Abstract
Background: Flower-shaped nuclei in plasma cells are rare in multiple myeloma. Case presentation: We report on an 88-year-old male who presented with a mass lesion in the clavicular region. A biopsy of the mass revealed an increase in mature plasma cells with round nuclei. In contrast, a bone marrow examination showed increased plasma cells with flower-shaped nuclei. The patient tested negative for human T-lymphotropic virus type-1 and was diagnosed with multiple myeloma. Conclusions: While multiple myeloma is known for intra-tumor heterogeneity, reports of morphological heterogeneity based on the site of tumor sampling are limited. In this case, the presence of plasma cells with flower-shaped nuclei enabled the identification of site-dependent morphological tumor heterogeneity.
Multiple myeloma (MM) is a plasma cell neoplasm, and one of the diagnostic criteria is the clonal proliferation of plasma cells in the bone marrow [1]. Although the tumor cells of MM resemble normal plasma cells in most cases, unusual morphological variants are sometimes observed in MM, including small, anaplastic, and plasmablastic cells [2]. Multilobulated cells were also reported as flower-shaped plasma cells mimicking the flower cells of adult T-cell leukemia/lymphoma (ATLL) [3,4]. However, the characteristics of MM exhibiting a flower-shaped morphology remain unclear due to the rarity of this subtype. We report an MM patient with flower-shaped plasma cells with morphological heterogeneity depending on the site of tumor involvement.
An 88-year-old male presented with a tumor in the left clavicular region. He had suffered a left clavicle fracture one year earlier. A radiographic examination revealed a lytic bone lesion (Figure 1a). The gadolinium-enhanced magnetic resonance imaging of the left clavicular area revealed a 55 mm soft tissue mass (Figure 1b,c). A tumor biopsy of the left clavicular region revealed clusters of plasma cells, leading to the diagnosis of a plasma cell neoplasm. The majority of the plasma cells had round nuclei (Figure 2a). Immunohistochemical staining showed that the plasma cells were positive for CD138, CD56, and cyclin D1 (Figure 2b,c). In situ hybridization for immunoglobulin light chains showed lambda positivity, indicating a skewed expression of light chains (Figure 2d,e). No anemia, renal insufficiency, or hypercalcemia developed. Bone involvement was also observed in the sternum and ribs. The serum immunoglobulin G (IgG) level was elevated to 2727 mg/dL, and the IgG-λ monoclonal protein was detected. The serum free light-chain assay showed serum kappa—19.4 mg/L (reference range: 3.3–19.4), serum lambda—123.8 mg/L (reference range: 5.7–26.3), and a decreased kappa/lambda ratio of 0.16 (reference range: 0.26–1.65).
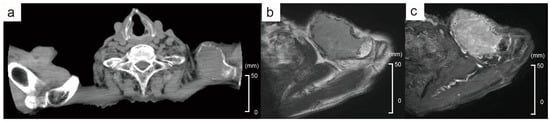
Figure 1.
Radiological findings of the left clavicular mass. (a) CT finding shows a lytic mass. (b) MRI T2-weighted image reveals a 55 mm soft tissue mass. (c) Gadolinium-enhanced MRI. The red arrows indicate the tumor.
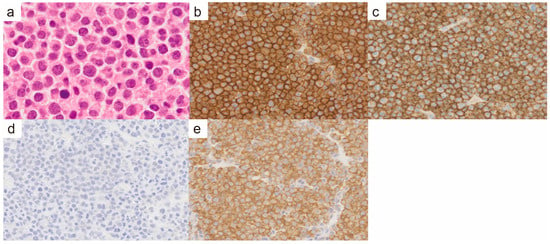
Figure 2.
Pathological findings of the left clavicular mass. (a) Hematoxylin and eosin staining (×400). There were very few plasma cells with cleaved nuclei, and the majority of plasma cells had round nuclei. (b) CD138 immunohistochemistry (×400). (c) CD56 immunohistochemistry (×400). (d) In situ hybridization for immunoglobulin light chain kappa (×400). (e) In situ hybridization for immunoglobulin light chain lambda (×400).
Bone marrow (BM) aspiration showed 43% of the abnormal cells with flower-shaped nuclei, morphologically distinct from the biopsy of the clavicular mass (Figure 3a,b). The abnormal cells observed in the bone marrow had a morphology typical of ATLL. However, a test for human T-lymphotropic virus type-1 (HTVL-1) antibodies was negative, ruling out a diagnosis of ATLL. The flow cytometry of the BM aspiration sample showed that the abnormal cells were positive for CD38, CD56, CD138, and cytoplasmic λ and negative for CD7, CD19, and CD20 (Figure 3c,d). The results indicated that the abnormal cells were of plasma cell origin. The cytogenetic analysis using fluorescence in situ hybridization showed that they were negative for TP53 deletion, 1q21 amplification, and FGFR3 and MAF translocations. The G-banding analysis revealed a normal karyotype. The patient was diagnosed with MM. The mass lesion in the left clavicular region was diagnosed as an extramedullary plasmacytoma of MM. He received a combination of bortezomib and dexamethasone considering his age and frailty. After two weekly doses of bortezomib, the mass in the left clavicular region had reduced in size, and the patient was transferred to a hospital near his home.
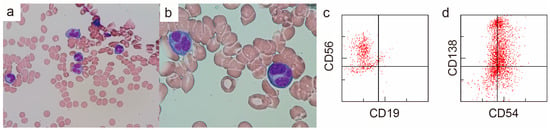
Figure 3.
Findings of bone marrow aspiration from the left iliac bone (a,b) Wright–Giemsa stain, (a) ×400, (b) ×1000. The majority of plasma cells had flower-shaped nuclei. (c,d) Surface antigen analysis by flow cytometry.
Although flower-shaped plasma cells are rare, several cases have been reported [3,4,5,6,7,8,9,10,11]. No studies have investigated the morphological differences across multiple sites within the same patient presenting flower-shaped plasma cells. MM is known to exhibit intra-patient and intra-tumor heterogeneity; however, few reports describe morphological diversity based on the sites of tumor involvement [8,12]. In our patient, a distinctive flower-shaped morphology was observed in the bone marrow, enabling the identification of morphological differences depending on the site of tumor involvement. Our case suggests that the morphology of plasma cells may vary according to the lesion site. On the other hand, it should be noted that the differences in the specimen-collection methods between biopsy and aspiration may also influence its morphological findings. Further investigations involving biopsies from multiple sites are warranted to explore the morphological heterogeneity of MM showing flower-shaped nuclei.
Author Contributions
Conceptualization, H.H.; methodology, H.H., M.T., R.I., and N.M.; investigation, H.H., R.I., and N.M.; resources, H.H., M.T., M.S., R.I., N.M., S.M., and T.M.; writing—original draft preparation, H.H.; writing—review and editing, M.T., S.M., T.M., A.N., S.-I.M., and T.S.; supervision, A.N., S.-I.M., and T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Japan Society for the Promotion of Science (JSPS) (KAKENHI, Grant Number 21K16248) and a grant from the Takeda Science Foundation to H.H.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki. As this was a single-case report, ethical review and approval were waived.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the patient and clinical staff at Wakayama Medical University Hospital for their participation in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rajkumar, S.V. Multiple myeloma: 2024 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2024, 99, 1802–1824. [Google Scholar] [CrossRef] [PubMed]
- El Hussein, S.; Medeiros, L.J.; Hu, S.; Lin, P.; Wang, W. The many faces of plasma cell neoplasms: Morphological and immunophenotypical variants of the great imitator. Pathology 2022, 54, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Shibusawa, M. Plasma cell leukaemia presenting as flower-shaped plasma cells mimicking adult T-cell leukaemia or lymphoma. Lancet Haematol. 2020, 7, e270. [Google Scholar] [CrossRef] [PubMed]
- De Miguel Sanchez, C.; Robles de Castro, D.; Pison Herrero, C.; Perez Persona, E.; Salcedo Cuesta, L.; Guinea de Castro, J.M. Primary plasma cell leukaemia presenting with flower-shaped nuclei. Br. J. Haematol. 2021, 193, 689. [Google Scholar] [CrossRef] [PubMed]
- Zukerberg, L.R.; Ferry, J.A.; Conlon, M.; Harris, N.L. Plasma cell myeloma with cleaved, multilobated, and monocytoid nuclei. Am. J. Clin. Pathol. 1990, 93, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.A.; Pappas, A.A.; Flick, J.T.; Butch, A.W. A case of aggressive multiple myeloma with cleaved, multilobated, and monocytoid nuclei, and no serum monoclonal gammopathy. Ann. Clin. Lab. Sci. 2000, 30, 283–288. [Google Scholar] [PubMed]
- Delhommeau, F.; Huguet, S.; Gachet, J.; van den Akker, J.; Lagrange, M. Primary plasma cell leukemia mimicking an adult T-cell leukemia-lymphoma: A case report. Acta Cytol. 2010, 54, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Hussain, N.; Rahman, K.; Nityanand, S. Plasma cell myeloma with unusual morphology—A series of 6 cases. Eur. J. Haematol. 2014, 93, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Fujimi, A.; Nagamachi, Y.; Yamauchi, N.; Kanisawa, Y. Morphological Transformation of Myeloma Cells into Multilobated Plasma Cell Nuclei within 7 Days in a Case of Secondary Plasma Cell Leukemia That Finally Transformed as Anaplastic Myeloma. Case Rep. Hematol. 2017, 2017, 5758368. [Google Scholar] [CrossRef] [PubMed]
- Sall, A.; Seck, M.; Samb, D.; Faye, B.; Gadji, M.; Diop, S.; Toure, A.O. Flower-Like Plasma Cell Nuclei in Multiple Myeloma. Turk. J. Haematol. 2021, 38, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Jakovic, L.; Jovanovic, J.; Kurtovic, N.K.; Fekete, M.D.; Bogdanovic, A. Primary plasma cell leukemia presented with atypical flower-like morphology. Int. J. Lab. Hematol. 2024, 46, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.J.; Kumar, S. High-risk multiple myeloma: Redefining genetic, clinical, and functional high-risk disease in the era of molecular medicine and immunotherapy. Am. J. Hematol. 2024, 99, 1560–1575. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).