, B.; van der Ploeg, A.T.; Pajkrt, D.; Nitz, E.; van den Hout, J.M.P.; van der Post, J.; et al. Neurofilament light protein as a biomarker for spinal muscular atrophy: A review and reference ranges. Clin. Chem. Lab. Med. 2024. [Google Scholar] [CrossRef]
- Benatar, M.; Ostrow, L.W.; Lewcock, J.W.; Bennett, F.; Shefner, J.; Bowser, R.; Larkin, P.; Bruijn, L.; Wuu, J. Biomarker Qualification for Neurofilament Light Chain in Amyotrophic Lateral Sclerosis: Theory and Practice. Ann. Neurol. 2024, 95, 211–216. [Google Scholar] [CrossRef]
- Gallingani, C.; Carbone, C.; Tondelli, M.; Zamboni, G. Neurofilaments Light Chain in Neurodegenerative Dementias: A Review of Imaging Correlates. Brain Sci. 2024, 14, 272. [Google Scholar] [CrossRef]
- Gauthier, A.; Viel, S.; Perret, M.; Brocard, G.; Casey, R.; Lombard, C.; Laurent-Chabalier, S.; Debouverie, M.; Edan, G.; Vukusic, S.; et al. Comparison of Simoa(TM) and Ella(TM) to assess serum neurofilament-light chain in multiple sclerosis. Ann. Clin. Transl. Neurol. 2021, 8, 1141–1150. [Google Scholar] [CrossRef]
- Giuffre, G.M.; Quaranta, D.; Costantini, E.M.; Citro, S.; Martellacci, N.; De Ninno, G.; Vita, M.G.; Guglielmi, V.; Rossini, P.M.; Calabresi, P.; et al. Cerebrospinal fluid neurofilament light chain and total-tau as biomarkers of neurodegeneration in Alzheimer’s disease and frontotemporal dementia. Neurobiol. Dis. 2023, 186, 106267. [Google Scholar] [CrossRef]
- Walia, N.; Eratne, D.; Loi, S.M.; Farrand, S.; Li, Q.X.; Malpas, C.B.; Varghese, S.; Walterfang, M.; Evans, A.H.; Parker, S.; et al. Cerebrospinal fluid neurofilament light and cerebral atrophy in younger-onset dementia and primary psychiatric disorders. Intern. Med. J. 2023, 53, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Bolsewig, K.; Hok, A.H.Y.S.; Sepe, F.N.; Boonkamp, L.; Jacobs, D.; Bellomo, G.; Paoletti, F.P.; Vanmechelen, E.; Teunissen, C.E.; Parnetti, L.; et al. A Combination of Neurofilament Light, Glial Fibrillary Acidic Protein, and Neuronal Pentraxin-2 Discriminates Between Frontotemporal Dementia and Other Dementias. J. Alzheimer’s Dis. 2022, 90, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.; Murotani, K.; Sato, N.; Tsuduki, T.; Hisada, T.; Shinohara, M.; Sugimoto, T.; Niida, S.; Toba, K.; Sakurai, T. Relationship Between Plasma Neurofilament Light Chain, Gut Microbiota, and Dementia: A Cross-Sectional Study. J. Alzheimer’s Dis. 2022, 86, 1323–1335. [Google Scholar] [CrossRef]
- Fang, T.; Dai, Y.; Hu, X.; Xu, Y.; Qiao, J. Evaluation of serum neurofilament light chain and glial fibrillary acidic protein in the diagnosis of Alzheimer’s disease. Front. Neurol. 2024, 15, 1320653. [Google Scholar] [CrossRef]
- Andersson, E.; Janelidze, S.; Lampinen, B.; Nilsson, M.; Leuzy, A.; Stomrud, E.; Blennow, K.; Zetterberg, H.; Hansson, O. Blood and cerebrospinal fluid neurofilament light differentially detect neurodegeneration in early Alzheimer’s disease. Neurobiol. Aging 2020, 95, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, J.; Barro, C.; Andreasson, U.; Derfuss, T.; Lindberg, R.; Sandelius, A.; Liman, V.; Norgren, N.; Blennow, K.; Zetterberg, H. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 2016, 54, 1655–1661. [Google Scholar] [CrossRef]
- Alagaratnam, J.; von Widekind, S.; De Francesco, D.; Underwood, J.; Edison, P.; Winston, A.; Zetterberg, H.; Fidler, S. Correlation between CSF and blood neurofilament light chain protein: A systematic review and meta-analysis. BMJ Neurol. Open 2021, 3, e000143. [Google Scholar] [CrossRef]
- Koini, M.; Pirpamer, L.; Hofer, E.; Buchmann, A.; Pinter, D.; Ropele, S.; Enzinger, C.; Benkert, P.; Leppert, D.; Kuhle, J.; et al. Factors influencing serum neurofilament light chain levels in normal aging. Aging (Albany NY) 2021, 13, 25729–25738. [Google Scholar] [CrossRef]
- Pifferi, F.; Laurent, B.; Plourde, M. Lipid transport and metabolism at the blood-brain interface: Implications in health and disease. Front. Physiol. 2021, 12, 645646. [Google Scholar] [CrossRef]
- Luigetti, M.; Primiano, G.; Basile, V.; Vitali, F.; Pignalosa, S.; Romano, A.; Sabino, A.; Marino, M.; Di Santo, R.; Ciasca, G.; et al. Serum Neurofilament and Free Light Chain Levels in Patients Undergoing Treatment for Chronic Inflammatory Demyelinating Polyneuropathy. Int. J. Mol. Sci. 2024, 25, 1254. [Google Scholar] [CrossRef]
- Khalil, M.; Pirpamer, L.; Hofer, E.; Voortman, M.M.; Barro, C.; Leppert, D.; Benkert, P.; Ropele, S.; Enzinger, C.; Fazekas, F.; et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat. Commun. 2020, 11, 812. [Google Scholar] [CrossRef] [PubMed]


| All (n = 30) | N-P Cognitive Impairment (n = 10) | AD (n = 10) | FTD (n= 10) | |||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| Age (years) | 61 | 54–66 | 51 | 43–56 | 60 | 56–66 | 67 | 63–68 |
| MMSE (score) | 27 | 23–28 | 29 | 27–30 | 25 | 24–27 | 23 | 19–27 |
| Disease duration (months) | 24 | 13–45 | 29 | 12–57 | 14 | 11–38 | 24 | 18–43 |
| NfL CSF (pg/mL) | ||||||||
| EllaTM | 879.0 | 510.0–3641.0 | 411.0 | 286.0–510.0 | 879.0 | 690.0–1166.0 | 6235.5 | 3641.0–7309.0 |
| LumipulseTM | 735.5 | 365.0–2897.0 | 293.5 | 209.0–365.0 | 735.5 | 648.0–859.0 | 4785.0 | 2897.0–6443.0 |
| NfL serum (pg/mL) | ||||||||
| EllaTM | 21.00 | 13.30–58.40 | 11.15 | 7.69–17.40 | 21.60 | 15.80–27.70 | 109.00 | 62.60–137.00 |
| LumipulseTM | 21.26 | 15.54–43.69 | 14.64 | 13.78–16.53 | 20.53 | 16.73–25.55 | 63.82 | 43.69–91.65 |
| Ella™ Versus Lumipulse™ | ||
|---|---|---|
| r | 95% CI | |
| NfL CSF | 0.976 | 0.950, 0.989 |
| NfL serum | 0.923 | 0.842, 0.964 |
| Parameter | Estimate | 95% CI |
|---|---|---|
| NfL CSF | ||
| Intercept | −45.38 | −118.58, 29.42 |
| Slope | 0.8261 | 0.7038, 0.9289 |
| NfL serum | ||
| Intercept | 7.543 | 5.828, 8.935 |
| Slope | 0.5775 | 0.5372, 0.6254 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbano, T.; Maramotti, R.; Tondelli, M.; Gallingani, C.; Carbone, C.; Iacovino, N.; Vinceti, G.; Zamboni, G.; Chiari, A.; Bedin, R. Comparison of Serum and Cerebrospinal Fluid Neurofilament Light Chain Concentrations Measured by Ella™ and Lumipulse™ in Patients with Cognitive Impairment. Diagnostics 2024, 14, 2408. https://doi.org/10.3390/diagnostics14212408
Urbano T, Maramotti R, Tondelli M, Gallingani C, Carbone C, Iacovino N, Vinceti G, Zamboni G, Chiari A, Bedin R. Comparison of Serum and Cerebrospinal Fluid Neurofilament Light Chain Concentrations Measured by Ella™ and Lumipulse™ in Patients with Cognitive Impairment. Diagnostics. 2024; 14(21):2408. https://doi.org/10.3390/diagnostics14212408
Chicago/Turabian StyleUrbano, Teresa, Riccardo Maramotti, Manuela Tondelli, Chiara Gallingani, Chiara Carbone, Najara Iacovino, Giulia Vinceti, Giovanna Zamboni, Annalisa Chiari, and Roberta Bedin. 2024. "Comparison of Serum and Cerebrospinal Fluid Neurofilament Light Chain Concentrations Measured by Ella™ and Lumipulse™ in Patients with Cognitive Impairment" Diagnostics 14, no. 21: 2408. https://doi.org/10.3390/diagnostics14212408
APA StyleUrbano, T., Maramotti, R., Tondelli, M., Gallingani, C., Carbone, C., Iacovino, N., Vinceti, G., Zamboni, G., Chiari, A., & Bedin, R. (2024). Comparison of Serum and Cerebrospinal Fluid Neurofilament Light Chain Concentrations Measured by Ella™ and Lumipulse™ in Patients with Cognitive Impairment. Diagnostics, 14(21), 2408. https://doi.org/10.3390/diagnostics14212408







