Evaluation and Limitations of the Novel Chemiluminescent Enzyme Immunoassay Technique for Measuring Total Tau Protein in the Cerebrospinal Fluid of Patients with Human Prion Disease: A 10-Year Prospective Study (2011–2020)
Abstract
1. Background
2. Methods
2.1. Aim, Design, and Setting of the Study
2.2. Ethical Statement
2.3. Patients
2.4. CLEA Measurement of T-Tau Protein in the CSF of 60 Patients
2.5. ELISA of T-Tau Protein in the CSF of 60 Patients
2.6. Biochemical Analysis of CSF Samples (T-Tau Protein by ELISA and 14-3-3 Protein Using Western Blotting and RT-QuIC Assay)
2.7. Statistical Analysis
- -
- In terms of reproducibility, the standard deviation (SD) ranged from 10.7 to 26.4, with a coefficient of variation (CV) ranging from 1.2 to 3.7%, consistently remaining below the threshold of 4.2.
- -
- For future reproducibility, the SD values ranged from 10.1 to 31.7, with a CV range between 1.9 and 4.2%, indicating consistent CV values below the threshold of 5.3.
- -
- Regarding storage stability at different temperatures, T-tau protein measurements remained relatively stable for up to 4 days at −20 °C and −80 °C but decreased by approximately 5% after storing for 29 days compared to measurements taken at 14 °C.
- -
- Dilution capability varied among different types; however, most dilution ratios fell within the range of 43 to 49%, with deionized water being closest to a 50% dilution ratio.
- -
- Resistance against blood contamination: even in cases where there is up to 10% whole blood contamination present, CLEIA detection remains highly sensitive in identifying cerebrospinal fluid samples with variations lower than CV (3%).
3. Results
3.1. Patient Profiles
3.2. Simultaneous Reproducibility of T-Tau Protein Measurements with CLEA in the Same Aliquots of the Control and Test Samples
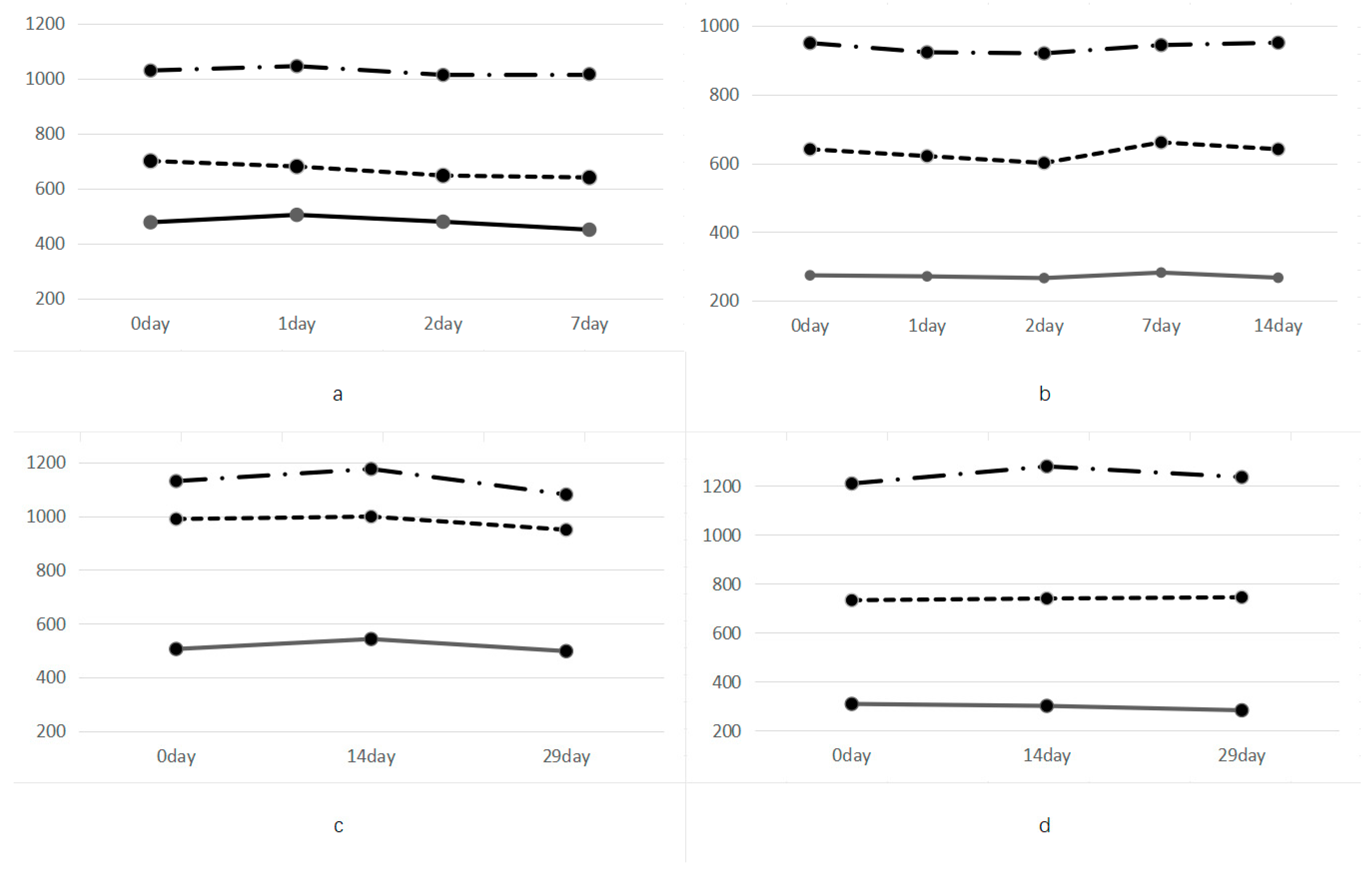
3.3. Stability of T-Tau Protein CLEA Measurements in the Control and Test Samples
3.4. Storage Stability of CSF Samples
3.5. Reproducibility of the Results with T-Tau Protein Dilution in CSF Samples
3.6. Similarities between ELISA and CLEA
3.7. Results of Biomarker Measurements in the CSF of All Patients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| AUC | relative area under the curve |
| CI | confidence interval |
| CJD | Creutzfeldt–Jakob disease |
| CLEA | chemiluminescent enzyme immunoassay |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| CV | coefficient of variation |
| Diff | difference |
| DNA | deoxyribonucleic acid |
| EEG | electroencephalogram |
| ELISA | enzyme-linked immunosorbent assay |
| FFI | fatal familial insomnia |
| gCJD | genetic CJD |
| HPD | human prion disease |
| NPV | negative predictive value |
| PBS | phosphate-buffered saline |
| PCR | polymerase chain reaction |
| PPV | positive predictive value |
| prion PrP | protein |
| PrPC | cellular prion protein |
| PrPSc | scrapie prion protein |
| Rcf | relative centrifugal force rel. |
| Ref | reference |
| Rfu | relative fluorescence units |
| ROC | receiver operating characteristic |
| Rpm | revolutions per minute |
| RT-QuIC | real-time quaking-induced conversion |
| sCJD | sporadic CJD |
| SD | standard deviation |
| SEM | Standard error of the mean |
References
- McGuire, L.I.; Poleggi, A.; Poggiolini, I.; Suardi, S.; Grznarova, K.; Shi, S.; Satoh, K.; Cheng, K.; Cramm, M.; Fairfoul, G.; et al. Cerebrospinal fluid real-time quaking-induced conversion is a robust and reliable test for sporadic Creutzfeldt–Jakob disease: An international study. Ann Neurol. 2016, 80, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. Molecular biology of prion diseases. Science 1991, 252, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Atarashi, R.; Nishida, N. Real-time quaking-induced conversion for diagnosis of prion disease. Methods Mol. Biol. 2017, 1658, 305–310. [Google Scholar] [PubMed]
- Wang, Z.B.; Tan, L.; Gao, P.Y.; Ma, Y.H.; Fu, Y.; Sun, Y.; Yu, J.T. Associations of the A/T/N profiles in PET, CSF, and plasma biomarkers with Alzheimer’s disease neuropathology at autopsy. Alzheimer’s Dement. 2023, 19, 4421–4435. [Google Scholar] [CrossRef] [PubMed]
- Terzapulo, X.; Kassenova, A.; Bukasov, R. Immunoassays: Analytical and clinical performance, challenges, and perspectives of SERS detection in comparison with fluorescent spectroscopic detection. Int. J. Mol. Sci. 2024, 24, 2080. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, J.; Eremin, S.; Dias, A.C.P.; Zhang, X. Development of ELISA and chemiluminescence enzyme immunoassay for quantification of histamine in drug products and food samples. Anal. Bioanal. Chem. 2020, 412, 4739–4747. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.; Iha, K.; Yoshimura, T.; Nakaishi, K.; Watabe, S. Early diagnosis with ultrasensitive ELISA. Adv. Clin. Chem. 2021, 101, 121–133. [Google Scholar]
- Faustini, S.E.; Jossi, S.E.; Perez-Toledo, M.; Shields, A.M.; Allen, J.D.; Watanabe, Y.; Newby, M.L.; Cook, A.; Willcox, C.R.; Salim, M.; et al. Development of a high-sensitivity ELISA detecting IgG, IgA and IgM antibodies to the SARS-CoV-2 spike glycoprotein in serum and saliva. Immunology 2021, 164, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Paciotti, S.; Sepe, F.N.; Eusebi, P.; Farotti, L.; Cataldi, S.; Gatticchi, L.; Parnetti, L. Diagnostic performance of a fully automated chemiluminescent enzyme immunoassay for Alzheimer’s disease diagnosis. Clin. Chim. Acta 2019, 494, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Dakterzada, F.; López-Ortega, R.; Arias, A.; Riba-Llena, I.; Ruiz-Julián, M.; Huerto, R.; Tahan, N.; Piñol-Ripoll, G. Assessment of the concordance and diagnostic accuracy between Elecsys and Lumipulse fully automated platforms and innotest. Front. Aging Neurosci. 2021, 13, 604119. [Google Scholar] [CrossRef] [PubMed]
- Tapari, A.; Braliou, G.G.; Papaefthimiou, M.; Mavriki, H.; Kontou, P.I.; Nikolopoulos, G.K.; Bagos, P.G. Performance of antigen detection tests for SARS-CoV-2: A systematic review and meta-analysis. Diagnostics 2022, 12, 1388. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, R.; Satoh, K.; Sano, K.; Fuse, T.; Yamaguchi, N.; Ishibashi, D.; Matsubara, T.; Nakagaki, T.; Yamanaka, H.; Shirabe, S.; et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat. Med. 2011, 17, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K. CSF biomarkers for prion diseases. Neurochem. Int. 2022, 155, 105306. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Satoh, K.; Atarashi, R.; Takashima, H.; Iwasaki, Y.; Yoshida, M.; Sanjo, N.; Murai, H.; Mizusawa, H.; Schmitz, M.; et al. Early detection of abnormal prion protein in genetic human prion diseases now possible using real-time QUIC assay. PLoS ONE 2013, 8, e54915. [Google Scholar]
- Cramm, M.; Schmitz, M.; Karch, A.; Mitrova, E.; Kuhn, F.; Schroeder, B.; Raeber, A.; Varges, D.; Kim, Y.S.; Satoh, K.; et al. Stability and reproducibility underscore utility of RT-QuIC for diagnosis of Creutzfeldt-Jakob disease. Mol. Neurobiol. 2016, 53, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Takemura, M.; Sato, M.; Yamada, Y.; Nakagawa, T.; Horibe, T.; Hoshi, M.; Otaki, H.; Ito, H.; Seishima, M.; et al. Comparison of chemiluminescence enzyme immunoassay (CLEIA) with ELISA for the determination of anti-cyclic citrullinated peptide antibodies. Clin. Chim. Acta 2010, 411, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.T.T.; Satoh, K. The latest research on RT-QuIC assays—A literature review. Pathogens 2021, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.; Appleby, B.; Brandel, J.-P.; Caughey, B.; Collins, S.; Geschwind, M.D.; Green, A.; Haïk, S.; Kovacs, G.G.; Ladogana, A.; et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 2021, 20, 235–246. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Sanjo, N.; Ae, R.; Nakamura, Y.; Sakai, K.; Takao, M.; Murayama, S.; Iwasaki, Y.; Satoh, K.; Murai, H.; et al. MM2-type sporadic Creutzfeldt-Jakob disease: New diagnostic criteria for MM2-cortical type. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1158–1165. [Google Scholar] [CrossRef]
- Higuma, M.; Sanjo, N.; Satoh, K.; Shiga, Y.; Sakai, K.; Nozaki, I.; Hamaguchi, T.; Nakamura, Y.; Kitamoto, T.; Shirabe, S.; et al. Relationships between clinicopathological features and cerebrospinal fluid biomarkers in Japanese patients with genetic prion diseases. PLoS ONE 2013, 8, e60003. [Google Scholar] [CrossRef]

| Control Samples | Test Samples | |||||
|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | |
| 1 | 311 | 511 | 822 | 452 | 660 | 1246 |
| 2 | 320 | 535 | 855 | 440 | 693 | 1249 |
| 3 | 330 | 533 | 854 | 444 | 685 | 1230 |
| 4 | 342 | 546 | 829 | 450 | 707 | 1299 |
| 5 | 325 | 539 | 861 | 457 | 687 | 1242 |
| 6 | 346 | 532 | 857 | 446 | 708 | 1213 |
| Number | 6 | 6 | 6 | 6 | 6 | 6 |
| Min | 311 | 511 | 822 | 440 | 660 | 1213 |
| Max | 346 | 546 | 861 | 457 | 708 | 1299 |
| Mean | 329.0 | 532.7 | 846.3 | 448.2 | 690.0 | 1246.5 |
| SD | 12.1 | 10.7 | 15.0 | 5.6 | 16.1 | 26.4 |
| CV (%) | 3.7 | 2.0 | 1.8 | 1.2 | 2.3 | 2.1 |
| Control Samples | Test Samples | |||||
|---|---|---|---|---|---|---|
| Aliquot 1 | Aliquot 2 | Aliquot 3 | Aliquot 4 | Aliquot 5 | Aliquot 6 | |
| Day 1-1 | 302 | 515 | 817 | 347 | 877 | 1066 |
| Day 1-2 | 307 | 512 | 843 | 390 | 916 | 1157 |
| Day 2-1 | 315 | 520 | 818 | 346 | 884 | 1114 |
| Day 2-2 | 320 | 515 | 793 | 347 | 897 | 1092 |
| Day 3-1 | 312 | 488 | 805 | 348 | 855 | 1067 |
| Day 3-2 | 321 | 496 | 824 | 374 | 892 | 1107 |
| Day 4-1 | 333 | 536 | 833 | 372 | 876 | 1119 |
| Day 4-2 | 333 | 543 | 876 | 380 | 897 | 1103 |
| Day 5-1 | 308 | 535 | 829 | 353 | 913 | 1152 |
| Day 5-2 | 327 | 531 | 836 | 361 | 889 | 1154 |
| n | 10 | 10 | 10 | 10 | 10 | 10 |
| Min | 302 | 488 | 793 | 346 | 855 | 1066 |
| Max | 333 | 543 | 876 | 390 | 916 | 1157 |
| Mean | 317.8 | 519.1 | 827.4 | 361.8 | 889.6 | 1113.1 |
| SD | 10.3 | 16.8 | 21.5 | 15.3 | 17.1 | 31.7 |
| CV (%) | 3.2 | 3.2 | 2.6 | 4.2 | 1.9 | 2.9 |
| (a) | ||||
| Total tau protein measured using CLEA without dilution | 1/2 dilution with PBS | 1/2 dilution with pure water | 1/2 dilution with dissolving solution contained in the kit | |
| #1 | 533 | 240 (0.450) | 250 (0.469) | 243 (0.456) |
| #2 | 628 | 295 (0.470) | 301 (0.479) | 308 (0.490) |
| #3 | 629 | 273 (0.434) | 289 (0.459) | 287 (0.456) |
| (b) | ||||
| Total tau protein measured using CLEA without dilution | 1/2 dilution with pure water | 1/4 dilution with pure water | 1/8 dilution with pure water | |
| #4 | 1806 | 875 (0.484) | 416 (0.230) | 177 (0.098) |
| #5 | 1826 | 892 (0.488) | 419 (0.229 | 187 (0.102) |
| #6 | 1876 | 915 (0.484) | 435 (0.232) | 187 (0.100) |
| RT-QuIC Assay | 14-3-3 Protein by Western Blotting | Total Tau Protein by ELISA | Total Tau Protein by CLEA | ||
|---|---|---|---|---|---|
| First Generation | Second Generation | (Cutoff: 1300 pg/mL) | (Cutoff: 1000 pg/mL) | ||
| Sensitivity | 100% | 100% | 100% | 100% | 100% |
| Specificity | 100% | 100% | 100% | 100% | 97.8% |
| Group | t0 | t1 | t2 | |
| 1 | 694 | 682 | 739 | |
| 2 | 678 | 705 | 738 | |
| t0 | t1 | t2 | ||
| N | valid | 2 | 2 | 2 |
| invalid | 0 | 0 | 0 | |
| Mean | 686 | 693.5 | 738.5 | |
| SEM | 8 | 11.5 | 0.5 | |
| Median | 686 | 693.5 | 738.5 | |
| SD | 11.314 | 16.263 | 0.707 | |
| CV (%) | 1.649 | 2.345 | 0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weijie, K.; Nonaka, T.; Satoh, K. Evaluation and Limitations of the Novel Chemiluminescent Enzyme Immunoassay Technique for Measuring Total Tau Protein in the Cerebrospinal Fluid of Patients with Human Prion Disease: A 10-Year Prospective Study (2011–2020). Diagnostics 2024, 14, 1520. https://doi.org/10.3390/diagnostics14141520
Weijie K, Nonaka T, Satoh K. Evaluation and Limitations of the Novel Chemiluminescent Enzyme Immunoassay Technique for Measuring Total Tau Protein in the Cerebrospinal Fluid of Patients with Human Prion Disease: A 10-Year Prospective Study (2011–2020). Diagnostics. 2024; 14(14):1520. https://doi.org/10.3390/diagnostics14141520
Chicago/Turabian StyleWeijie, Kong, Toshiaki Nonaka, and Katsuya Satoh. 2024. "Evaluation and Limitations of the Novel Chemiluminescent Enzyme Immunoassay Technique for Measuring Total Tau Protein in the Cerebrospinal Fluid of Patients with Human Prion Disease: A 10-Year Prospective Study (2011–2020)" Diagnostics 14, no. 14: 1520. https://doi.org/10.3390/diagnostics14141520
APA StyleWeijie, K., Nonaka, T., & Satoh, K. (2024). Evaluation and Limitations of the Novel Chemiluminescent Enzyme Immunoassay Technique for Measuring Total Tau Protein in the Cerebrospinal Fluid of Patients with Human Prion Disease: A 10-Year Prospective Study (2011–2020). Diagnostics, 14(14), 1520. https://doi.org/10.3390/diagnostics14141520





