Bone-Targeting Radionuclides in the Treatment of Metastatic Castration-Resistant Prostate Cancer: A Review on Radium-223 Chloride (Alpharadin) in Combination with Other Therapies
Abstract
1. Introduction
1.1. Prostate Cancer as a Global Health Challenge
1.2. Significance of mCRPC
1.3. Overview of Current Treatments and Recent Advances
2. Current Treatment Landscape for mCRPC
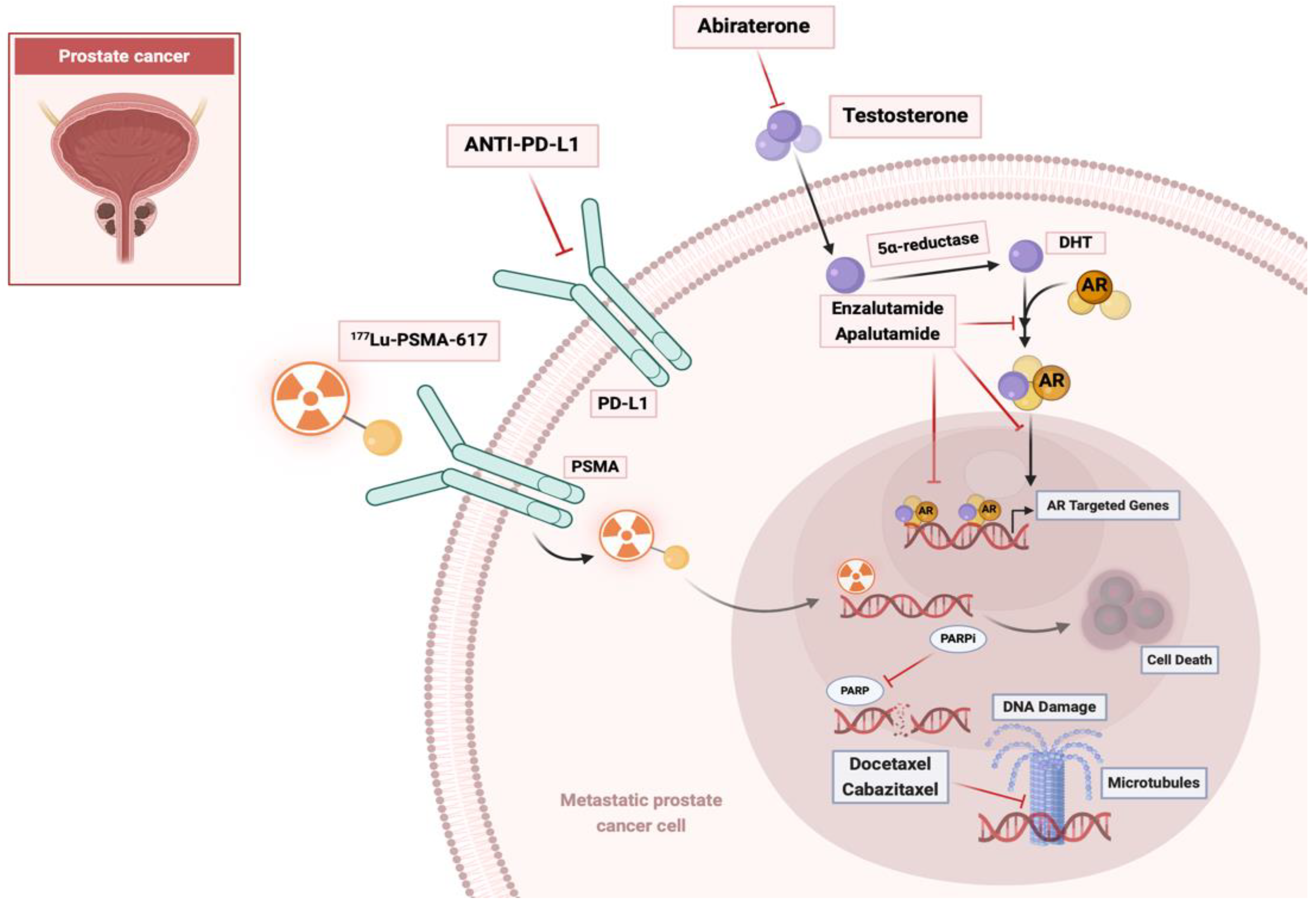
2.1. Hormonal Therapies Targeting the Androgen Receptor Axis
2.2. Chemotherapy
2.3. Immunotherapy
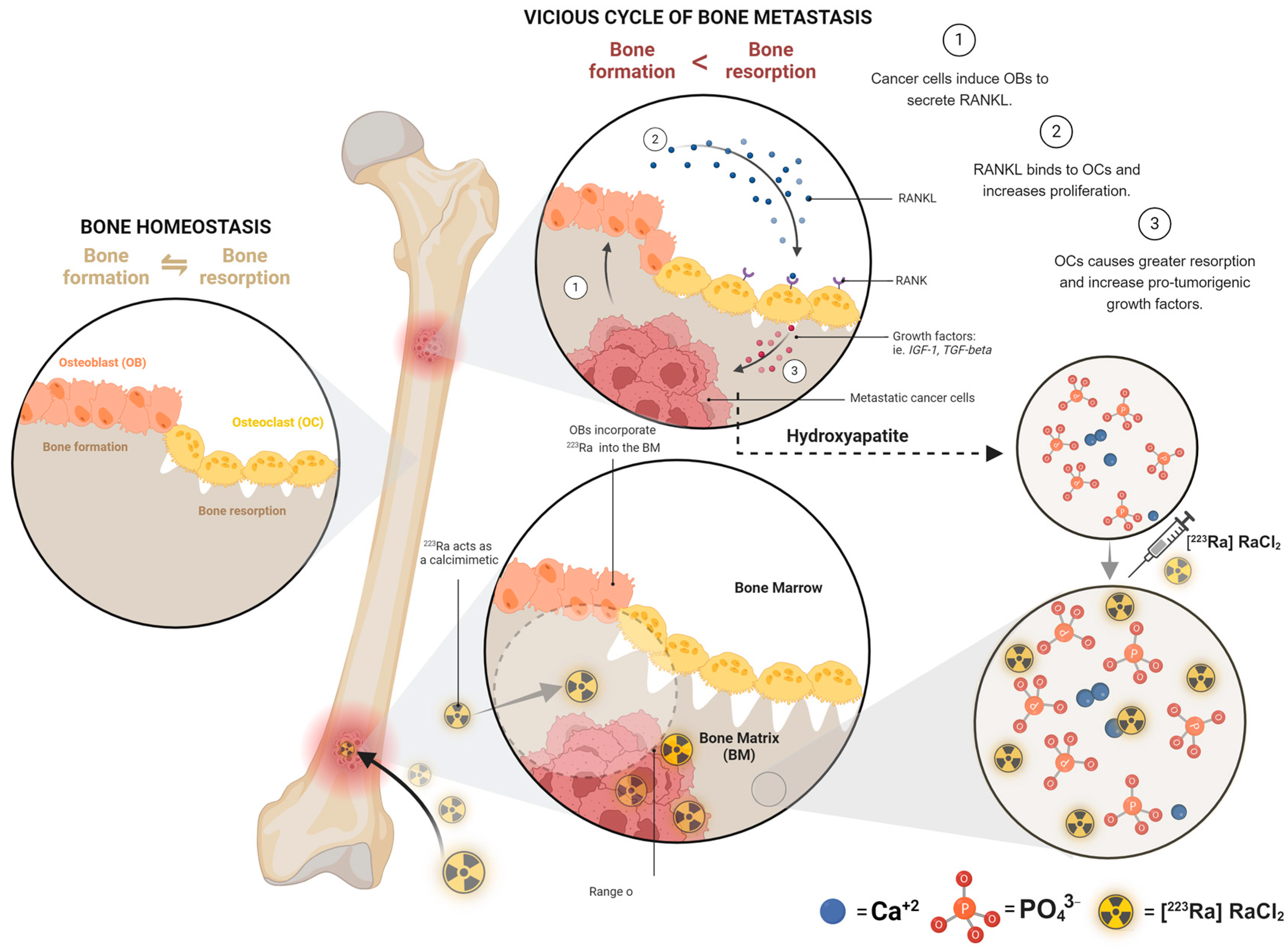
2.4. Bone-Targeting Therapy
2.5. Novel Treatments
3. Radium-223 in Clinical Trials
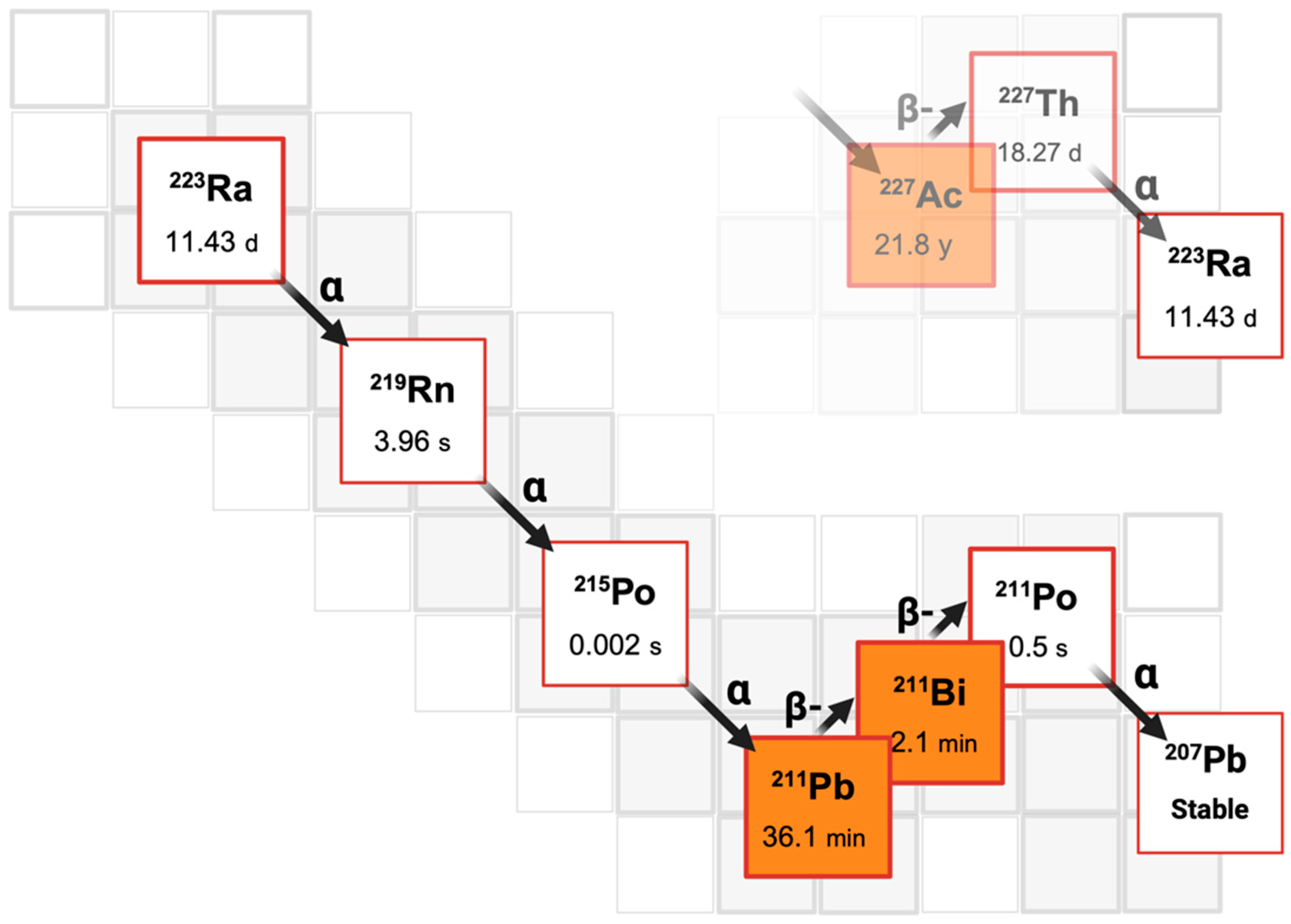
3.1. Mechanism of Action
3.2. Clinical Trials and Evidence Supporting the Use of Radium-22
3.3. Safety and Efficacy Considerations
4. Combination Therapies Involving Radium-223
4.1. Radium-223 with Hormonal Therapy
4.2. Radium-223 with Chemotherapy
4.3. Radium-223 with External Beam Radiotherapy
4.4. Radium-223 with Immunotherapy
4.5. Radium-223 with Bone-Protecting Agents
5. Ongoing Research and Future Directions
5.1. Investigating Novel Combinations and Sequencing Strategies
5.2. Addressing Unanswered Questions and Optimizing Treatment Approaches
- Investigate the long-term efficacy and safety of radium-223 in combination with other therapies through dedicated prospective clinical trials, focusing on diverse patient populations and treatment settings.
- Explore the potential of emerging biomarkers, such as circulating tumor DNA, and molecular imaging techniques to predict treatment response and guide personalized therapeutic approaches in mCRPC.
- Address challenges in implementing and optimizing new combination or sequencing strategies in clinical practice, including the development of standardized protocols, validation of biomarkers, and integration into routine care pathways.
- Investigate the economic implications, including cost-effectiveness and healthcare resource use, of new treatment strategies for mCRPC to inform decision-making and resource allocation.
- Conduct patient-centered research to understand the impact of combination therapies on quality of life, treatment adherence, and survivorship outcomes by incorporating patient-reported outcomes and real-world evidence into study designs.
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Leaning, D.; Kaur, G.; Morgans, A.K.; Ghouse, R.; Mirante, O.; Chowdhury, S. Treatment landscape and burden of disease in metastatic castration-resistant prostate cancer: Systematic and structured literature reviews. Front. Oncol. 2023, 13, 1240864. [Google Scholar] [CrossRef] [PubMed]
- Cursano, M.C.; Valsecchi, A.A.; Pantano, F.; Di Maio, M.; Procopio, G.; Berruti, A.; Bertoldo, F.; Tucci, M.; De Giorgi, U.; Santini, D. Bone health and body composition in prostate cancer: Meet-URO and AIOM consensus about prevention and management strategies. ESMO Open 2024, 9, 103484. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Heidenreich, A.; Nilsson, S.; Shore, N. Current approaches to incorporation of radium-223 in clinical practice. Prostate Cancer Prostatic Dis. 2018, 21, 37–47. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.M.; Carles, J.; Cathomas, R.; Gomez-Iturriaga, A.; Heinrich, D.; Kramer, G.; Ost, P.; van Oort, I.; Tombal, B. Radium-223 within the evolving treatment options for metastatic castration-resistant prostate cancer: Recommendations from a European Expert Working Group. Eur. Urol. Oncol. 2020, 3, 455–463. [Google Scholar] [CrossRef]
- Bultijnck, R.; De Laere, L.; De Grande, R.; Develter, T.; Vantieghem, S.; Uvin, P.; Ghysel, C.; De Laere, B.; on Behalf of the Patient Organization Think Blue Vlaanderen VZW. Androgen deprivation therapy in advanced prostate cancer: Insights from a real-world patient survey on health-related quality of life and information and communication sources. Qual. Life Res. 2024, 33, 2553–2562. [Google Scholar] [CrossRef]
- Fatima, H.; Rangwala, H.S.; Riaz, F.; Ali, L.M.; Abbas, S.R.M.; Haque, S.U.M. Castration resistant prostate cancer: Recent advances in novel therapeutic treatments. IJS Glob. Health 2024, 7, e0400. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, W.; Yan, S.; Zhang, K.; Wu, H.; Chen, H.; Shi, M.; Zhou, T. Novel Therapeutic Targets on the Horizon: An Analysis of Clinical Trials on Therapies for Bone Metastasis in Prostate Cancer. Cancers 2024, 16, 627. [Google Scholar] [CrossRef]
- Hoskin, P.; Sartor, O.; O’Sullivan, J.M.; Johannessen, D.C.; Helle, S.I.; Logue, J.; Bottomley, D.; Nilsson, S.; Vogelzang, N.J.; Fang, F.; et al. Efficacy and safety of radium-223 dichloride in CRPC patients with symptomatic bone metastases, with or without previous docetaxel use: A prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014, 15, 1397–1406. [Google Scholar] [CrossRef]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA-223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- O’Sullivan, J.M.; McKay, R.R.; Rahbar, K.; Fizazi, K.; George, D.J.; Tombal, B.; Schmall, A.; Sandström, P.; Verholen, F.; Shore, N. Real-world effectiveness, long-term safety and treatment pathway integration of radium-223 therapy in patients with metastatic castration-resistant prostate cancer. Front. Med. 2022, 9, fmed-09. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Kawada, T.; Rajwa, P.; Mostafaei, H.; Motlagh, R.S.; Quhal, F.; Laukhtina, E.; König, F.; Pallauf, M.; Pradere, B.; et al. Sequencing impact and prognostic factors in metastatic castration-resistant prostate cancer patients treated with cabazitaxel: A systematic review and meta-analysis. Urol. Oncol.-Semin. Orig. Investig. 2023, 41, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Higano, C.S.; Dizdarevic, S.; Logue, J.; Richardson, T.; George, S.; de Jong, I.; Tomaszewski, J.J.; Saad, F.; Miller, K.; Meltzer, J.; et al. Safety and effectiveness of the radium-223–taxane treatment sequence in patients with metastatic castration-resistant prostate cancer in a global observational study (REASSURE). Cancer 2024, 130, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; De Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Miederer, M.; Benešová-Schäfer, M.; Mamat, C.; Kästner, D.; Pretze, M.; Michler, E.; Brogsitter, C.; Kotzerke, J.; Kopka, K.; Scheinberg, D.A.; et al. Alpha-emitting radionuclides: Current status and future perspectives. Pharmaceuticals 2024, 17, 76. [Google Scholar] [CrossRef]
- Franchi, S.; Asti, M.; DiMarco, V.; Tosato, M. The Curies’ element: State of the art and perspectives on the use of radium in nuclear medicine. EJNMMI Radiopharm. Chem. 2023, 8, 38. [Google Scholar] [CrossRef]
- Mansi, L.; Lisa Bodei, J.; Lewis, B. (Eds.) Radiopharmaceutical Therapy; Springer Nature: Cham, Switzerland, 2024; pp. 934–935. [Google Scholar]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Atkinson, S.; Pearson, R.; Leaning, D.; Cumming, S.; Burns, A.; Azzabi, A.; Frew, J.; McMenemin, R.; Pedley, I.; et al. Optimising radium 223 therapy for metastatic castration-resistant prostate cancer–5-year real-world outcome: Focusing on treatment sequence and quality of life. Clin. Oncol. 2020, 32, e177–e187. [Google Scholar] [CrossRef]
- Choudhury, A.D.; Kwak, L.; Cheung, A.; Tripathi, A.; Pace, A.F.; Van Allen, E.M.; Kilbridge, K.L.; Wei, X.X.; McGregor, B.A.; Pomerantz, M.; et al. Randomized Phase II Study Evaluating the Addition of Pembrolizumab to Radium-223 in Metastatic Castration-resistant Prostate Cancer. Cancer Immunol. Res. 2024, 12, 704–718. [Google Scholar] [CrossRef]
- Shore, N.; Higano, C.S.; George, D.J.; Sternberg, C.N.; Saad, F.; Tombal, B.; Miller, K.; Kalinovsky, J.; Jiao, X.; Tangirala, K.; et al. Concurrent or layered treatment with radium-223 and enzalutamide or abiraterone/prednisone: Real-world clinical outcomes in patients with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2020, 23, 680–688. [Google Scholar] [CrossRef]
- Maughan, B.L.; Kessel, A.; McFarland, T.R.; Sayegh, N.; Nussenzveig, R.; Hahn, A.W.; Hoffman, J.M.; Morton, K.; Sirohi, D.; Kohli, M.; et al. Radium-223 plus enzalutamide versus enzalutamide in metastatic castration-refractory prostate cancer: Final safety and efficacy results. Oncologist 2021, 26, 1006–e2129. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; Vogelzang, N.J.; Sweeney, C.; Fernandez, D.C.; Almeida, F.; Iagaru, A.; Brown, A.; Smith, M.R.; Agrawal, M.; Dicker, A.P.; et al. Radium-223 safety, efficacy, and concurrent use with abiraterone or enzalutamide: First US experience from an expanded access program. Oncologist 2018, 23, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Dai, J.D.; Zhang, X.M.; Zhao, J.G.; Sun, G.X.; Zeng, Y.H.; Zeng, H.; Xu, N.W.; Zeng, H.; Shen, P.F. The safety of radium-223 combined with new-generation hormonal agents in bone metastatic castration-resistant prostate cancer: A systematic review and network meta-analysis. Asian J. Androl. 2023, 25, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Anido-Herranz, U.; Fernandez-Calvo, O.; Ruiz-Bañobre, J.; Martinez-Breijo, S.; Fernandez-Nuñez, N.; Nogareda-Seoane, Z.; Garrido-Pumar, M.; Casas-Nebra, J.; Muñiz-Garcia, G.; Portela-Pereira, P.; et al. Outcomes and patterns of use of Radium-223 in metastatic castration-resistant prostate cancer. Front. Oncol. 2024, 14, 1385466. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.Á.; Pérez-Valderrama, B.; Mellado, B.; Parra, E.M.F.; Calvo, O.F.; de Olza, M.O.; Romay, L.M.; Anido, U.; Domenech, M.; Polo, S.H.; et al. Weekly cabazitaxel plus prednisone is effective and less toxic for ‘unfit’metastatic castration-resistant prostate cancer: Phase II Spanish Oncology Genitourinary Group (SOGUG) trial. Eur. J. Cancer 2017, 87, 30–37. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Chen, Y.H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef]
- Clarke, N.W.; Ali, A.; Ingleby, F.C.; Hoyle, A.; Amos, C.L.; Attard, G.; Brawley, C.D.; Calvert, J.; Chowdhury, S.; Cook, A.; et al. Addition of docetaxel to hormonal therapy in low-and high-burden metastatic hormone sensitive prostate cancer: Long-term survival results from the STAMPEDE trial. Ann. Oncol. 2019, 30, 1992–2003. [Google Scholar] [CrossRef]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef]
- Morris, M.J.; Loriot, Y.; Sweeney, C.J.; Fizazi, K.; Ryan, C.J.; Shevrin, D.H.; Antonarakis, E.S.; Pandit-Taskar, N.; Deandreis, D.; Jacene, H.A.; et al. Radium-223 in combination with docetaxel in patients with castration-resistant prostate cancer and bone metastases: A phase 1 dose escalation/randomised phase 2a trial. Eur. J. Cancer 2019, 114, 107–116. [Google Scholar] [CrossRef]
- Sarnelli, A.; Belli, M.L.; Azzali, I.; Loi, E.; Severi, S.; Strigari, L. Alpha-Emitter Radiopharmaceuticals and External Beam Radiotherapy: A Radiobiological Model for the Combined Treatment. Cancers 2022, 14, 1077. [Google Scholar] [CrossRef]
- Turner, P.G.; Jain, S.; Cole, A.; Grey, A.; Mitchell, D.; Prise, K.M.; Hounsell, A.R.; McGarry, C.K.; Biggart, S.; O’Sullivan, J.M. Toxicity and efficacy of concurrent androgen deprivation therapy, pelvic radiotherapy, and radium-223 in patients with de novo metastatic hormone-sensitive prostate cancer. Clin. Cancer Res. 2021, 27, 4549–4556. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, S.E.; Michalski, J.M.; O’Sullivan, J.M.; Parker, C.; Garcia-Vargas, J.E.; Sartor, A.O. External beam radiation therapy use and safety with radium-223 in patients with castration-resistant prostate cancer and symptomatic bone metastases from the ALSYMPCA trial. J. Clin. Oncol. 2015, 33, 182. [Google Scholar] [CrossRef]
- Sartor, O.; Coleman, R.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014, 15, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G. Dosimetry, radiobiology and synthetic lethality: Radiopharmaceutical therapy (RPT) with alpha-particle-emitters. Nucl. Med. Semin. 2020, 50, 124–132. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- López-Campos, F.; Gajate, P.; Romero-Laorden, N.; Zafra-Martín, J.; Juan, M.; Polo, S.H.; Moreno, A.C.; Couñago, F. Immunotherapy in advanced prostate cancer: Current knowledge and future directions. Biomedicines 2022, 10, 537. [Google Scholar] [CrossRef]
- Morris, M.J.; Corey, E.; Guise, T.A.; Gulley, J.L.; Kelly, W.K.; Quinn, D.I.; Scholz, A.; Sgouros, G. Radium-223 mechanism of action: Implications for use in treatment combinations. Nat. Rev. Urol. 2019, 16, 745–756. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Gameiro, S.R.; Ardiani, A.; Kwilas, A.; Hodge, J.W. Radiation-induced survival responses promote immunogenic modulation to enhance immunotherapy in combinatorial regimens. Oncoimmunology 2014, 3, e28643. [Google Scholar] [CrossRef]
- Stattin, P.; Westerberg, M.; Lissbrant, I.F.; Eriksson, M.H.; Kjellman, A.; Ullén, A.; Vassilev, Z.; Sandstrom, P.; Weinrib, R.; Martinez, D.; et al. Real world outcomes in patients with metastatic, castration-resistant prostate cancer treated with radium-223 in routine clinical practice in Sweden. Clin. Genitourin. Cancer 2023, 21, 107.e1. [Google Scholar] [CrossRef]
- Kim, S.I.; Szeto, A.H.; Morgan, K.P.; Brower, B.; Dunn, M.W.; Khandani, A.H.; Godley, P.A.; Rose, T.L.; Basch, E.M.; Milowsky, M.I.; et al. A real-world evaluation of radium-223 in combination with abiraterone or enzalutamide for the treatment of metastatic castration-resistant prostate cancer. PloS ONE 2021, 16, e0253021. [Google Scholar] [CrossRef] [PubMed]
- Trieu, J.; Chang, M.; Rojas, V.; Varada, N.; Cao, Y.; Anderson, M.; Vogelzang, N.J. Lower fracture rates in patients treated with radium-223, abiraterone or enzalutamide, when given concurrently with bone health agents: A real-world analysis. Clin. Genitourin. Cancer 2022, 20, 399–403. [Google Scholar] [CrossRef]
- Gillessen, S.; Choudhury, A.; Rodriguez-Vida, A.; Nole, F.; Diaz, E.G.; Roumeguere, T.A.; Daugaard, G.; Loriot, Y.; Saad, F.; McDermott, R.S.; et al. Decreased fracture rate by mandating bone protecting agents in the EORTC-1333/PEACEIII trial combining Ra223 with enzalutamide versus enzalutamide alone: An updated safety analysis. J. Clin. Oncol. 2021, 39, 5002. [Google Scholar] [CrossRef]
- Fizazi, K.; González, A.; Mustafa, Ö.; Skoneczna, I.A.; Krissel, H.; Uema, D.; Chen, H.; Wagner, V.J.; Boegemann, M. Radium-223 versus novel antihormone therapy for progressive metastatic castration-resistant prostate cancer after 1 line of NAH: RADIANT, an international phase 4, randomized, open-label study. J. Clin. Oncol. 2021, 39, TPS5093. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Phase I Radium 223 mCRPC-PEACE III (PEACE III). ClinicalTrials.gov Identifier NCT02194842. 2014. Available online: https://www.clinicaltrials.gov/study/NCT02194842?term=NCT02194842&rank=1 (accessed on 1 September 2024).
- ClinicalTrials.gov. A Study to Test Radium-223 with Docetaxel in Patients with Prostate Cancer. Clinicaltrials.gov Identifier NCT03574571. 2018. Available online: https://www.clinicaltrials.gov/study/NCT03574571?term=NCT03574571&rank=1 (accessed on 1 September 2024).
- ClinicalTrials.gov. Combination of Radium-223 and Lutetium-177 PSMAI&T in Men with Metastatic Castration-Resistant Prostate Cancer (AlphaBet). ClinicalTrials.gov Identifier: NCT05383079. 2022. Available online: https://www.clinicaltrials.gov/study/NCT05383079?term=NCT05383079&rank=1 (accessed on 1 September 2024).
- ClinicalTrials.gov. Study of Nivolumab in Combination with Radium223 in Men with Metastatic Castration Resistant Prostate Cancer (Rad2Nivo). ClinicalTrials.gov Identifier: NCT04109729. 2019. Available online: https://www.clinicaltrials.gov/study/NCT04109729?term=NCT04109729&rank=1 (accessed on 1 September 2024).
- ClinicalTrials.gov. Observational Study for the Evaluation of Long-Term Safety of Radium-223 Used for the Treatment of Metastatic Castration Resistant Prostate Cancer (REASSURE). ClinicalTrials.gov Identifier: NCT02141438. 2014. Available online: https://www.clinicaltrials.gov/study/NCT02141438 (accessed on 1 September 2024).
- Rahbar, K.; Essler, M.; Eiber, M.; La Fougere, C.; Prasad, V.; Fendler, W.P.; Rassek, P.; Hasa, E.; Dittmann, H.; Bundschuh, R.A.; et al. Safety and survival outcomes in patients with metastatic castration-resistant prostate cancer treated with lutetium-177–prostate-specific membrane antigen after radium-223: Interim analysis of the RALU study. J. Clin. Oncol. 2022, 40, 5040. [Google Scholar] [CrossRef]
- Slootbeek, P.H.; Tolmeijer, S.H.; Mehra, N.; Schalken, J.A. Therapeutic biomarkers in metastatic castration-resistant prostate cancer: Does the state matter? Crit. Rev. Clin. Lab. Sci. 2024, 61, 178–204. [Google Scholar] [CrossRef]
- Conteduca, V.; Mosca, A.; Brighi, N.; de Giorgi, U.; Rescigno, P. New prognostic biomarkers in metastatic castration-resistant prostate cancer. Cells 2021, 10, 193. [Google Scholar] [CrossRef]
| Drug | Action | Mechanism |
|---|---|---|
| Abiraterone | Inhibition of androgen synthesis | Inhibits CYP17, reduces androgen production [15] |
| Enzalutamide | Antagonization of androgen action | Androgen receptor inhibitor blocks testosterone effects [16] |
| Bicalutamide | Blockade of AR [17] | |
| Apalutamide | Prevents AR translocation, DNA binding, and AR-mediated transcription [18] | |
| Docetaxel | Inhibition of mitosis | Tubulin inhibition [19] |
| Cabazitaxel | ||
| Radium-223 | Alpha radiation, gamma rays | Targets bone metastases, emits alpha particles [20] |
| 177Lu-PSMA-617 | Inhibition of growth signals | Binding and internalization of PSMA ligands triggers cell death [21] |
| MEDI3726 | ||
| Ipilimumab | Checkpoint (CTLA-4) inhibitor | Increases antitumor T-cell responses [22] |
| Pembrolizumab | PD-1 inhibitor | Regulates T-cell activation [23] |
| Sipuleucel-T | Immunotherapy | Autologous vaccine [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, A.H.D. Bone-Targeting Radionuclides in the Treatment of Metastatic Castration-Resistant Prostate Cancer: A Review on Radium-223 Chloride (Alpharadin) in Combination with Other Therapies. Diagnostics 2024, 14, 2407. https://doi.org/10.3390/diagnostics14212407
Alshehri AHD. Bone-Targeting Radionuclides in the Treatment of Metastatic Castration-Resistant Prostate Cancer: A Review on Radium-223 Chloride (Alpharadin) in Combination with Other Therapies. Diagnostics. 2024; 14(21):2407. https://doi.org/10.3390/diagnostics14212407
Chicago/Turabian StyleAlshehri, Ali H. D. 2024. "Bone-Targeting Radionuclides in the Treatment of Metastatic Castration-Resistant Prostate Cancer: A Review on Radium-223 Chloride (Alpharadin) in Combination with Other Therapies" Diagnostics 14, no. 21: 2407. https://doi.org/10.3390/diagnostics14212407
APA StyleAlshehri, A. H. D. (2024). Bone-Targeting Radionuclides in the Treatment of Metastatic Castration-Resistant Prostate Cancer: A Review on Radium-223 Chloride (Alpharadin) in Combination with Other Therapies. Diagnostics, 14(21), 2407. https://doi.org/10.3390/diagnostics14212407





