Lung Ultrasound Assessment of Regional Distribution of Pulmonary Edema and Atelectasis in Infants with Evolving Bronchopulmonary Dysplasia
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guaman, M.C.; Gien, J.; Baker, C.D.; Zhang, H.; Austin, E.D.; Collaco, J.M. Point prevalence, clinical characteristics, and treatment variation for infants with severe bronchopulmonary dysplasia. Am. J. Perinatol. 2015, 32, 960–967. [Google Scholar] [PubMed]
- Abman, S.H.; Bancalari, E.; Jobe, A. The evolution of bronchopulmonary dysplasia after 50 years. Am. J. Respir. Crit. Care Med. 2017, 195, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Anderson, P.J. Long-term outcomes of bronchopulmonary dysplasia. Semin. Fetal Neonatal Med. 2009, 14, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, D.; Zaghloul, N.; Watkins, L.; Liu, J. Neonatal lung ultrasound exam guidelines. J. Perinatol. 2018, 38, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Copetti, R.; Sorantin, E.; Lovrenski, J.; Rodriguez-Fanjul, J.; Kurepa, D.; Feng, X.; Cattarossi, L.; Zhang, H.; Hwang, M.; et al. Protocol and Guidelines for Point-of-Care Lung Ultrasound in Diagnosing Neonatal Pulmonary Diseases Based on International Expert Consensus. J. Vis. Exp. 2019, 145, e58990. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, S.W.; Liu, F.; Wang, Y.; Kong, X.Y.; Li, Q.P.; Huang, J.J. BPD, not BPD, or iatrogenic BPD: Findings of lung ultrasound examinations. Medicine 2014, 93, e133. [Google Scholar] [CrossRef]
- Kasniya, G.; Weinberger, B.; Cerise, J.; Pulju, M.; Boyar, V.; Frunza, F.; Kurepa, D. Lung ultrasound assessment of pulmonary edema in neonates with chronic lung disease before and after diuretic therapy. Pediatr. Pulmonol. 2022, 57, 3145–3150. [Google Scholar] [CrossRef] [PubMed]
- Dollery, C.T.; Hugh-Jones, P.; Matthews, C.M. Use of radioactive xenon for studies of regional lung function. A comparison with oxygen-15. Br. Med. J. 1962, 2, 1006–1016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- West, J.; Luks, A. West’s Respiratory Physiology the Essentials, 11th ed.; Walters Kluwer: Alphen aan den Rijn, The Netherlands, 2020. [Google Scholar]
- Tingay, D.G.; Togo, A.; Pereira-Fantini, P.M.; Miedema, M.; E McCall, K.; Perkins, E.J.; Thomson, J.; Dowse, G.; Sourial, M.; Dellacà, R.L.; et al. Aeration strategy at birth influences the physiological response to surfactant in preterm lambs. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F587–F593. [Google Scholar] [CrossRef] [PubMed]
- Bamat, N.; Fierro, J.; Wang, Y.; Millar, D.; Kirpalani, H. Positive end-expiratory pressure for preterm infants requiring conventional mechanical ventilation for respiratory distress syndrome or bronchopulmonary dysplasia. Cochrane Database Syst. Rev. 2019, 2, CD004500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heinrich, S.; Schiffmann, H.; Frerichs, A.; Klockgether-Radke, A.; Frerichs, I. Body and head position effects on regional lung ventilation in infants: An electrical impedance tomography study. Intensive Care Med. 2006, 32, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Hough, J.L.; Johnston, L.; Brauer, S.G.; Woodgate, P.G.; Pham, T.M.; Schibler, A. Effect of body position on ventilation distribution in preterm infants on continuous positive airway pressure. Pediatr. Crit. Care Med. 2012, 13, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.; Rüegger, C.M.; Perkins, E.J.; Pereira-Fantini, P.M.; Farrell, O.; Owen, L.S.; Tingay, D.G. Regional ventilation characteristics during non-invasive respiratory support in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Riedel, T.; Kyburz, M.; Latzin, P.; Thamrin, C.; Frey, U. Regional and overall ventilation inhomogeneities in preterm and term-born infants. Intensive Care Med. 2009, 35, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Lupton-Smith, A.; Argent, A.; Rimensberger, P.; Frerichs, I.; Morrow, B. Prone Positioning Improves Ventilation Homogeneity in Children with Acute Respiratory Distress Syndrome. Pediatr. Crit. Care Med. 2017, 18, e229–e234. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, V.D.; Waldmann, A.D.; Davis, P.G.; Bassler, D.; Springer, L.; Thomson, J.; Tingay, D.G.; Rüegger, C.M. Lung volume distribution in preterm infants on non-invasive high-frequency ventilation. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 551–557. [Google Scholar] [CrossRef] [PubMed]
- O’Brodovich, H.M. Immature epithelial Na+ channel expression is one of the pathogenetic mechanisms leading to human neonatal respiratory distress syndrome. Proc. Assoc. Am. Physicians 1996, 108, 345–355. [Google Scholar] [PubMed]
- Jain, L.; Dudell, G.G. Respiratory transition in infants delivered by cesarean section. Semin. Perinatol. 2006, 30, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.B.; Te Pas, A.B.; Kitchen, M.J. Respiratory transition in the newborn: A three-phase process. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F266–F271. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.; Belen, K.; Farooqui, M.; Idiong, N.; Amer, R.; Hussain, A.; ElSayed, Y. Prone versus Supine Position for Lung Ultrasound in Neonates with Respiratory Distress. Am. J. Perinatol. 2021, 38, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y.; Arai, J.; Hirono, K.; Maruo, K.; Kajikawa, D.; Yukitake, Y.; Hinata, A.; Miura, R. Gravity-induced loss of aeration and atelectasis development in the preterm lung: A serial sonographic assessment. J. Perinatol. 2022, 42, 231–236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahtu, M.; Frerichs, I.; Pokka, T.; Becher, T.; Peltoniemi, O.; Kallio, M. Effect of body position on ventilation distribution in healthy newborn infants: An observational study. Arch. Dis. Child. Fetal Neonatal Ed. 2024, 109, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, I.; Amato, M.B.P.; van Kaam, A.H.; Tingay, D.G.; Zhao, Z.; Grychtol, B.; Bodenstein, M.; Gagnon, H.; Böhm, S.H.; Teschner, E.; et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2017, 72, 83–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hough, J.; Trojman, A.; Schibler, A. Effect of time and body position on ventilation in premature infants. Pediatr. Res. 2016, 80, 499–504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raimondi, F.; Migliaro, F.; Corsini, I.; Meneghin, F.; Pierri, L.; Salomè, S.; Perri, A.; Aversa, S.; Nobile, S.; Lama, S.; et al. Neonatal Lung Ultrasound and Surfactant Administration: A Pragmatic, Multicenter Study. Chest 2021, 160, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Loi, B.; Vigo, G.; Baraldi, E.; Raimondi, F.; Carnielli, V.P.; Mosca, F.; De Luca, D. Lung Ultrasound to Monitor Extremely Preterm Infants and Predict Bronchopulmonary Dysplasia: A Multicenter Longitudinal Cohort Study. Am. J. Respir. Crit. Care Med. 2021, 203, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Hülskamp, G.; Pillow, J.J.; Dinger, J.; Stocks, J. Lung function tests in neonates and infants with chronic lung disease of infancy: Functional residual capacity. Pediatr. Pulmonol. 2006, 41, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Thome, U.; Töpfer, A.; Schaller, P.; Pohlandt, F. Comparison of lung volume measurements by antero-posterior chest X-ray and the SF6 washout technique in mechanically ventilated infants. Pediatr. Pulmonol. 1998, 26, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Tonson la Tour, A.; Spadola, L.; Sayegh, Y.; Combescure, C.; Pfister, R.; Argiroffo, C.B.; Rochat, I. Chest CT in bronchopulmonary dysplasia: Clinical and radiological correlations. Pediatr. Pulmonol. 2013, 48, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Dyke, J.P.; Voskrebenzev, A.; Blatt, L.K.; Vogel-Claussen, J.; Grimm, R.; Worgall, S.; Perlman, J.M.; Kovanlikaya, A. Assessment of lung ventilation of premature infants with bronchopulmonary dysplasia at 1.5 Tesla using phase-resolved functional lung magnetic resonance imaging. Pediatr. Radiol. 2023, 53, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.W.; Harrison, M.C.; Counsell, S.J.; Allsop, J.M.; Kennea, N.L.; Hajnal, J.V.; Thornton, A.S.; Duggan, P.; Edwards, A.D. Increased lung water and tissue damage in bronchopulmonary dysplasia. J. Pediatr. 2004, 145, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.K.; Gómez-Laberge, C.; Rettig, J.S.; Vargas, S.O.; Smallwood, C.D.; Prabhu, S.P.; Vitali, S.H.; Zurakowski, D.; Arnold, J.H. Mechanical ventilation guided by electrical impedance tomography in experimental acute lung injury. Crit. Care Med. 2013, 41, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Schibler, A.; Yuill, M.; Parsley, C.; Pham, T.; Gilshenan, K.; Dakin, C. Regional ventilation distribution in non-sedated spontaneously breathing newborns and adults is not different. Pediatr. Pulmonol. 2009, 44, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.M.; Yuill, M.; Dakin, C.; Schibler, A. Regional ventilation distribution in the first 6 months of life. Eur. Respir. J. 2011, 37, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Sett, A.; Kenna, K.R.; Sutton, R.J.; Perkins, E.J.; Sourial, M.; Chapman, J.D.; Donath, S.M.; Sasi, A.; Rogerson, S.R.; Manley, B.J.; et al. Lung ultrasound of the dependent lung detects real-time changes in lung volume in the preterm lamb. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 51–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sett, A.; Foo, G.W.C.; Kenna, K.R.; Sutton, R.J.; Perkins, E.J.; Sourial, M.; Rogerson, S.R.; Manley, B.J.; Davis, P.G.; Pereira-Fantini, P.M.; et al. Quantitative lung ultrasound detects dynamic changes in lung recruitment in the preterm lamb. Pediatr. Res. 2023, 93, 1591–1598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, L.L.H.; Foo, G.; Kenna, K.R.; Douglas, E.; Fatmous, M.; Sutton, R.J.; Perkins, E.J.; Sourial, M.; Pereira-Fantini, P.M.; Tingay, D.G.; et al. Lung ultrasound detects regional aeration inhomogeneity in ventilated preterm lambs. Pediatr. Res. 2023, 95, 129–134. [Google Scholar] [CrossRef] [PubMed]

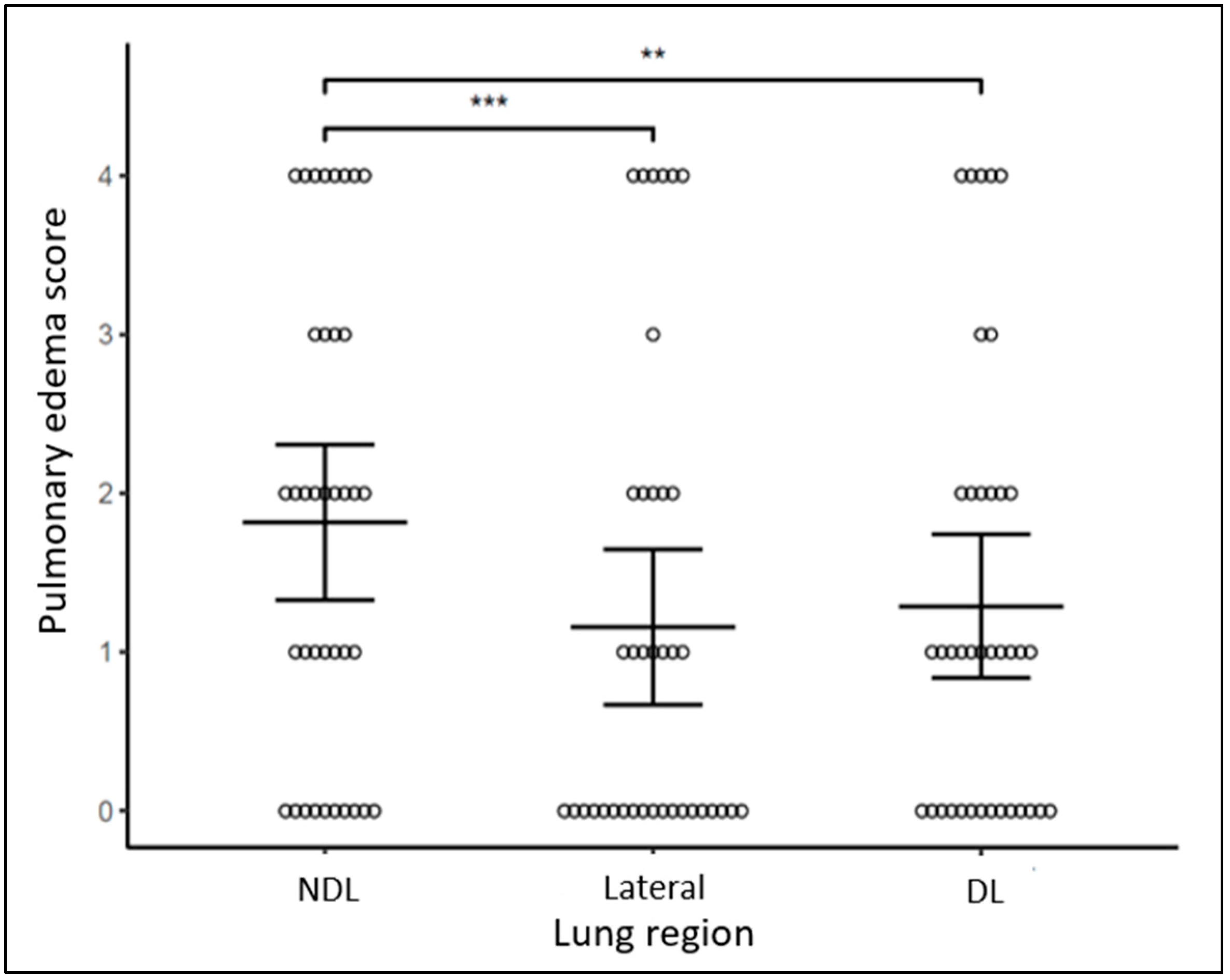

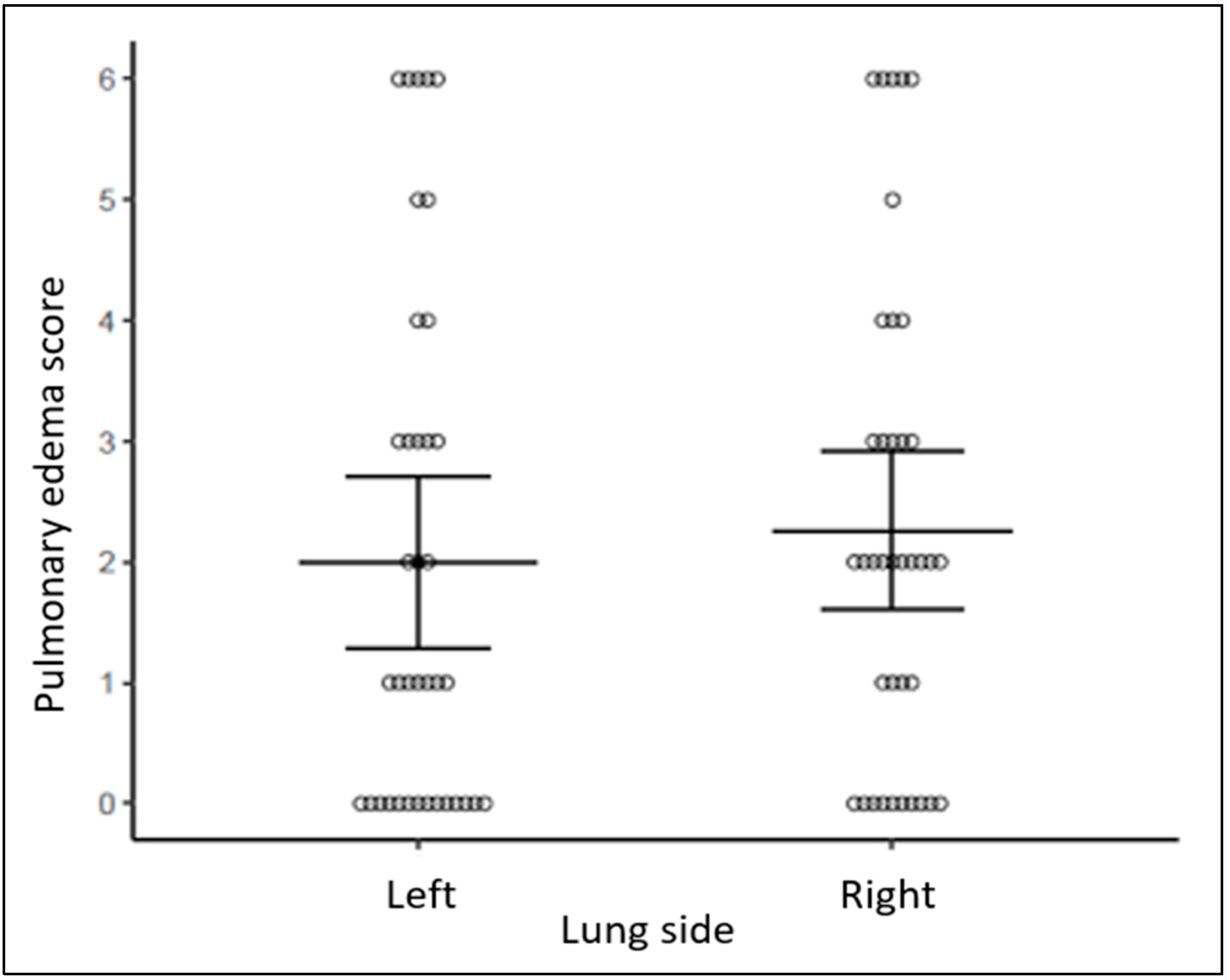
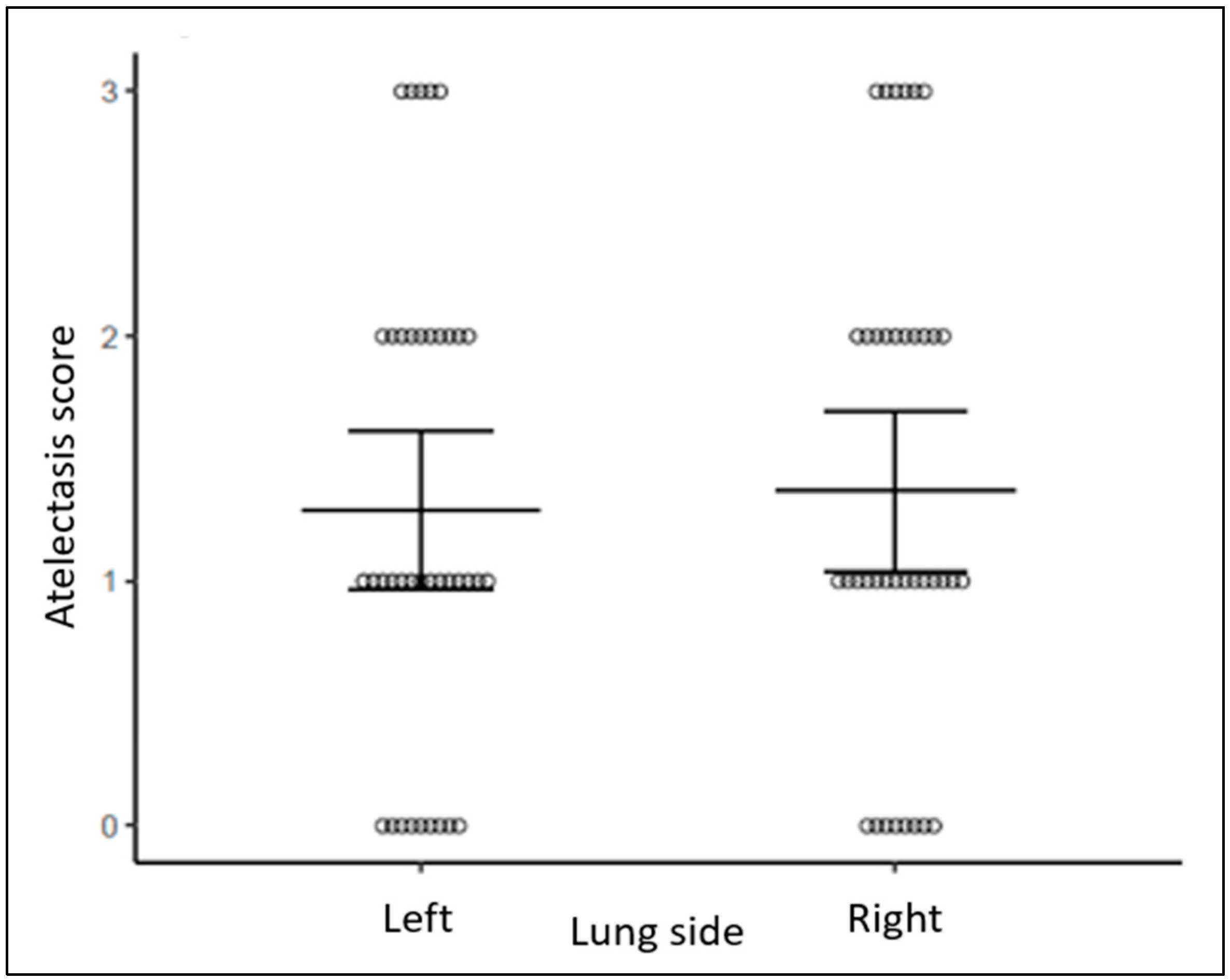
| Infant Characteristics | Cohort (N = 38) ^ |
|---|---|
| Gestational age (weeks) | 27.02 ± 1.73 |
| Birth weight (g) | 898.16 ± 304.28 |
| Sex (male) | 18 (47) |
| Small for gestational age (n) | 12 (32) |
| Antenatal steroids (n) | 28 (74) |
| Chorioamnionitis (n) | 8 (21) |
| Delivery type (Cesarean section) | 29 (76) |
| Apgar 5 min | 8 (6–8) |
| Day of life at LUS exam (days) | 53 ± 15.5 |
| Gestational age at LUS exam (weeks) | 35 ± 2.2 |
| FiO2 (%) | 26.2 ± 6.4 |
| Respiratory rate (bpm) | 56.4 ± 7.3 |
| CPAP (n) | 20 (53) |
| CPAP level (mm Hg) | 5.6 ± 1.6 |
| NC flow (lpm) | 2.3 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, J.; Weinberger, B.; Pulju, M.; Galanti, S.G.; Kasniya, G.; Gupta, V.; Kurepa, D. Lung Ultrasound Assessment of Regional Distribution of Pulmonary Edema and Atelectasis in Infants with Evolving Bronchopulmonary Dysplasia. Diagnostics 2024, 14, 2341. https://doi.org/10.3390/diagnostics14202341
Patel J, Weinberger B, Pulju M, Galanti SG, Kasniya G, Gupta V, Kurepa D. Lung Ultrasound Assessment of Regional Distribution of Pulmonary Edema and Atelectasis in Infants with Evolving Bronchopulmonary Dysplasia. Diagnostics. 2024; 14(20):2341. https://doi.org/10.3390/diagnostics14202341
Chicago/Turabian StylePatel, Jimikumar, Barry Weinberger, Margaret Pulju, Stephanie G. Galanti, Gangajal Kasniya, Venkata Gupta, and Dalibor Kurepa. 2024. "Lung Ultrasound Assessment of Regional Distribution of Pulmonary Edema and Atelectasis in Infants with Evolving Bronchopulmonary Dysplasia" Diagnostics 14, no. 20: 2341. https://doi.org/10.3390/diagnostics14202341
APA StylePatel, J., Weinberger, B., Pulju, M., Galanti, S. G., Kasniya, G., Gupta, V., & Kurepa, D. (2024). Lung Ultrasound Assessment of Regional Distribution of Pulmonary Edema and Atelectasis in Infants with Evolving Bronchopulmonary Dysplasia. Diagnostics, 14(20), 2341. https://doi.org/10.3390/diagnostics14202341






