Hypoxia-Regulated Proteins: Expression in Endometrial Cancer and Their Association with Clinicopathologic Features
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. TMA and Immunohistochemical Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
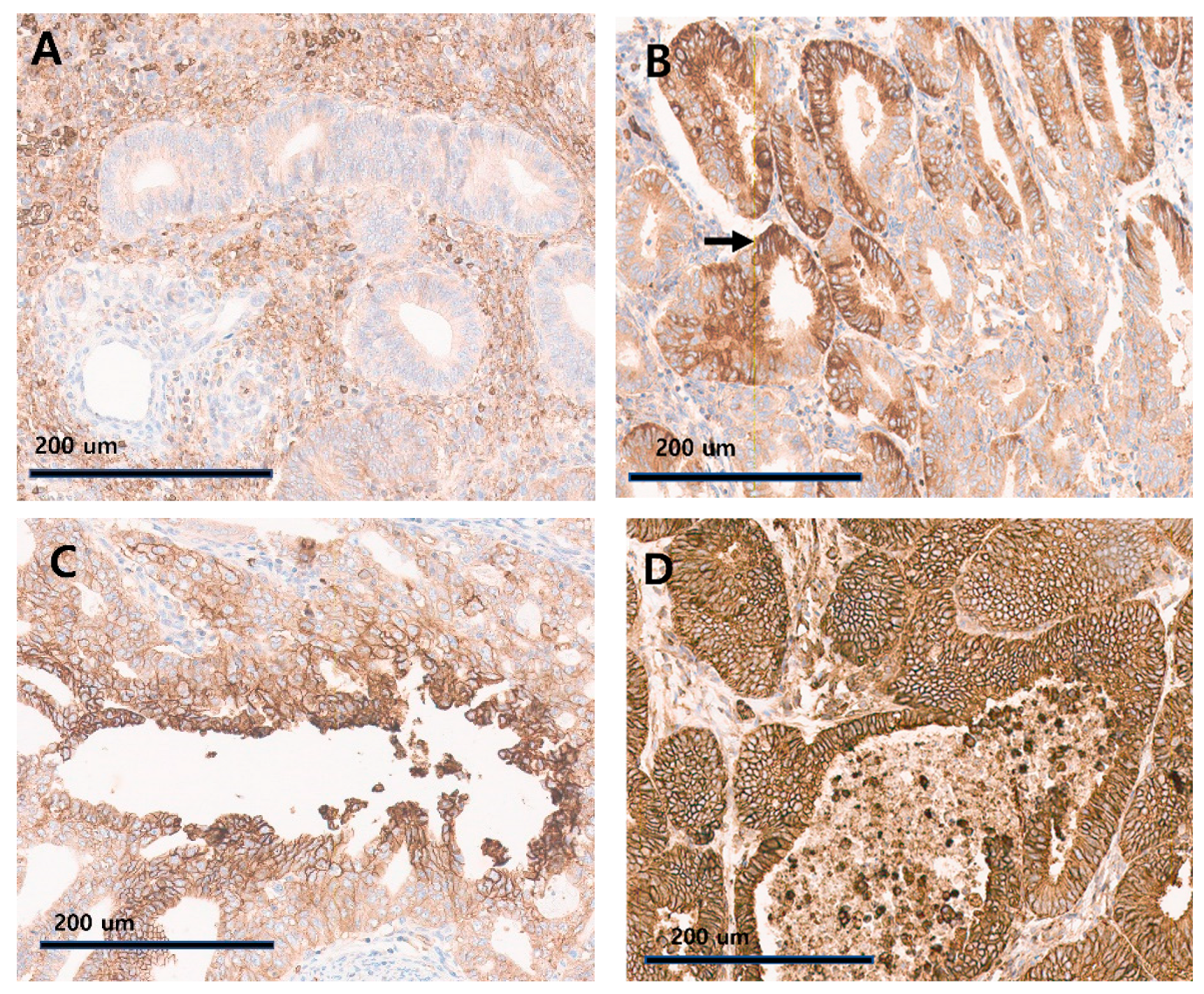
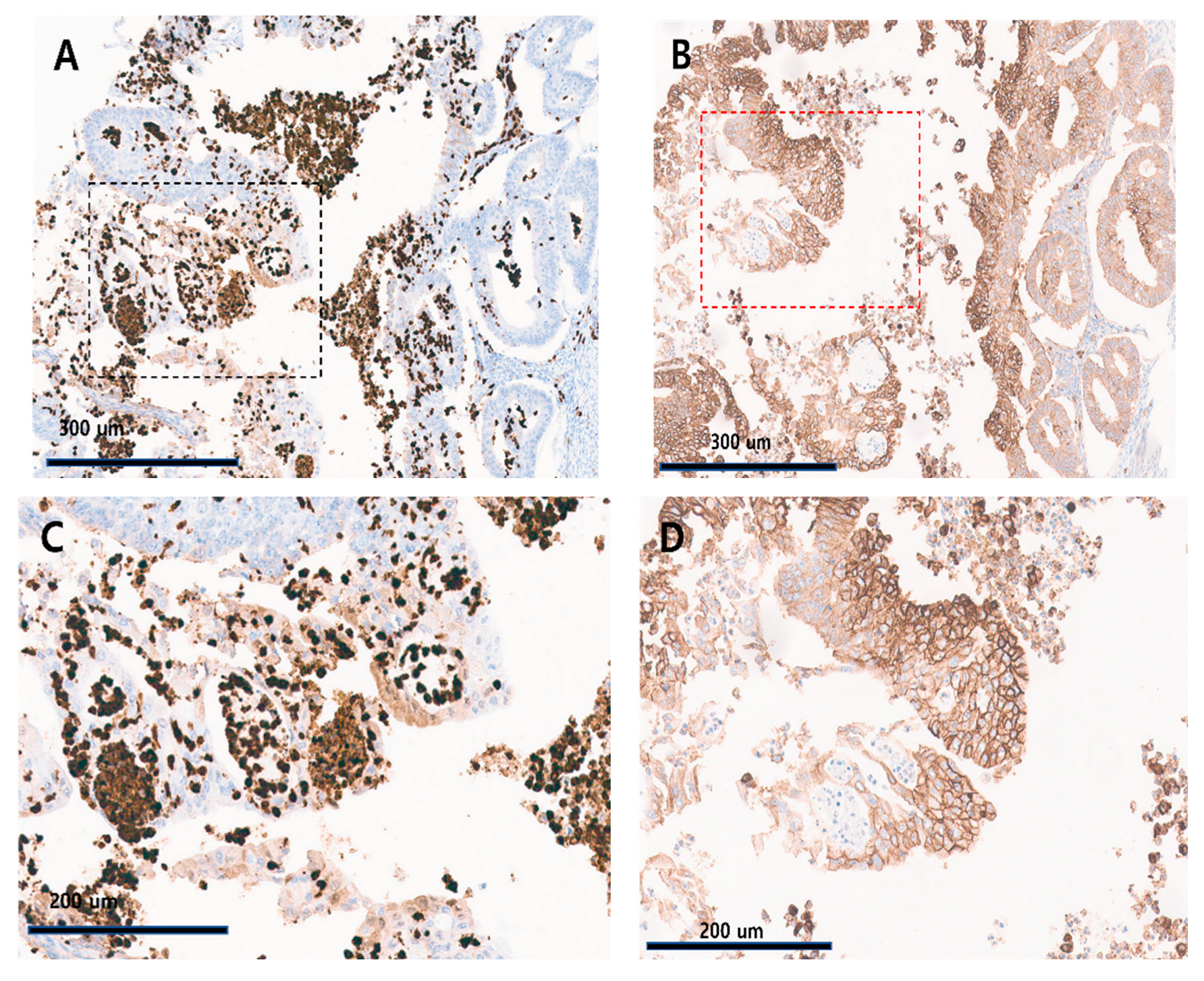
3.2. Association of GLUT-1, HIF-1α Expression, and FIGO Histologic Grade
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.I.; Chang, H.K.; Park, S.J.; Lim, J.; Won, Y.J.; Lim, M.C. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea Central Cancer Registry. Obstet. Gynecol. Sci. 2021, 64, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Bae, S.H.; Jeong, J.W.; Kim, S.H.; Kim, K.W. Hypoxia-inducible factor (HIF-1)alpha: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29 (Suppl. S16), 15–18. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Robey, I.F.; Lien, A.D.; Welsh, S.J.; Baggett, B.K.; Gillies, R.J. Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia 2005, 7, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.T.; Wang, Z.; Nuyten, D.S.; Rodriguez, E.H.; Schaner, M.E.; Salim, A.; Wang, Y.; Kristensen, G.B.; Helland, A.; Borresen-Dale, A.L.; et al. Gene expression programs in response to hypoxia: Cell type specificity and prognostic significance in human cancers. PLoS Med. 2006, 3, e47. [Google Scholar] [CrossRef]
- Chen, C.; Pore, N.; Behrooz, A.; Ismail-Beigi, F.; Maity, A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J. Biol. Chem. 2001, 276, 9519–9525. [Google Scholar] [CrossRef]
- Hayashi, M.; Sakata, M.; Takeda, T.; Yamamoto, T.; Okamoto, Y.; Sawada, K.; Kimura, A.; Minekawa, R.; Tahara, M.; Tasaka, K.; et al. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells. J. Endocrinol. 2004, 183, 145–154. [Google Scholar] [CrossRef]
- Thorens, B.; Mueckler, M. Glucose transporters in the 21st Century. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E141–E145. [Google Scholar] [CrossRef] [PubMed]
- Airley, R.E.; Mobasheri, A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: Novel pathways and targets for anticancer therapeutics. Chemotherapy 2007, 53, 233–256. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 2009, 121, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Ohba, S.; Fujii, H.; Ito, S.; Fujimaki, M.; Matsumoto, F.; Furukawa, M.; Yokoyama, J.; Kusunoki, T.; Ikeda, K.; Hino, O. Overexpression of GLUT-1 in the invasion front is associated with depth of oral squamous cell carcinoma and prognosis. J. Oral Pathol. Med. 2010, 39, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Krzeslak, A.; Wojcik-Krowiranda, K.; Forma, E.; Jozwiak, P.; Romanowicz, H.; Bienkiewicz, A.; Brys, M. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers. Pathol. Oncol. Res. 2012, 18, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Han, Y.; Li, Q.; Lin, R.; Zhang, J.; Yang, Y.; Wang, X.; Zhang, L. Prognostic impact of the combination of HIF-1alpha and GLUT1 in patients with oesophageal squamous cell carcinoma. Oncol. Lett. 2023, 26, 404. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Li, M.; Xu, X.J.; Xuan, L.; Huang, G.N.; Song, X.L.; Liu, Q.F. HIF-1alpha and GLUT1 gene expression is associated with chemoresistance of acute myeloid leukemia. Asian Pac. J. Cancer Prev. 2014, 15, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Shen, L.; Ren, Q.; Zeng, Q.; He, X. Prognostic and Clinicopathological Significance of Hypoxia-Inducible Factor-1alpha in Endometrial Cancer: A Meta-Analysis. Front. Oncol. 2020, 10, 587420. [Google Scholar] [CrossRef] [PubMed]
- Ozbudak, I.H.; Karaveli, S.; Simsek, T.; Erdogan, G.; Pestereli, E. Neoangiogenesis and expression of hypoxia-inducible factor 1alpha, vascular endothelial growth factor, and glucose transporter-1 in endometrioid type endometrium adenocarcinomas. Gynecol. Oncol. 2008, 108, 603–608. [Google Scholar] [CrossRef]
- Khabaz, M.N.; Qureshi, I.A.; Al-Maghrabi, J.A. GLUT 1 expression is a supportive mean in predicting prognosis and survival estimates of endometrial carcinoma. Ginekol. Pol. 2019, 90, 582–588. [Google Scholar] [CrossRef]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF transcription factors, inflammation, and immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Bertero, L.; Massa, F.; Metovic, J.; Zanetti, R.; Castellano, I.; Ricardi, U.; Papotti, M.; Cassoni, P. Eighth Edition of the UICC Classification of Malignant Tumours: An overview of the changes in the pathological TNM classification criteria-What has changed and why? Virchows Arch. 2018, 472, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Nakahama, K.; Osawa, M.; Izumi, M.; Yoshimoto, N.; Sugimoto, A.; Nagamine, H.; Ogawa, K.; Matsumoto, Y.; Sawa, K.; Tani, Y.; et al. SP142 evaluation contributes to the prediction of immune checkpoint inhibitor efficacy in non-small cell lung cancer with high PD-L1 expression assessed by 22C3. Transl. Lung Cancer Res. 2022, 11, 2438–2451. [Google Scholar] [CrossRef]
- Sigurjonsdottir, G.; De Marchi, T.; Ehinger, A.; Hartman, J.; Bosch, A.; Staaf, J.; Killander, F.; Nimeus, E. Comparison of SP142 and 22C3 PD-L1 assays in a population-based cohort of triple-negative breast cancer patients in the context of their clinically established scoring algorithms. Breast Cancer Res. 2023, 25, 123. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Shang, X.; Yan, M.; Li, X.; Wang, W.; Wang, Q.; Zhang, C. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990–2019. Gynecol. Oncol. 2021, 161, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278. [Google Scholar] [CrossRef]
- Zorzato, P.C.; Uccella, S.; Biancotto, G.; Bosco, M.; Festi, A.; Franchi, M.; Garzon, S. Intrauterine manipulator during hysterectomy for endometrial cancer: A systematic review and meta-analysis of oncologic outcomes. Am. J. Obstet. Gynecol. 2024, 230, 185–198.e4. [Google Scholar] [CrossRef]
- Bremond, A.; Bataillard, A.; Thomas, L.; Achard, J.L.; Fervers, B.; Fondrinier, E.; Lansac, J.; Bailly, C.; Hoffstetter, S.; Basuyau, J.P.; et al. Cancer of the endometrium. Br. J. Cancer 2001, 84 (Suppl. S2), 31–36. [Google Scholar] [CrossRef]
- McCluggage, W.G.; Singh, N.; Gilks, C.B. Key changes to the World Health Organization (WHO) classification of female genital tumours introduced in the 5th edition (2020). Histopathology 2022, 80, 762–778. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee; et al. FIGO staging of endometrial cancer. J. Gynecol. Oncol. 2023, 34, e85. [Google Scholar] [CrossRef] [PubMed]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Sarwar, M.; Thorat, N.D. Targeting the tumor microenvironment: Potential strategy for cancer therapeutics. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166746. [Google Scholar] [CrossRef] [PubMed]
- Eales, K.L.; Hollinshead, K.E.; Tennant, D.A. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016, 5, e190. [Google Scholar] [CrossRef] [PubMed]
- Moar, K.; Pant, A.; Saini, V.; Maurya, P.K. Potential biomarkers in endometrial cancer: A narrative review. Biomarkers 2023, 28, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Karpel, H.; Slomovitz, B.; Coleman, R.L.; Pothuri, B. Biomarker-driven therapy in endometrial cancer. Int. J. Gynecol. Cancer 2023, 33, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.W.; Lautner, M.H.; Schutze, A.; Taubert, H.; Schubert, J.; Bilkenroth, U. Coexpression of hypoxia-inducible factor-1alpha and glucose transporter-1 is associated with poor prognosis in oral squamous cell carcinoma patients. Histopathology 2011, 58, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Barlin, J.N.; Soslow, R.A.; Lutz, M.; Zhou, Q.C.; St Clair, C.M.; Leitao, M.M., Jr.; Iasonos, A.; Hensley, M.L.; Barakat, R.R.; Matias-Guiu, X.; et al. Redefining stage I endometrial cancer: Incorporating histology, a binary grading system, myometrial invasion, and lymph node assessment. Int. J. Gynecol. Cancer 2013, 23, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71. [Google Scholar] [CrossRef]
- Peyssonnaux, C.; Datta, V.; Cramer, T.; Doedens, A.; Theodorakis, E.A.; Gallo, R.L.; Hurtado-Ziola, N.; Nizet, V.; Johnson, R.S. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J. Clin. Investig. 2005, 115, 1806–1815. [Google Scholar] [CrossRef]
- Mughees, M.; Sengupta, A.; Khowal, S.; Wajid, S. Mechanism of tumour microenvironment in the progression and development of oral cancer. Mol. Biol. Rep. 2021, 48, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Xi, N.; Song, J.; Huang, W.; Song, S.; Liu, Y.; Liu, X.; Xie, Y. Partial Oxygen Pressure Affects the Expression of Prognostic Biomarkers HIF-1 Alpha, Ki67, and CK20 in the Microenvironment of Colorectal Cancer Tissue. Oxid. Med. Cell. Longev. 2016, 2016, 1204715. [Google Scholar] [CrossRef]
- Rose, P.G.; Ali, S.; Moslemi-Kebria, M.; Simpkins, F. Paclitaxel, Carboplatin, and Bevacizumab in Advanced and Recurrent Endometrial Carcinoma. Int. J. Gynecol. Cancer 2017, 27, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novak, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Raybuck, A.L.; Blagih, J.; Kemboi, E.; Haase, V.H.; Jones, R.G.; Boothby, M.R. Hypoxia-inducible factors in CD4(+) T cells promote metabolism, switch cytokine secretion, and T cell help in humoral immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 8975–8984. [Google Scholar] [CrossRef] [PubMed]
- Palazon, A.; Tyrakis, P.A.; Macias, D.; Velica, P.; Rundqvist, H.; Fitzpatrick, S.; Vojnovic, N.; Phan, A.T.; Loman, N.; Hedenfalk, I.; et al. An HIF-1alpha/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell 2017, 32, 669–683.e665. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Zhang, C.; Shan, W.; Qiu, X. T-Cell Exhaustion Status Under High and Low Levels of Hypoxia-Inducible Factor 1alpha Expression in Glioma. Front. Pharmacol. 2021, 12, 711772. [Google Scholar]
| Variables | Value (Median or Proportion) | ||
|---|---|---|---|
| Age | 35~78 (51) | ||
| Tumor size (cm) | 0.2~10 (3) | ||
| Invasion depth (mm) | 0.1~40 (3) | ||
| LVSI, n (%) | Negative | 46 (90.2%) | |
| Positive | 5 (9.8%) | ||
| T stage | 1a | 35 (68.6%) | |
| 1b | 11 (21.6%) | ||
| 2 | 3 (5.9%) | ||
| 3a | 1 (2%) | ||
| 3b | 1 (2%) | ||
| N stage | 0 | 46 (90.2%) | |
| 1 | 3 (5.9%) | ||
| 2 | 2 (3.9%) | ||
| FIGO stage, n (%) | IA | 34 (66.7%) | |
| IB | 9 (17.7%) | ||
| II | 2 (3.9%) | ||
| 3A | 1 (2.0%) | ||
| 3C | 5 (9.8%) | ||
| FIGO histologic grade | G1 | 34 (66.6%) | |
| G2 | 12 (23.5%) | ||
| G3 | 5 (9.8%) | ||
| GLUT-1 pattern * | Mosaic | 6 (12.0%) | |
| Central | 28 (56.0%) | ||
| Diffuse | 16 (32.0%) | ||
| HIF-1 α ** | TC a | ≤1% | 32 (65.3%) |
| ≤5% | 40 (81.6%) | ||
| IC b | ≤1% | 16 (32.7%) | |
| ≤5% | 31 (63.3%) |
| Non-Tumorous Epithelium | Endometrioid Carcinoma | |||||
|---|---|---|---|---|---|---|
| G1 c | G2 | G3 | p-Value | |||
| GLUT-1 pattern | Mosaic | 0 * | 6 | 0 | 0 | 0.365 |
| Central | 0 | 18 | 6 | 4 | ||
| Diffuse | 0 | 10 | 5 | 1 | ||
| TCs a of HIF-1α | ≤1% | 3 ** | 23 | 5 | 4 | 0.263 |
| >1% | 0 | 10 | 6 | 1 | ||
| ICs b of HIF-1α | ≤1% | 3 | 14 | 0 | 2 | 0.032 |
| >1% | 0 | 19 | 11 | 3 | ||
| The Proportion of Cytoplasmic HIF-1α-Positive Immune Cells | |||||||
|---|---|---|---|---|---|---|---|
| ≤1% | >1% | p-Value | ≤5% | >5% | p-Value | ||
| Age (years) | ≤51 | 9 | 16 | 0.610 | 16 | 9 | 0.913 |
| >51 | 7 | 17 | 15 | 9 | |||
| Tumor size (cm) | ≤3 | 7 | 15 | 0.910 | 14 | 8 | 0.961 |
| >3 | 9 | 18 | 17 | 10 | |||
| MI | <1/2 | 9 | 13 | 0.386 | 16 | 6 | 0.140 |
| ≥1/2 | 5 | 13 | 9 | 9 | |||
| Histologic grade | 1 | 14 | 19 | 0.036 | 24 | 9 | 0.048 |
| 2 or 3 | 2 | 14 | 7 | 9 | |||
| GLUT-1 pattern | Central, mosaic | 12 | 21 | 0.426 | 24 | 9 | 0.048 |
| Diffuse | 4 | 12 | 7 | 9 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.H.; Jo, J.Y.; Kim, C.H.; Kim, M.H.; Cho, I.A.; Shin, J.K.; Choi, W.J.; Baek, J.C. Hypoxia-Regulated Proteins: Expression in Endometrial Cancer and Their Association with Clinicopathologic Features. Diagnostics 2024, 14, 1735. https://doi.org/10.3390/diagnostics14161735
Song DH, Jo JY, Kim CH, Kim MH, Cho IA, Shin JK, Choi WJ, Baek JC. Hypoxia-Regulated Proteins: Expression in Endometrial Cancer and Their Association with Clinicopathologic Features. Diagnostics. 2024; 14(16):1735. https://doi.org/10.3390/diagnostics14161735
Chicago/Turabian StyleSong, Dae Hyun, Jae Yoon Jo, Cho Hee Kim, Min Hye Kim, In Ae Cho, Jeong Kyu Shin, Won Jun Choi, and Jong Chul Baek. 2024. "Hypoxia-Regulated Proteins: Expression in Endometrial Cancer and Their Association with Clinicopathologic Features" Diagnostics 14, no. 16: 1735. https://doi.org/10.3390/diagnostics14161735
APA StyleSong, D. H., Jo, J. Y., Kim, C. H., Kim, M. H., Cho, I. A., Shin, J. K., Choi, W. J., & Baek, J. C. (2024). Hypoxia-Regulated Proteins: Expression in Endometrial Cancer and Their Association with Clinicopathologic Features. Diagnostics, 14(16), 1735. https://doi.org/10.3390/diagnostics14161735






